Abstract
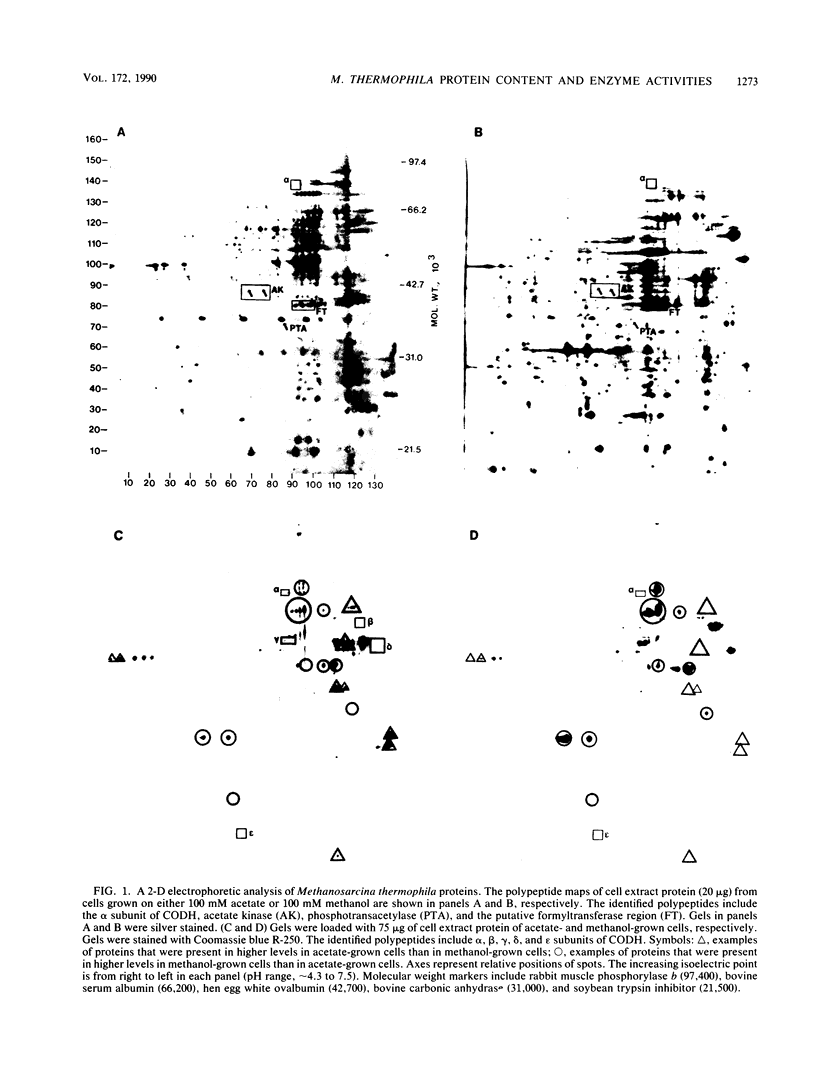
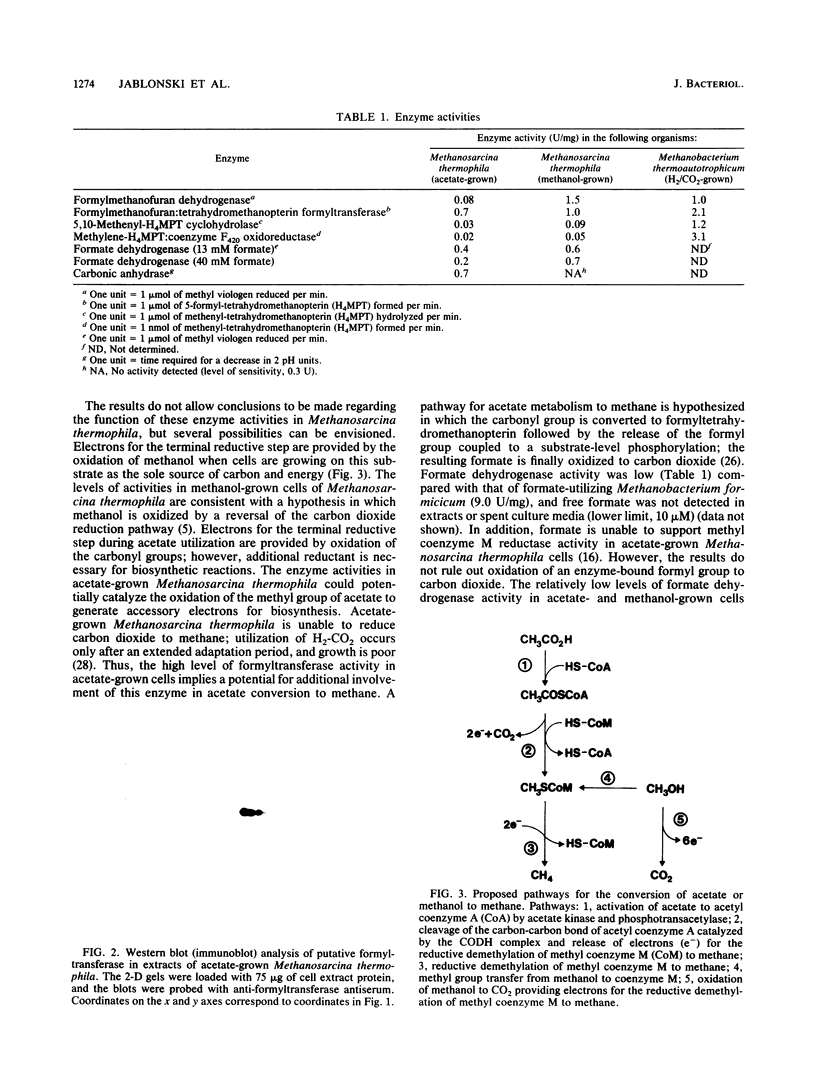
The cell extract protein content of acetate- and methanol-grown Methanosarcina thermophila TM-1 was examined by two-dimensional polyacrylamide gel electrophoresis. More than 100 mutually exclusive spots were present in acetate- and methanol-grown cells. Spots corresponding to acetate kinase, phosphotransacetylase, and the five subunits of the carbon monoxide dehydrogenase complex were identified in acetate-grown cells. Activities of formylmethanofuran dehydrogenase, formylmethanofuran:tetrahydromethanopterin formyltransferase, 5,10-methenyltetrahydromethanopterin cyclohydrolase, methylene tetrahydromethanopterin:coenzyme F420 oxidoreductase, formate dehydrogenase, and carbonic anhydrase were examined in acetate- and methanol-grown Methanosarcina thermophila. Levels of formyltransferase in either acetate- or methanol-grown Methanosarcina thermophila were approximately half the levels detected in H2-CO2-grown Methanobacterium thermoautotrophicum. All other enzyme activities were significantly lower in acetate- and methanol-grown Methanosarcina thermophila.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aceti D. J., Ferry J. G. Purification and characterization of acetate kinase from acetate-grown Methanosarcina thermophila. Evidence for regulation of synthesis. J Biol Chem. 1988 Oct 25;263(30):15444–15448. [PubMed] [Google Scholar]
- Anthony R. S., Spector L. B. A phosphoenzyme intermediary in acetate kinase action. J Biol Chem. 1970 Dec 25;245(24):6739–6741. [PubMed] [Google Scholar]
- Bloch P. L., Phillips T. A., Neidhardt F. C. Protein identifications of O'Farrell two-dimensional gels: locations of 81 Escherichia coli proteins. J Bacteriol. 1980 Mar;141(3):1409–1420. doi: 10.1128/jb.141.3.1409-1420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K. J. Protein damage and degradation by oxygen radicals. I. general aspects. J Biol Chem. 1987 Jul 15;262(20):9895–9901. [PubMed] [Google Scholar]
- DiMarco A. A., Donnelly M. I., Wolfe R. S. Purification and properties of the 5,10-methenyltetrahydromethanopterin cyclohydrolase from Methanobacterium thermoautotrophicum. J Bacteriol. 1986 Dec;168(3):1372–1377. doi: 10.1128/jb.168.3.1372-1377.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly M. I., Wolfe R. S. The role of formylmethanofuran: tetrahydromethanopterin formyltransferase in methanogenesis from carbon dioxide. J Biol Chem. 1986 Dec 15;261(35):16653–16659. [PubMed] [Google Scholar]
- Hartzell P. L., Zvilius G., Escalante-Semerena J. C., Donnelly M. I. Coenzyme F420 dependence of the methylenetetrahydromethanopterin dehydrogenase of Methanobacterium thermoautotrophicum. Biochem Biophys Res Commun. 1985 Dec 31;133(3):884–890. doi: 10.1016/0006-291x(85)91218-5. [DOI] [PubMed] [Google Scholar]
- Jaenicke R., Bernhardt G., Lüdemann H. D., Stetter K. O. Pressure-Induced Alterations in the Protein Pattern of the Thermophilic Archaebacterium Methanococcus thermolithotrophicus. Appl Environ Microbiol. 1988 Oct;54(10):2375–2380. doi: 10.1128/aem.54.10.2375-2380.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundie L. L., Jr, Ferry J. G. Activation of acetate by Methanosarcina thermophila. Purification and characterization of phosphotransacetylase. J Biol Chem. 1989 Nov 5;264(31):18392–18396. [PubMed] [Google Scholar]
- Nelson M. J., Ferry J. G. Carbon monoxide-dependent methyl coenzyme M methylreductase in acetotrophic Methosarcina spp. J Bacteriol. 1984 Nov;160(2):526–532. doi: 10.1128/jb.160.2.526-532.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rouvière P. E., Wolfe R. S. Novel biochemistry of methanogenesis. J Biol Chem. 1988 Jun 15;263(17):7913–7916. [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G. Properties of formate dehydrogenase in Methanobacterium formicicum. J Bacteriol. 1982 Apr;150(1):1–7. doi: 10.1128/jb.150.1.1-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner R. S., McInerney M. J., Nagle D. P., Jr Formate auxotroph of Methanobacterium thermoautotrophicum Marburg. J Bacteriol. 1989 Dec;171(12):6534–6538. doi: 10.1128/jb.171.12.6534-6538.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlesky K. C., Nelson M. J., Ferry J. G. Isolation of an enzyme complex with carbon monoxide dehydrogenase activity containing corrinoid and nickel from acetate-grown Methanosarcina thermophila. J Bacteriol. 1986 Dec;168(3):1053–1058. doi: 10.1128/jb.168.3.1053-1058.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Zinder S. H., Mah R. A. Isolation and Characterization of a Thermophilic Strain of Methanosarcina Unable to Use H(2)-CO(2) for Methanogenesis. Appl Environ Microbiol. 1979 Nov;38(5):996–1008. doi: 10.1128/aem.38.5.996-1008.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beelen P., Labro J. F., Keltjens J. T., Geerts W. J., Vogels G. D., Laarhoven W. H., Guijt W., Haasnoot C. A. Derivatives of methanopterin, a coenzyme involved in methanogenesis. Eur J Biochem. 1984 Mar 1;139(2):359–365. doi: 10.1111/j.1432-1033.1984.tb08014.x. [DOI] [PubMed] [Google Scholar]
- van der Meijden P., Heythuysen H. J., Pouwels A., Houwen F., van der Drift C., Vogels G. D. Methyltransferases involved in methanol conversion by Methanosarcina barkeri. Arch Microbiol. 1983 Jun;134(3):238–242. doi: 10.1007/BF00407765. [DOI] [PubMed] [Google Scholar]