Abstract
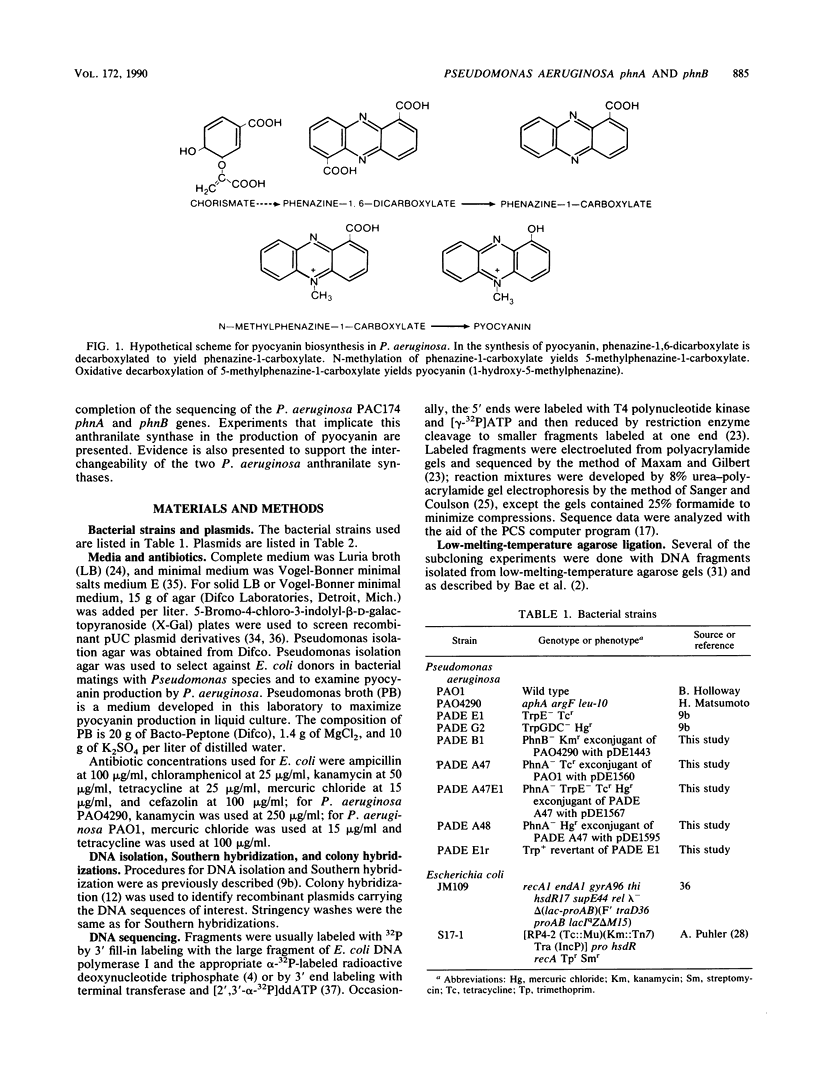
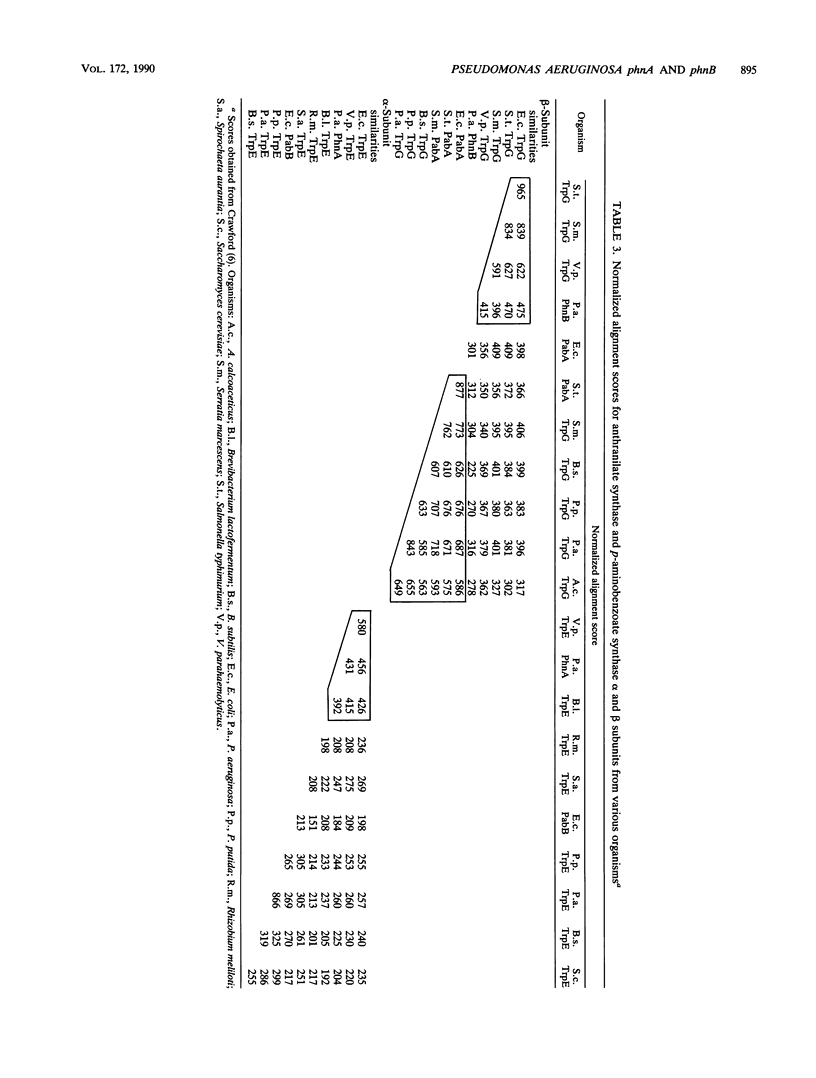
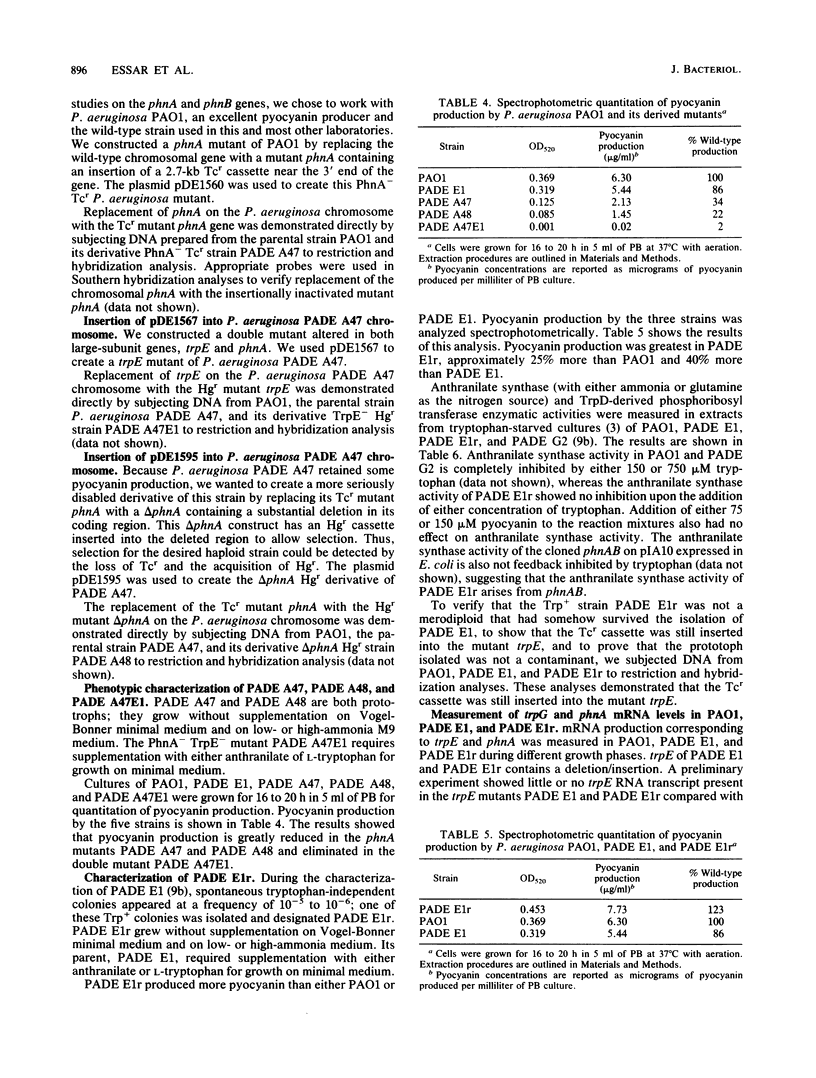
Two anthranilate synthase gene pairs have been identified in Pseudomonas aeruginosa. They were cloned, sequenced, inactivated in vitro by insertion of an antibiotic resistance gene, and returned to P. aeruginosa, replacing the wild-type gene. One anthranilate synthase enzyme participates in tryptophan synthesis; its genes are designated trpE and trpG. The other anthranilate synthase enzyme, encoded by phnA and phnB, participates in the synthesis of pyocyanin, the characteristic phenazine pigment of the organism. trpE and trpG are independently transcribed; homologous genes have been cloned from Pseudomonas putida. The phenazine pathway genes phnA and phnB are cotranscribed. The cloned phnA phnB gene pair complements trpE and trpE(G) mutants of Escherichia coli. Homologous genes were not found in P. putida PPG1, a non-phenazine producer. Surprisingly, PhnA and PhnB are more closely related to E. coli TrpE and TrpG than to Pseudomonas TrpE and TrpG, whereas Pseudomonas TrpE and TrpG are more closely related to E. coli PabB and PabA than to E. coli TrpE and TrpG. We replaced the wild-type trpE on the P. aeruginosa chromosome with a mutant form having a considerable portion of its coding sequence deleted and replaced by a tetracycline resistance gene cassette. This resulted in tryptophan auxotrophy; however, spontaneous tryptophan-independent revertants appeared at a frequency of 10(-5) to 10(6). The anthranilate synthase of these revertants is not feedback inhibited by tryptophan, suggesting that it arises from PhnAB. phnA mutants retain a low level of pyocyanin production. Introduction of an inactivated trpE gene into a phnA mutant abolished residual pyocyanin production, suggesting that the trpE trpG gene products are capable of providing some anthranilate for pyocyanin synthesis.
Full text
PDF

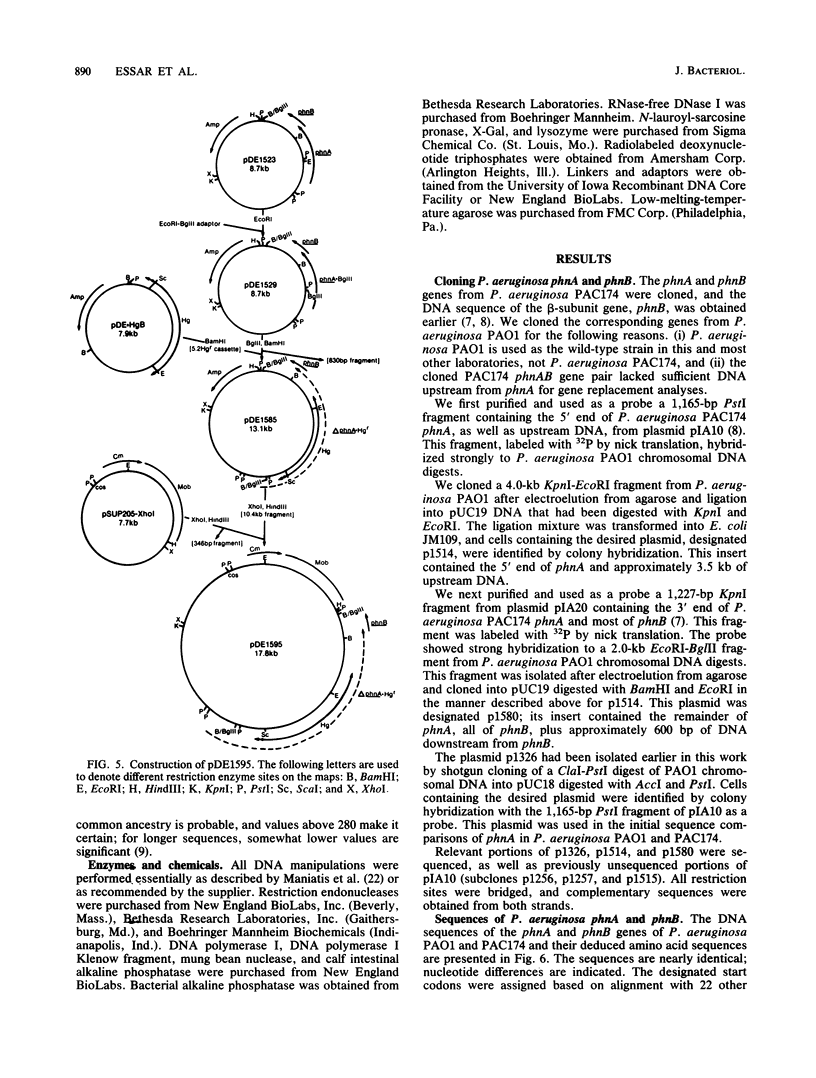

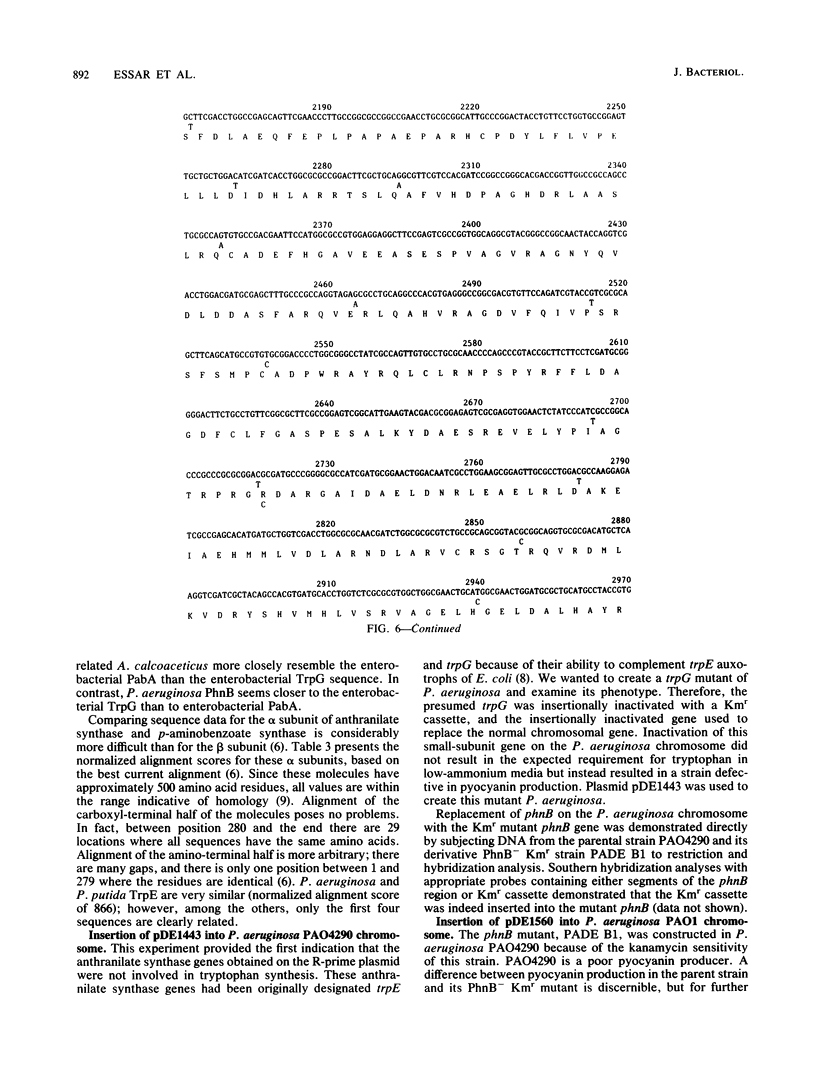
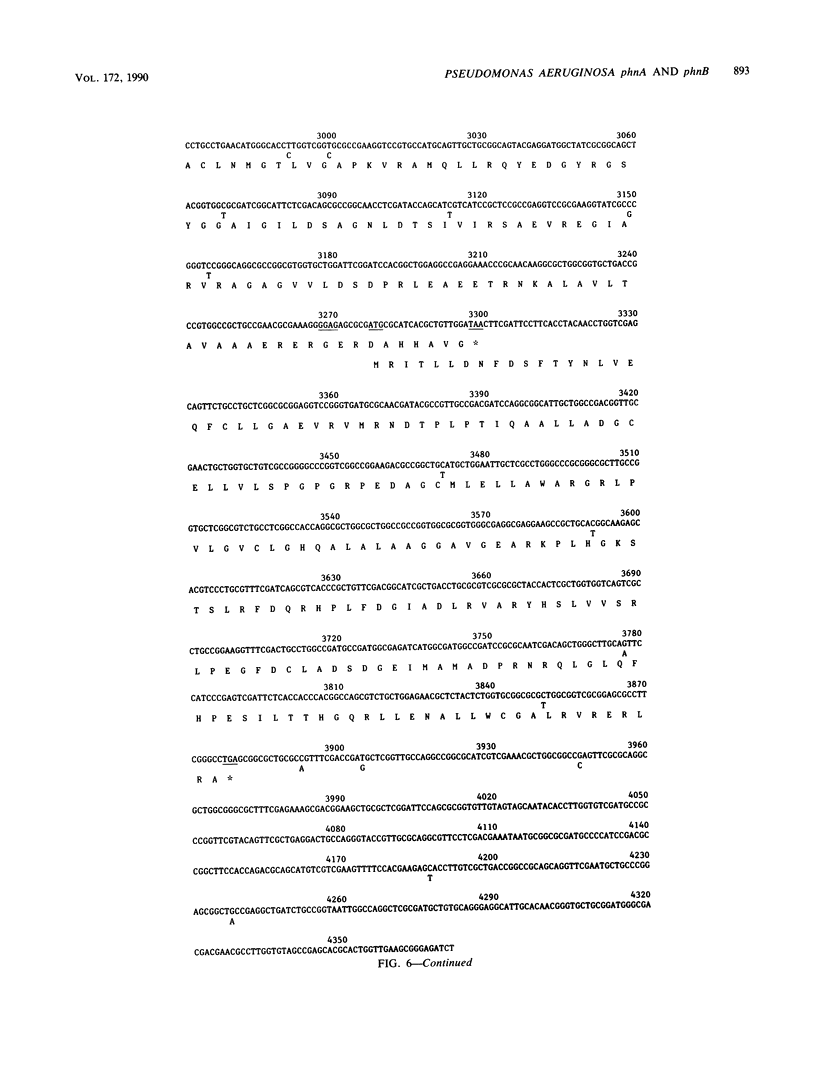
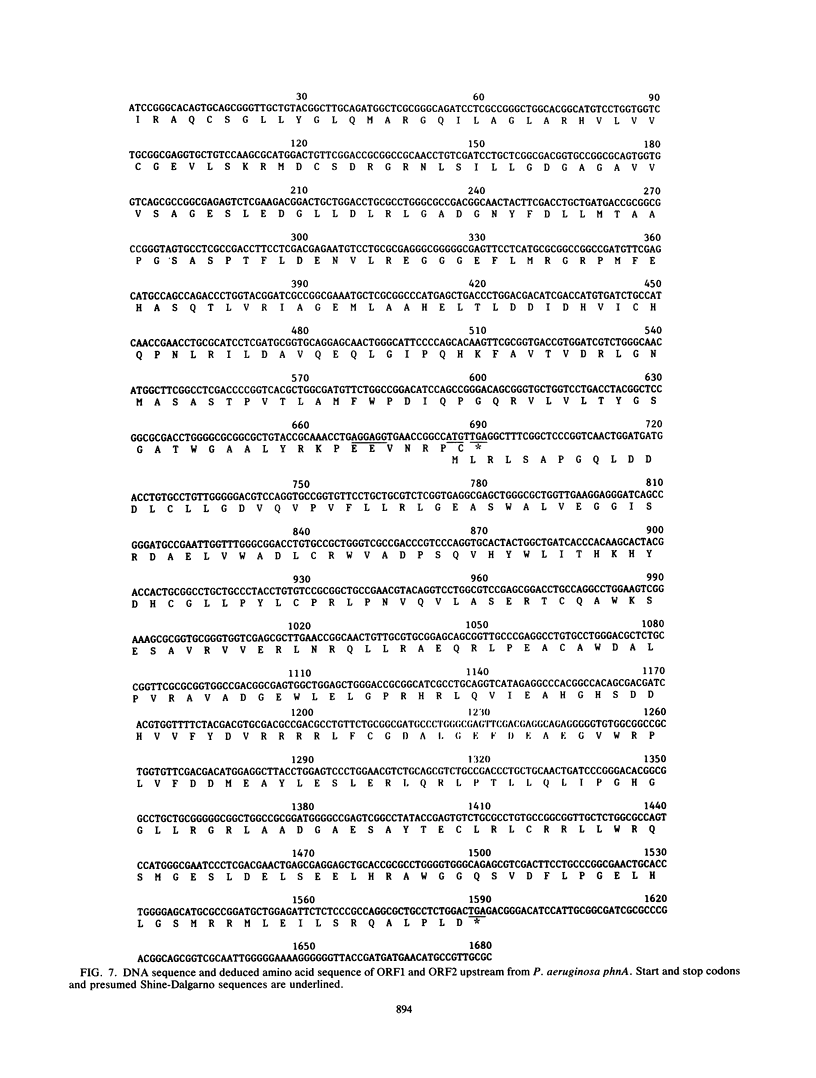




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Bae Y. M., Holmgren E., Crawford I. P. Rhizobium meliloti anthranilate synthase gene: cloning, sequence, and expression in Escherichia coli. J Bacteriol. 1989 Jun;171(6):3471–3478. doi: 10.1128/jb.171.6.3471-3478.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun D. H., Pierson D. L., Jensen R. A. The regulation of tryptophan biosynthesis in Pseudomonas aeruginosa. Mol Gen Genet. 1973 Mar 1;121(2):117–132. doi: 10.1007/BF00277526. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Englund P. T. Specific labeling of 3' termini with T4 DNA polymerase. Methods Enzymol. 1980;65(1):39–43. doi: 10.1016/s0076-6879(80)65008-3. [DOI] [PubMed] [Google Scholar]
- Chang M., Hadero A., Crawford I. P. Sequence of the Pseudomonas aeruginosa trpI activator gene and relatedness of trpI to other procaryotic regulatory genes. J Bacteriol. 1989 Jan;171(1):172–183. doi: 10.1128/jb.171.1.172-183.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Eberly L. Structure and regulation of the anthranilate synthase genes in Pseudomonas aeruginosa: I. Sequence of trpG encoding the glutamine amidotransferase subunit. Mol Biol Evol. 1986 Sep;3(5):436–448. doi: 10.1093/oxfordjournals.molbev.a040408. [DOI] [PubMed] [Google Scholar]
- Crawford I. P. Evolution of a biosynthetic pathway: the tryptophan paradigm. Annu Rev Microbiol. 1989;43:567–600. doi: 10.1146/annurev.mi.43.100189.003031. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Wilde A., Yelverton E. M., Figurski D., Hedges R. W. Structure and regulation of the anthranilate synthase genes in Pseudomonas aeruginosa: II. Cloning and expression in Escherichia coli. Mol Biol Evol. 1986 Sep;3(5):449–458. doi: 10.1093/oxfordjournals.molbev.a040409. [DOI] [PubMed] [Google Scholar]
- Essar D. W., Eberly L., Crawford I. P. Evolutionary differences in chromosomal locations of four early genes of the tryptophan pathway in fluorescent pseudomonads: DNA sequences and characterization of Pseudomonas putida trpE and trpGDC. J Bacteriol. 1990 Feb;172(2):867–883. doi: 10.1128/jb.172.2.867-883.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essar D. W., Eberly L., Han C. Y., Crawford I. P. DNA sequences and characterization of four early genes of the tryptophan pathway in Pseudomonas aeruginosa. J Bacteriol. 1990 Feb;172(2):853–866. doi: 10.1128/jb.172.2.853-866.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambill B. D., Summers A. O. Versatile mercury-resistant cloning and expression vectors. Gene. 1985;39(2-3):293–297. doi: 10.1016/0378-1119(85)90326-9. [DOI] [PubMed] [Google Scholar]
- Goncharoff P., Nichols B. P. Nucleotide sequence of Escherichia coli pabB indicates a common evolutionary origin of p-aminobenzoate synthetase and anthranilate synthetase. J Bacteriol. 1984 Jul;159(1):57–62. doi: 10.1128/jb.159.1.57-62.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M., Keim P. S., Goto Y., Zalkin H., Heinrikson R. L. Anthranilate synthetase component II from Pseudomonas putida. Covalent structure and identification of the cysteine residue involved in catalysis. J Biol Chem. 1978 Jul 10;253(13):4659–4668. [PubMed] [Google Scholar]
- Lagrimini L. M., Brentano S. T., Donelson J. E. A DNA sequence analysis package for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):605–614. doi: 10.1093/nar/12.1part2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisinger T., Margraff R. Secondary metabolites of the fluorescent pseudomonads. Microbiol Rev. 1979 Sep;43(3):422–442. doi: 10.1128/mr.43.3.422-442.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya T., Shiga S., Kageyama M. Genetic determinant of pyocin R2 in Pseudomonas aeruginosa PAO. I. Localization of the pyocin R2 gene cluster between the trpCD and trpE genes. Mol Gen Genet. 1983;189(3):375–381. doi: 10.1007/BF00325898. [DOI] [PubMed] [Google Scholar]
- Simon R., O'Connell M., Labes M., Pühler A. Plasmid vectors for the genetic analysis and manipulation of rhizobia and other gram-negative bacteria. Methods Enzymol. 1986;118:640–659. doi: 10.1016/0076-6879(86)18106-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomashow L. S., Weller D. M. Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. J Bacteriol. 1988 Aug;170(8):3499–3508. doi: 10.1128/jb.170.8.3499-3508.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. M., Messenger A. J. Occurrence, biochemistry and physiology of phenazine pigment production. Adv Microb Physiol. 1986;27:211–275. doi: 10.1016/s0065-2911(08)60306-9. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yousaf S. I., Carroll A. R., Clarke B. E. A new and improved method for 3'-end labelling DNA using [alpha-32P]ddATP. Gene. 1984 Mar;27(3):309–313. doi: 10.1016/0378-1119(84)90075-1. [DOI] [PubMed] [Google Scholar]


