Abstract
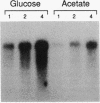
Growth rate-dependent regulation of the level of Escherichia coli glucose 6-phosphate dehydrogenase, encoded by zwf, and 6-phosphogluconate dehydrogenase, encoded by gnd, is similar during steady-state growth and after nutritional upshifts. To determine whether the mechanism regulating zwf expression is like that of gnd, which involves a site of posttranscriptional control located within the structural gene, we prepared and analyzed a set of zwf-lacZ protein fusions in which the fusion joints are distributed across the glucose 6-phosphate dehydrogenase coding sequence. Expression of beta-galactosidase from the protein fusions was as growth rate dependent as that of glucose 6-phosphate dehydrogenase itself, indicating that regulation does not involve an internal regulatory region. The level of beta-galactosidase in zwf-lac operon fusion strains and the level of zwf mRNA from a wild-type strain increased with increasing growth rate, which suggests that growth rate control is exerted on the mRNA level. The half-life of the zwf mRNA mass was 3.0 min during growth on glucose and 3.4 min during growth on acetate. Thus, zwf transcription appears to be the target for growth rate control of the glucose 6-phosphate dehydrogenase level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H. V., 2nd, Wolf R. E., Jr Essential site for growth rate-dependent regulation within the Escherichia coli gnd structural gene. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7669–7673. doi: 10.1073/pnas.81.24.7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H. V., 2nd, Wolf R. E., Jr Growth rate-dependent regulation of 6-phosphogluconate dehydrogenase level in Escherichia coli K-12: beta-galactosidase expression in gnd-lac operon fusion strains. J Bacteriol. 1983 Feb;153(2):771–781. doi: 10.1128/jb.153.2.771-781.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcak G. J., Wolf R. E., Jr Growth-rate-dependent expression and cloning of gnd alleles from natural isolates of Escherichia coli. J Bacteriol. 1988 Jan;170(1):365–371. doi: 10.1128/jb.170.1.365-371.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhout B., van Duin J. Mechanism of translational coupling between coat protein and replicase genes of RNA bacteriophage MS2. Nucleic Acids Res. 1985 Oct 11;13(19):6955–6967. doi: 10.1093/nar/13.19.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carter-Muenchau P., Wolf R. E., Jr Growth-rate-dependent regulation of 6-phosphogluconate dehydrogenase level mediated by an anti-Shine-Dalgarno sequence located within the Escherichia coli gnd structural gene. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1138–1142. doi: 10.1073/pnas.86.4.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis N., Corcos D., Kruh J., Kitzis A. A rapid and accurate method for quantitating total RNA transferred during Northern blot analysis. Nucleic Acids Res. 1988 Mar 25;16(5):2354–2354. doi: 10.1093/nar/16.5.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrish E. E., Baker H. V., 2nd, Wolf R. E., Jr Different control circuits for growth rate-dependent regulation of 6-phosphogluconate dehydrogenase and protein components of the translational machinery in Escherichia coli. J Bacteriol. 1982 Nov;152(2):584–594. doi: 10.1128/jb.152.2.584-594.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Greenberg J. T., Demple B. A global response induced in Escherichia coli by redox-cycling agents overlaps with that induced by peroxide stress. J Bacteriol. 1989 Jul;171(7):3933–3939. doi: 10.1128/jb.171.7.3933-3939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. T., Monach P., Chou J. H., Josephy P. D., Demple B. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6181–6185. doi: 10.1073/pnas.87.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Kao S. M., Hassan H. M. Biochemical characterization of a paraquat-tolerant mutant of Escherichia coli. J Biol Chem. 1985 Sep 5;260(19):10478–10481. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Min Jou W., Haegeman G., Ysebaert M., Fiers W. Nucleotide sequence of the gene coding for the bacteriophage MS2 coat protein. Nature. 1972 May 12;237(5350):82–88. doi: 10.1038/237082a0. [DOI] [PubMed] [Google Scholar]
- Mitchell D. H., Reznikoff W. S., Beckwith J. R. Genetic fusions defining trp and lac operon regulatory elements. J Mol Biol. 1975 Apr 15;93(3):331–350. doi: 10.1016/0022-2836(75)90281-8. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C. Long-range translational coupling in the rplJL-rpoBC operon of Escherichia coli. J Mol Biol. 1989 Mar 20;206(2):323–332. doi: 10.1016/0022-2836(89)90482-8. [DOI] [PubMed] [Google Scholar]
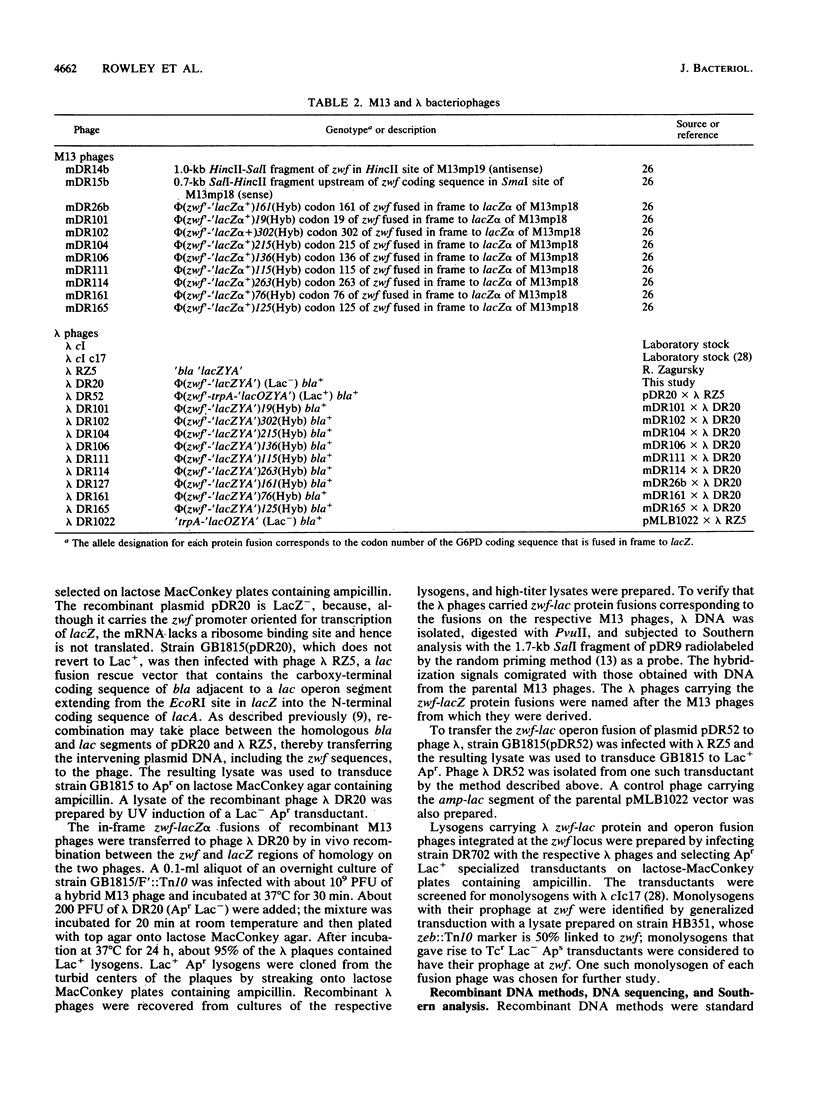
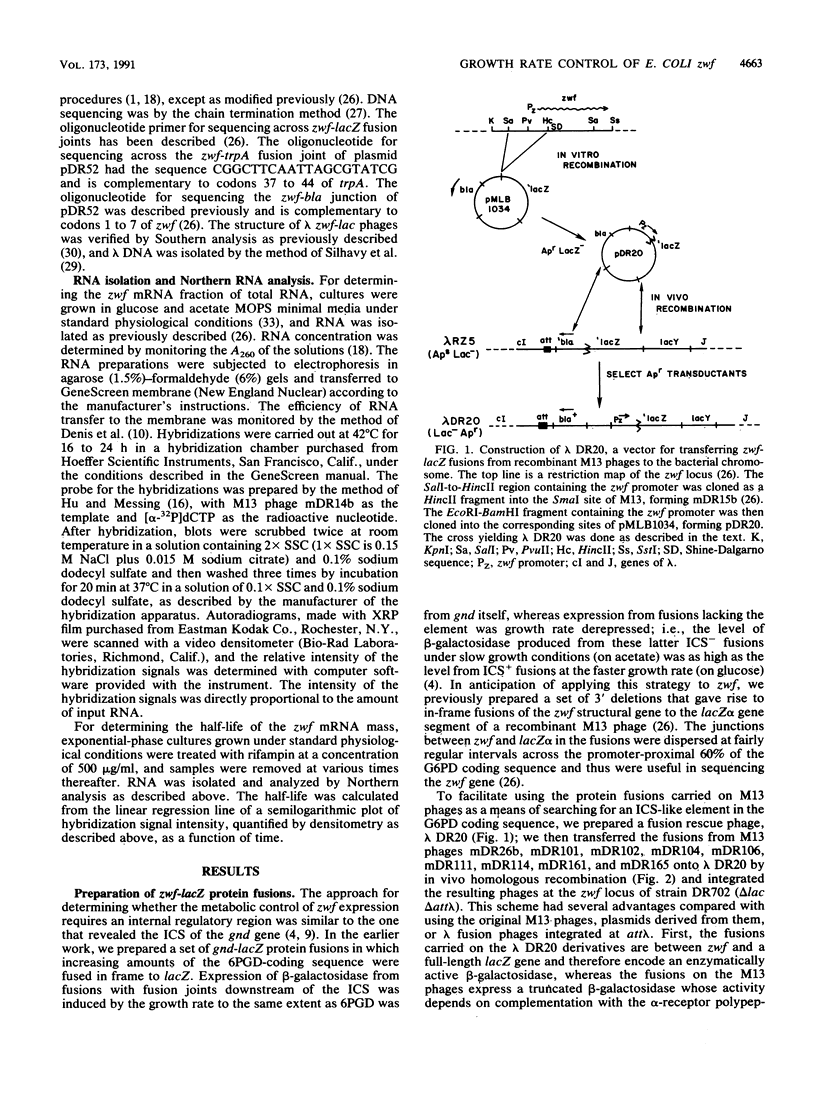
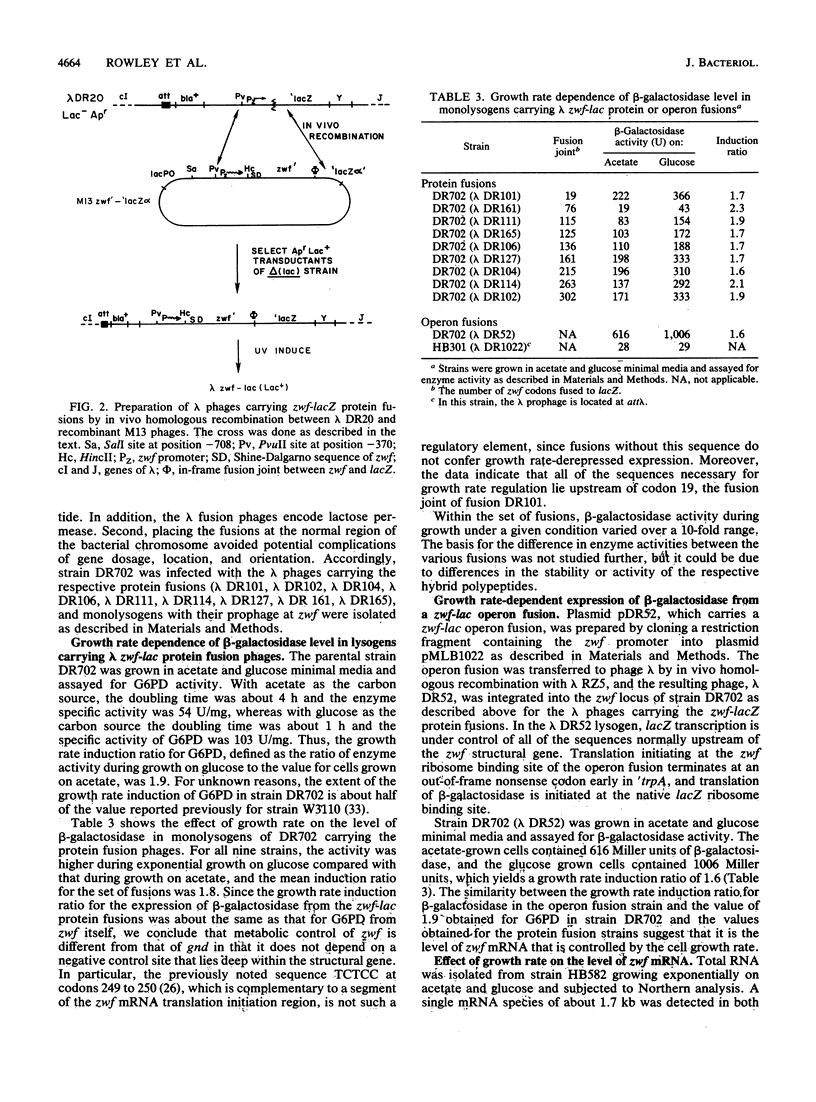
- Rowley D. L., Wolf R. E., Jr Molecular characterization of the Escherichia coli K-12 zwf gene encoding glucose 6-phosphate dehydrogenase. J Bacteriol. 1991 Feb;173(3):968–977. doi: 10.1128/jb.173.3.968-977.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J Mol Biol. 1972 Feb 14;63(3):483–503. doi: 10.1016/0022-2836(72)90443-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wolf R. E., Jr, Prather D. M., Shea F. M. Growth-rate-dependent alteration of 6-phosphogluconate dehydrogenase and glucose 6-phosphate dehydrogenase levels in Escherichia coli K-12. J Bacteriol. 1979 Sep;139(3):1093–1096. doi: 10.1128/jb.139.3.1093-1096.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. M., Munson L. M., Reznikoff W. S. Molecular cloning and sequence analysis of trp-lac fusion deletions. J Mol Biol. 1984 Jan 25;172(3):355–362. doi: 10.1016/s0022-2836(84)80032-7. [DOI] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]