Abstract
We have developed methods to transiently and selectably transform the human-infective protist Trichomonas vaginalis. This parasite, a common cause of vaginitis worldwide, is one of the earlier branching eukaryotes studied to date. We have introduced three heterologous genes into T. vaginalis by electroporation and have used the 5′ and 3′ untranslated regions of the endogenous gene α-succinyl CoA synthetase B (α-SCSB) to drive transcription of these genes. Transient expression of two reporter proteins, chloramphenicol acetyltransferase (CAT) or luciferase, was detected when electroporating in the presence of 50 μg closed-circular construct. Optimal levels of expression were observed using ≈2.5 × 108 T. vaginalis cells and 350 volts, 960 μFd for electroporation; however, other conditions also led to significant reporter gene expression. A time course following the expression of CAT in T. vaginalis transient transformants revealed the highest level of expression 8–21 hr postelectroporation and showed that CAT activity is undetectable using TLC by 99 hr postelectroporation. The system we established to obtain selectable transformants uses the neomycin phosphotransferase (neo) gene as the selectable marker. Cells electroporated with 20 μg of the NEO construct were plated in the presence of 50 μg/ml paromomycin and incubated in an anaerobic chamber. The paromomycin-resistant colonies that formed within 3–5 days were cultivated in the presence of drug and DNA was isolated for analyses. The NEO construct was shown to be maintained episomally, as a closed-circle, at between 10–30 copies per cell. The ability to transiently and selectably transform T. vaginalis should greatly enhance research on this important human parasite.
Keywords: electroporation, chloramphenicol acetyltransferase, luciferase, neomycin phosphotransferase, parasite
Trichomonas vaginalis is one of the earliest-diverging eukaryotes studied to date (1, 2). This organism, a flagellated protist belonging to the group Parabasalia, is parasitic to humans. T. vaginalis is transmitted from host to host by sexual intercourse and is a common cause of vaginitis worldwide. In addition to its medical importance, a number of unusual properties of trichomonads have captured the attention of scientists interested in energy metabolism, organelle biogenesis, gene transcription, drug-resistance, and pathogenesis. The appeal of Trichomonas from a biological viewpoint stems, in large part, from properties that reflect both its primitive nature and its parasitic lifestyle. For example, trichomonads lack two organelles typically found in eukaryotes, the mitochondrion and the peroxisome, but instead contain an organelle involved in carbohydrate metabolism called the hydrogenosome (3, 4). Interestingly, the hydrogenosome and mitochondrion appear to have evolved from a common endosymbiont (5–8). Recent molecular analyses have also revealed two unusual features regarding gene transcription in trichomonads. All examined protein-coding genes have a conserved motif that surrounds the start site of transcription, which appears to play a critical role in transcription initiation (9). Also, trichomonads contain an atypical RNA polymerase II and transcription of protein-coding genes is α-amanitin insensitive, unlike that observed for most eukaryotes (10).
Given its evolutionary status, T. vaginalis could provide an excellent model system for examining the evolution of eukaryotes. Although the application of recombinant DNA technology during the last decade has greatly increased our knowledge of trichomonads, the lack of a genetic system still hinders research progress. Here we describe the development of both transient and selectable transformation assays for this important human parasite. We demonstrate the applicability of two reporter genes, chloramphenicol acetyltransferase (cat) and luciferase (luc), for studies using transient transformation. We have also established a selectable transformation procedure that allows rapid isolation of clonal populations expressing the reporter gene neomycin phosphotransferase (neo). The ability to use transformation to study the variety of intriguing biochemical processes presented by trichomonads promises to expand our understanding of the biology of early diverging eukaryotes.
MATERIALS AND METHODS
Plasmid Construction.
Three plasmids were constructed using a genomic clone of the α-succinyl CoA synthetase B (α-SCSB) gene (11) to provide 5′ and 3′ untranslated regions (UTRs) of 1660 and 449 nucleotides, respectively. Primers corresponding to 5′ and 3′ ends of the α-SCSB gene with either a KpnI (5′ end primer) or a BamHI (3′ end primer) restriction enzyme site added at their 5′ ends were used in an inverse PCR to amplify the 5′ and 3′ α-SCSB UTRs and the pBluescript vector. These primers were 5′-GATCGGTACCTTGGATAACAACTCTTGT-3′ and 5′-GATCGGATCCATCTACTGCTTACTTTAA-3′. The 5.15-kb product of the inverse PCR, called T. vaginalis expression vector 1, was purified and digested with KpnI and BamHI. The coding regions of cat, luc, and neo were derived from pBLCAT3 (12), pDR100 (13), and pKm2 (14), respectively. Each plasmid was used as a template in PCR using primers with a KpnI (5′ end primer) or a BamHI (3′ end primer) restriction enzyme site added at their 5′ ends. Primers used to amplify the cat gene were 5′-GGCCGGTACCATGGAGAAAAAAATCACT-3′ and 5′-GATCGGATCCTTACGCCCCGCCCTGCCA-3′. Primers used to amplify the luc gene were 5′-GGCCGGTACCATGGAAGACGCCAAAAAC-3′ and 5′-GATCGGATCCTTACAATTTGGACTTTCC-3′. Primers used to amplify the neo gene were 5′-GATCGGTACCATGATTGAACAAGATGGATTG-3′ and 5′-GATCGGATCCTCAGAAGAACTCGTCAAGAAG-3′. The resulting PCR products were digested with KpnI and BamHI and ligated into T. vaginalis expression vector 1 (see above). The derived constructs were named α-SCSB-CAT, α-SCSB-LUC, and α-SCSB-NEO, accordingly. Plasmid DNAs were purified (Maxi Kit, Qiagen, Chatsworth, CA) and the entire 5′ and 3′ UTRs and roughly the first 100 nucleotides of the reporter genes were sequenced (Sequenase, United States Biochemical).
Electroporation.
T. vaginalis strain C1 (ATCC no. 30001) was grown in Diamond’s medium (15) and cells were harvested by centrifugation at 1,500 × g for 10 min at 4°C. The pellet was weighed and resuspended in cold Diamond’s medium to a final concentration of 1 g cells/1.5 ml medium. Three hundred microliters of the cell suspension (≈2.5 × 108 cells) and closed-circular DNA constructs were electroporated using 350 volts and 960 μFd (unless otherwise stated) in 0.4 cm electrocuvettes using a Bio-Rad gene pulser and capacitance extender. DNA (50 μg) was used for electroporations with both α-SCSB-CAT and α-SCSB-LUC constructs and 20 μg of DNA was used for electroporations with the α-SCSB-NEO construct. Following electroporation with either the α-SCSB-CAT or the α-SCSB-LUC construct, cells were incubated on ice for 10 min and then diluted into 50 ml Diamond’s medium and incubated for 21 hr (unless otherwise stated) at 37°C. Cells were then harvested by centrifugation. Selectable transformants were obtained from cells electroporated with the α-SCSB-NEO construct as described below.
CAT Assay.
Transformants were tested for CAT activity by resuspending cells harvested 21 hr postelectroporation in 250 μl of 250 mM Tris, pH 7.8. Cells were lysed by three freeze-thaw cycles, alternating between incubation in dry ice/ethanol and at 37°C for 10 min each. Samples were heated to 65–67°C for 10 min to inactivate endogenous proteases in the extract. The extract was then centrifuged at 16,000 × g in a microcentrifuge at 4°C for 10 min, the supernatant was collected and a 20 μl sample was removed to determine protein concentration. The remainder of the extract was stored at −80°C. For the CAT assay, 75 μl of the heat-treated extract was added to 75 μl of 250 mM Tris (pH 7.8) followed by the addition of 3 μl 14C chloramphenicol (0.075 μCi, Amersham, 50–60 mCi/mmol; 1 Ci = 37 GBq) and 20 μl 4 mM acetyl CoA (Pharmacia) and the reaction was incubated at 37°C overnight. The reaction was then extracted with 1 ml ethyl acetate. Nine hundred microliters of the upper phase was transferred to a fresh tube and dried-down using a Speed Vac. The dried sample was resuspended in 25 μl ethyl acetate and spotted repeatedly in 5 μl aliquots onto a TLC plate (J. T. Baker, Phillipsburg, NJ). The TLC tank was pre-equilibrated with 95% chloroform:5% methanol. Upon addition of the 20-cm plate to the tank, the solvent was allowed to run up to 5 cm from the top. Plates were then dried and exposed to x-ray film. To quantitate the data, acetylated and unacetylated spots of 14C-labeled-chloramphenicol were cut from the TLC plate and subjected to liquid scintillation counting. The activity of the extracts was calculated and expressed as % acetylation/25 μg protein added to the CAT assay. Protein concentrations were determined using the Bradford assay (Bio-Rad).
Luciferase Assays.
Cells were assayed for luciferase activity using the luciferase Assay system from Promega with the following modifications. Cells were washed using PBS and resuspended in lysis buffer (Promega) supplemented with 50 μg/ml leupeptin (Boehringer Mannheim) at a concentration of 7.5 × 107 cells/ml buffer. The samples were frozen at −80°C for a minimum of 1 hr and then spun at 1,500 × g for 5 min.The supernatant (20 μl) was mixed with 100 μl of luciferase assay reagent (Promega) and light production was measured using a Monolight 2010 luminometer (Analytical Luminiscence Laboratory, San Diego).
Selectable Transformants.
To obtain selectable transformants, cells were electroporated using 20 μg of the α-SCSB-NEO construct as described above. After electroporation, the cells were resuspended in 50 ml Diamond’s medium (15) and incubated at 37°C for 4 hr without drug. Paromomycin sulfate (Sigma) was then added to a final concentration of 50 μg/ml and incubation was continued for another 17 hr. 25 ml of the culture was harvested by centrifugation at 1,500 × g for 7 min, the supernatant was removed and the pellet was gently resuspended in the residual supernatant. Resuspended cells were added to 50 ml of molten modified Diamond’s medium containing 0.36% agar, 10% horse serum (16), and 50 μg/ml paromomycin at 40°C. The solution was inverted twice and plated. Plates were sealed with stretchable sealing tape (Diversified Biotech) and incubated without inversion in an anaerobic chamber for 4–5 days at 37°C to allow colony formation inside the agar. Fifty to three hundred colonies per 4 μg α-SCSB-NEO construct per transformation can be obtained using this procedure. To assure cloning, colonies were replated. An isolated colony was then transferred into 0.5 ml liquid media containing 50 μg/ml paromomycin with a sterile transfer pipet and incubated overnight at 37°C. The culture was scaled-up to larger volumes as cell density increased. Transformed cells can be frozen in Diamond’s medium containing 10% dimethyl sulfoxide and stored in liquid nitrogen.
Analysis of Selectable Transformants.
High molecular weight DNA was extracted from 100 ml cultures of transformed clones grown in the presence of 50 μg/ml paromomycin using DNAzol (GIBCO/BRL). Southern blots were prepared and hybridized with a 32P-labeled neo probe as previously described (17). To determine the copy number of the NEO construct in selectable transformants, the amount of the neo gene (800 bp) that would represent one copy per T. vaginalis genome was calculated using the genome size of 2.5 × 107 bp (18). Accordingly, 32, 160, 320, 480, 640, 800, 960, and 1120 pg of the neo gene was used to represent 1, 5, 10, 15, 20, 25, 30, and 35 copies per genome when compared with 1 μg total T. vaginalis DNA. 0.5 μg and 1 μg DNA from clones 1 and 2 were used for comparison. DNA samples were denatured in 0.4N NaOH for 10 min at room temperature, neutralized in 1M NH4OAc and vacuum transferred onto nitrocellulose using a slot-blot apparatus. Blots were then hybridized and washed as before. After exposing to x-ray film, radioactive signals were quantitated using liquid scintillation counting.
RESULTS AND DISCUSSION
To establish a transient transformation assay for T. vaginalis, we prepared two constructs containing either the reporter gene cat or luc in pBluescript (Fig. 1) These genes are flanked by 1660 and 449 nucleotides of the 5′ and 3′ UTR, respectively, of the T. vaginalis α-SCSB gene (11). Additionally, DNA encoding the first 27 amino acids of α-SCSB are fused in-frame to the reporter genes. We chose to flank cat and luc genes with α-SCSB UTRs, as the α-SCSB gene is highly expressed (11) and was expected to provide a strong promoter to drive the expression of these reporter genes.
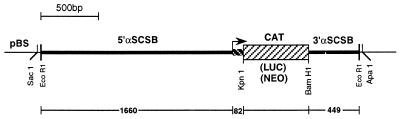
Figure 1.
Schematic diagram of the plasmids α-SCSB-CAT, α-SCSB-NEO and α-SCSB-LUC used to transform T. vaginalis. Sequences of the 5′ and 3′ ends of the α-SCSB UTRs are represented by a heavy line. → Represents the start of the α-SCSB coding region, which is followed by the first 82 bp of the α-SCSB gene(▪) fused in-frame to the reporter gene (▨). The coding regions of cat, neo, and luc are flanked by KpnI/BamHI sites that were used to prepare the constructs using the pBluescript (pBS) vector. Relevant restriction enzyme sites are shown.
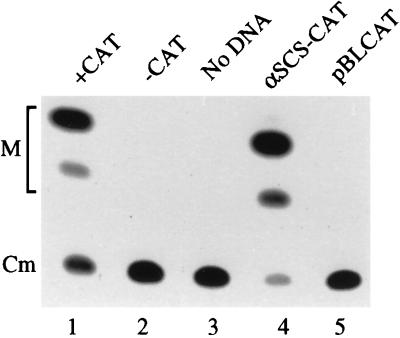
50 μg of either the closed-circular cat or luc construct was introduced into ≈2.5 × 108 T. vaginalis cells by electroporation in Diamond’s medium. The cells were then cultured in Diamond’s medium for 21 hr, harvested and assayed for the presence of reporter activity. Relatively high levels of CAT activity were detectable by TLC in cells subjected to electroporation at 350 volts and 960 μFd (Fig. 2, lane 4). In contrast, CAT activity was undetectable in either nonelectroporated T. vaginalis extracts (lane 2) or T. vaginalis extracts prepared from cells electroporated in the absence of DNA (lane 3), showing that neither endogenous nor contaminating CAT activity is responsible for the activity detected upon electroporation with the α-SCSB-CAT construct. Furthermore, no CAT activity is detected when the cells are electroporated with a CAT construct (pBLCAT) that lacks both 5′ and 3′ α-SCSB UTRs (lane 5) demonstrating the requirement of flanking T. vaginalis DNA for gene expression. T. vaginalis cells electroporated with the α-SCSB-CAT construct were plated on Luria–Bertani broth and no bacterial colonies were observed, ruling-out the possibility that the observed CAT activity derives from bacterial contamination of the T. vaginalis cultures.
Figure 2.
CAT expression in T. vaginalis cells transformed by electroporation at 350 volts and 960 μFd. TLC demonstrating unacetylated 14-C chloramphenicol (Cm) and monoacetylated forms of the substrate (M) derived following incubation of T. vaginalis extracts with 14-C chloramphenicol and acetyl CoA. The following T. vaginalis extracts were used in CAT assays: Lane 1, nonelectroporated extracts containing 5 × 10 units of commercially available CAT (Pharmacia) as a positive control; Lane 2, nonelectroporated extracts without addition of commercially available CAT; Lane 3, extracts electroporated in the absence of DNA; Lane 4, extracts electroporated in the presence of the α-SCSB-CAT construct; Lane 5, extracts electroporated in the presence of the plasmid pBLCAT.
T. vaginalis cells electroporated using the α-SCSB-LUC construct likewise express high levels of luciferase activity, ranging from 40,000 to 450,000 light units (data not shown). Unlike that observed for CAT activity, luciferase activity requires the addition of the cysteine protease inhibitor, leupeptin, to a concentration of ≈30 mM to cell extracts to prevent proteolysis. Moreover, we found levels of luciferase to vary over 10-fold in individual experiments where the same conditions and reagents were used. For these reasons, we chose to optimize conditions for transient expression of proteins in T. vaginalis using the α-SCSB-CAT construct.
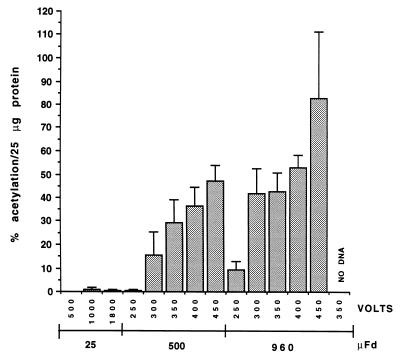
Fig. 3 shows the levels of CAT activity obtained using different electroporation conditions. Conditions typically used to transform bacteria (between 500 and 1800 volts and 25 μFd) yield barely detectable CAT activity in T. vaginalis extracts prepared 21 hr postelectroporation. On the other hand, CAT activity ranging from 29–82% acetylation per 25 μg protein is detected when electroporating at 350–450 volts and either 500 or 960 μFd. Electroporation using 350 volts and 960 μFd yields on average 43% acetylation per 25 μg protein. Electroporation at higher voltages (i.e., 400 or 450) results in even higher levels of acetylation, however, these conditions also lead to lower cell survival (higher kill rate) and severe cell aggregration. Taking all parameters into account, we have chosen 350 volts and 960 μFd as our standard electroporation conditions. Transformation experiments were conducted using ≈2.5 × 108 T. vaginalis cells resuspended in Diamond’s medium prior to electroporation. However, cells electroporated in Cytomix (19) and Zimmermann’s postfusion media (20) were also found to express comparatively high levels of CAT activity (data not shown).
Figure 3.
CAT expression in T. vaginalis using various electroporation conditions. The graph shows the percent acetylation of [14C]chloramphenicol per 25 μg protein in CAT assays from electroporations with conditions varying from 25 μFd (500–1800 volts), 500 μFd (250–450 volts) to 960 μFd (250–450 volts). Except for the no DNA control, cells were electroporated using 50 μg of the αSCSB-CAT construct, incubated 21 hr at 37°C and then assayed for cat expression. The average value ± SD for three experiments is shown.
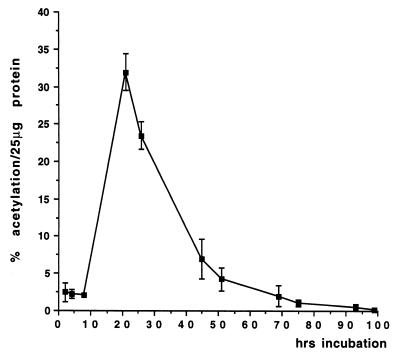
Levels of cat expression were examined from 2–165 hr postelectroporation to determine the optimal time for harvesting cells. CAT activity was found to peak between 8–21 hr postelectroporation (Fig. 4). Two percent acetylation (per 25 μg protein) was observed at 8 hr, rising to a maximum of 32% at 21 hr. Activity drops to 23.5% by 26 hr and by 51 hr posttransformation only 4% acetylation is observed. Between 90–99 hr posttransformation, CAT activity becomes undetectable using TLC. Cells harvested at later time points were passed into fresh medium and were healthy prior to being tested for CAT activity. Thus, loss of CAT activity between 90–99 hr is not due to cell death but probably results from turnover of the CAT protein and loss of the plasmid from the cells. A similar time course experiment using cells transformed with the α-SCSB-LUC construct also showed that LUC activity peaks between 8–21 hr postelectroporation (data not shown).
Figure 4.
Time course for CAT activity in T. vaginalis. Cells were electroporated, subcultured into fresh medium every 24 hr and harvested at various times postelectroporation and assayed for CAT activity. The results are the average ± SD of an experiment done in triplicate.
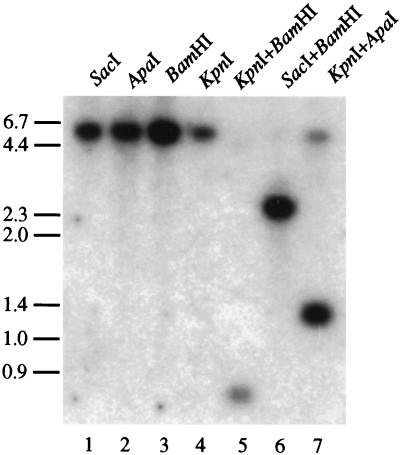
To increase the sensitivity of T. vaginalis transformation methods, allowing a broader range of biological properties to be studied, and to ultimately develop methods for knocking-out genes in this parasite, we have also established a system to obtain selectable transformants using neo as a selectable marker. The sensitivity of nontransformed T. vaginalis cells to paromomycin sulfate was found to be 50 μg/ml when cells are grown either on plates or in liquid medium. Under these conditions, no growth was observed on plates or cultures incubated up to 10 days. A construct containing the neo gene flanked by the α-SCSB UTRs (Fig. 1) was introduced into T. vaginalis as described for the α-SCSB-CAT construct, except that only 20 μg of DNA was used in the electroporation. Transformants were plated and incubated in an anerobic chamber. After 3–5 days of selection, colonies were replated to ensure clonality. Two of the resulting colonies (clones 1 and 2) were cultivated in liquid medium and total DNA was prepared to confirm the presence and to determine the physical structure of the α-SCSB-NEO construct. Restriction enzyme digestion and Southern blot analysis of total DNA reveals that the α-SCSB-NEO construct is maintained in the closed-circular form originally introduced into the cells (Fig. 5). Digestions of the DNA derived from clone 1 with four enzymes that cut only once in the construct (see Fig. 1) always result in detection of a linear band the size of the construct (≈5800 bp) upon Southern blotting and hybridization with a neo gene probe (lanes 1–4). Likewise fragments of the predicted size are observed when digesting with enzymes that cut at two locations in the construct (lanes 5–7), indicating no DNA rearrangements have occurred as would be predicted upon integration of the construct into the T. vaginalis genome. The same results are obtained upon analysis of the DNA from clone 2 (data not shown). These data demonstrate that the α-SCSB-NEO constructs isolated from T. vaginalis clones 1 & 2 are maintained as episomally closed-circular plasmids. Furthermore, when total DNA isolated from clone 1 was used to transform E. coli, a plasmid that is indistinguishable by restriction enzyme analysis from the original α-SCSB-NEO construct used to transform T. vaginalis was retrieved (data not shown). Also, Northern blot analysis of total RNA prepared from T. vaginalis clones 1 and 2 revealed the presence of a ≈850 nucleotide RNA when hybridized with a neo probe, consistent with the size predicted for a neo mRNA (data not shown). In preliminary experiments, we have also been able to select transformants of T. vaginalis using hygromycin phosphotransferase as a marker and 100 μg/ml hygromycin in the selection procedure (data not shown).
Figure 5.
Southern blot analysis of total T. vaginalis DNA from clone 1, transformed with the αSCSB-NEO construct and selected using 50 μg/ml paromomycin. DNA (1 μg) was digested with a single restriction enzyme, as indicated (lanes 1–4) or a combination of two restriction enzymes, as indicated (lanes 5–7), and hybridized with a 32P-labeled neo gene probe. See Fig. 1 for restriction enzyme map of the construct.
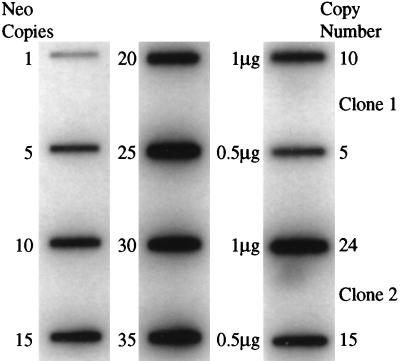
To determine the copy number of the α-SCSB-NEO construct in paromomycin-resistant clones 1 and 2, either 0.5 μg or 1 μg of total DNA isolated from the clones was slotted onto nitrocellulose and hybridized with the neo probe (Fig. 6). Varying amounts of the neo gene were also slotted onto the blot, corresponding to the amount of neo DNA that would be present per μg of total DNA if there were 1, 5, 10, 15, 20, 25, 30, or 35 copies of the construct per cell. Quantitation of the hybridization signals shown in Fig. 6 indicates that clone 1 harbors 10 copies of the construct per cell, whereas clone 2 contains between 24–30 copies per cell.
Figure 6.
Quantitation of the copy number of αSCSB-NEO construct copy number per cell. DNA from T. vaginalis clones 1 and 2, transformed with the αSCSB-NEO construct and selected using 50 μg/ml paromomycin was slotted onto nitrocellulose and probed with a 32P-labeled neo gene fragment. The amount of DNA equivalent to 1–35 copies of the αSCSB-NEO construct per cell when compared with 1 μg total T. vaginalis DNA was slotted in the left two lanes. T. vaginalis DNA (1 and 0.5 μg of total) from clones 1 and 2 was slotted in the far-right lane. Copy number was calculated by counting the radioactivity in each slot using a liquid scintillation counter.
The ability to transform T. vaginalis now provides the means to directly test the function and properties of a variety of molecules in vivo. Currently, we are using this new technology to study organelle biogenesis, drug-resistance and gene transcription. These transformation assays should provide invaluable tools for advancing our knowledge of the basic biology and pathogenesis of trichomonads, as similar methods developed in the past few years for other protozoan parasites (19–28) have done.
Acknowledgments
We thank Stephen Beverley, John Boothroyd and David Roos for helpful discussions and encouragement and Peter Bradley, Evelyn Plümper, Kirkwood Land, and Elizabeth Bui for advice and critical review of the manuscript. This work was supported by National Institutes of Health Grants AI30537 and AI27857 to P.J.J., a predoctoral training grant (U.S. Public Health Service National Research Service Award GM07185) to D.R.L., and a Burroughs Wellcome New Investigator in Molecular Parasitology Award to P.J.J.
ABBREVIATIONS
- CAT
chloramphenicol acetyltransferase
- LUC
luciferase, NEO, neomycin phosphotransferase, α-SCSB, α-succinyl CoA synthetase B
- UTR
untranslated region
References
- 1.Sogin M L. Curr Opin Genet Dev. 1991;1:457–463. doi: 10.1016/s0959-437x(05)80192-3. [DOI] [PubMed] [Google Scholar]
- 2.Leipe D D, Gunderson J H, Nerad T A, Sogin M L. Mol Biochem Parasitol. 1993;59:41–48. doi: 10.1016/0166-6851(93)90005-i. [DOI] [PubMed] [Google Scholar]
- 3.Johnson P J, Bradley P J, Lahti C J. In: Molecular Approaches to Parasitology. Boothroyd J C, Komuniecki R, editors. New York: Wiley-Liss; 1995. pp. 399–411. [Google Scholar]
- 4.Müller M. J Gen Microbiol. 1993;139:2879–2889. doi: 10.1099/00221287-139-12-2879. [DOI] [PubMed] [Google Scholar]
- 5.Bui E T N, Bradley P J, Johnson P J. Proc Natl Acad Sci USA. 1996;93:9651–9656. doi: 10.1073/pnas.93.18.9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horner D S, Hirt R P, Kivington D, Lloyd D, Embley T M. Proc R Soc London B. 1996;263:1053–1059. doi: 10.1098/rspb.1996.0155. [DOI] [PubMed] [Google Scholar]
- 7.Germot A, Philippe H, Le Guyader H. Proc Natl Acad Sci USA. 1996;93:14614–14617. doi: 10.1073/pnas.93.25.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roger A J, Clark C G, Doolittle W F. Proc Natl Acad Sci USA. 1996;93:14618–14622. doi: 10.1073/pnas.93.25.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quon D V, Delgadillo M G, Khachi A, Smale S T, Johnson P J. Proc Natl Acad Sci USA. 1994;91:4579–4583. doi: 10.1073/pnas.91.10.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quon D V K, Delgadillo M G, Johnson P J. J Mol Evol. 1996;43:253–262. doi: 10.1007/BF02338833. [DOI] [PubMed] [Google Scholar]
- 11.Lahti C J, Bradley P J, Johnson P J. Mol Biochem Parasitol. 1994;66:309–318. doi: 10.1016/0166-6851(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 12.Luckow B, Schutz G. Nucleic Acids Res. 1987;15:5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riggs C D, Chrispeels M J. Nucleic Acids Res. 1987;15:8115. doi: 10.1093/nar/15.19.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck E, Ludwig G, Auerswald E A, Reiss B, Schaller H. Gene. 1982;19:327–336. doi: 10.1016/0378-1119(82)90023-3. [DOI] [PubMed] [Google Scholar]
- 15.Diamond L S. J Parasitol. 1957;43:488–490. [PubMed] [Google Scholar]
- 16.Hollander D H. J Parasitol. 1976;62:826–828. [PubMed] [Google Scholar]
- 17.Johnson P J, d’Oliveira C E, Gorrell T E, Müller M. Proc Natl Acad Sci USA. 1990;87:6097–6101. doi: 10.1073/pnas.87.16.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang A L, Wang C C. Mol Biochem Parasitol. 1985;14:323–335. doi: 10.1016/0166-6851(85)90060-x. [DOI] [PubMed] [Google Scholar]
- 19.Soldati D, Boothroyd J C. Science. 1993;260:349–352. doi: 10.1126/science.8469986. [DOI] [PubMed] [Google Scholar]
- 20.Bellofatto V, Cross G A. Science. 1989;244:1167–1169. doi: 10.1126/science.2499047. [DOI] [PubMed] [Google Scholar]
- 21.Cruz A, Beverley S M. Nature (London) 1990;348:171–173. doi: 10.1038/348171a0. [DOI] [PubMed] [Google Scholar]
- 22.Donald R G, Roos D S. Proc Natl Acad Sci USA. 1993;90:11703–11707. doi: 10.1073/pnas.90.24.11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ten Asbroek A L, Ouellette M, Borst P. Nature (London) 1990;348:174–175. doi: 10.1038/348174a0. [DOI] [PubMed] [Google Scholar]
- 24.Goonewardene R, Daily J, Kaslow D, Sullivan T J, Duffy P, Carter R, Mendis K, Wirth D. Proc Natl Acad Sci USA. 1993;90:5234–5236. doi: 10.1073/pnas.90.11.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Y, Sifri C D, Lei H H, Su X Z, Wellems T E. Proc Natl Acad Sci USA. 1995;92:973–977. doi: 10.1073/pnas.92.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nickel R, Tannish E. Proc Natl Acad Sci USA. 1994;91:7095–7098. doi: 10.1073/pnas.91.15.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purdy J E, Mann B J, Pho L T, Petri W A. Proc Natl Acad Sci USA. 1994;91:7099–7103. doi: 10.1073/pnas.91.15.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yee J, Nash T E. Proc Natl Acad Sci USA. 1995;92:5615–5619. doi: 10.1073/pnas.92.12.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]