Abstract
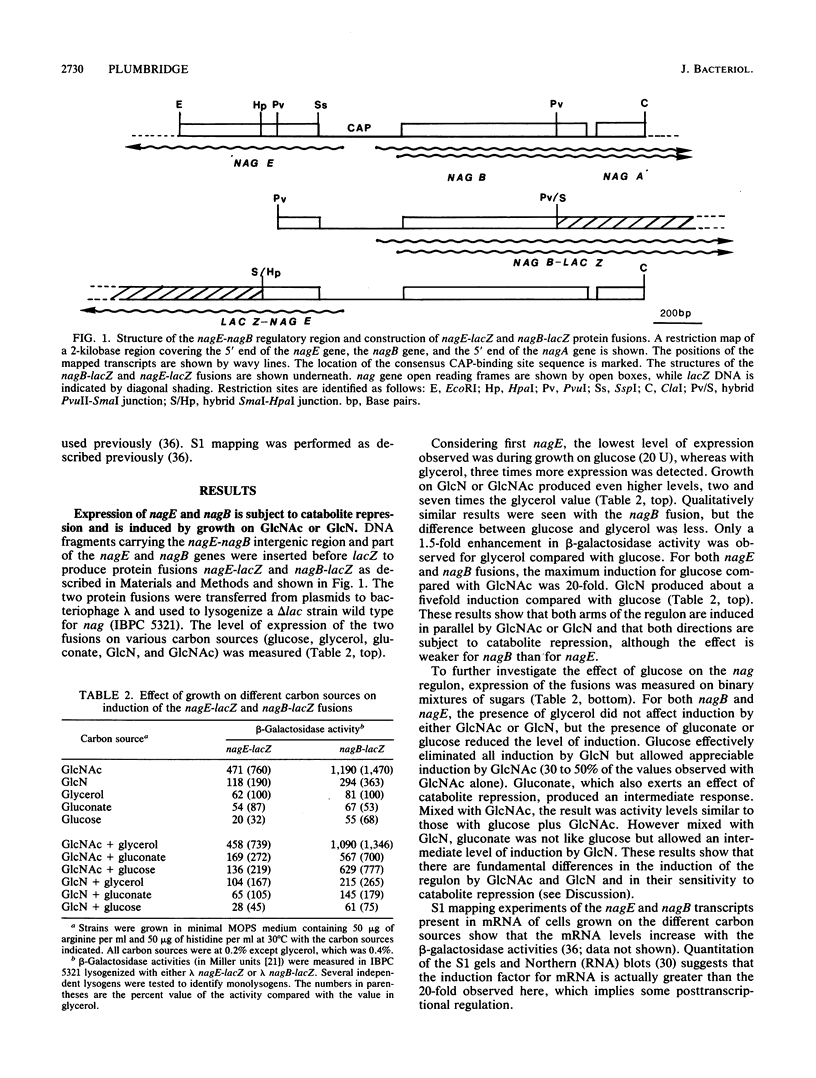
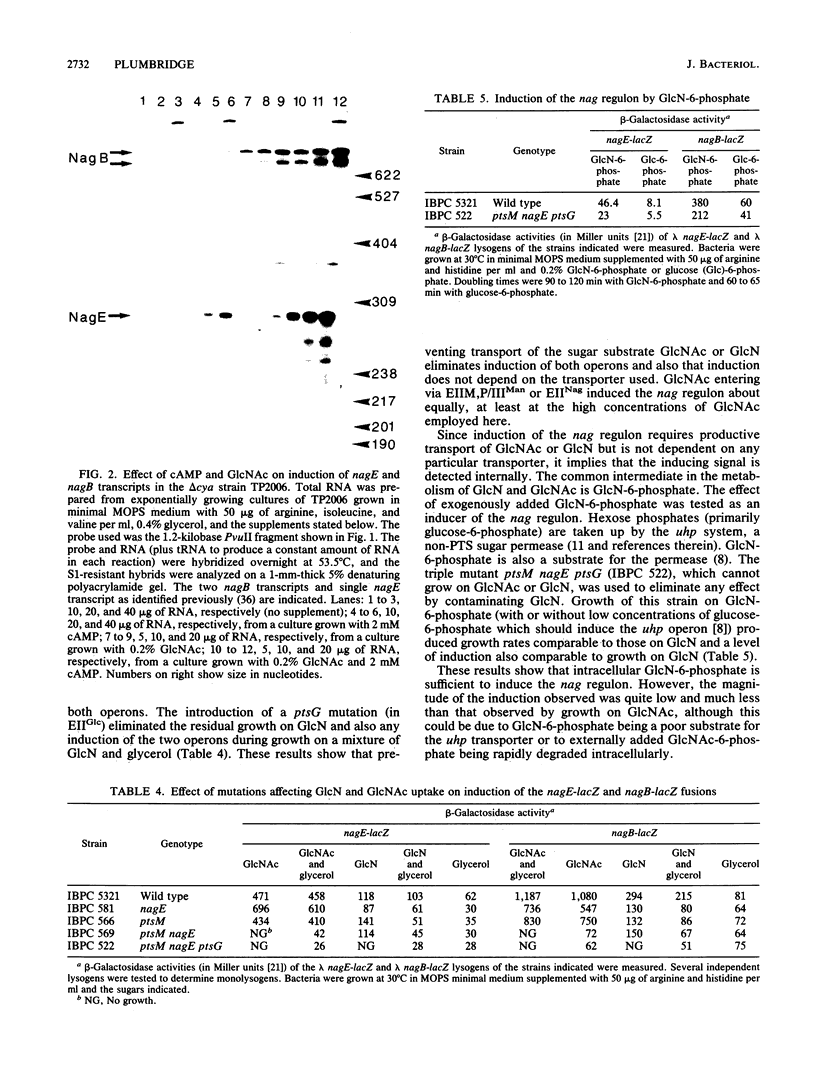
The divergent nag regulon located at 15.5 min on the Escherichia coli map encodes genes necessary for growth on N-acetylglucosamine and glucosamine. Full induction of the regulon requires both the presence of N-acetylglucosamine and a functional cyclic AMP (cAMP)-catabolite activator protein (CAP) complex. Glucosamine produces a lower level of induction of the regulon. A nearly symmetric consensus CAP-binding site is located in the intergenic region between nagE (encoding EIINag) and nagB (encoding glucosamine-6-phosphate deaminase). Expression of both nagE and nagB genes is stimulated by cAMP-CAP, but the effect is more pronounced for nagE. In fact, very little expression of nagE is observed in the absence of cAMP-CAP, whereas 50% maximum expression of nagB is observed with N-acetylglucosamine in the absence of cAMP-CAP. Two mRNA 5' ends separated by about 100 nucleotides were located before nagB, and both seem to be similarly subject to N-acetylglucosamine induction and cAMP-CAP stimulation. To induce the regulon, N-acetylglucosamine or glucosamine must enter the cell, but the particular transport mechanism used is not important.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapon C. Role of the catabolite activator protein in the maltose regulon of Escherichia coli. J Bacteriol. 1982 May;150(2):722–729. doi: 10.1128/jb.150.2.722-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. J., Epstein W. Phosphorylation of D-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J Bacteriol. 1975 Jun;122(3):1189–1199. doi: 10.1128/jb.122.3.1189-1199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T., Yamada M., Elgort M., Saier M. H., Jr Nucleotide sequence of the mannitol (mtl) operon in Escherichia coli. Mol Microbiol. 1988 May;2(3):405–412. doi: 10.1111/j.1365-2958.1988.tb00045.x. [DOI] [PubMed] [Google Scholar]
- De Reuse H., Danchin A. The ptsH, ptsI, and crr genes of the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system: a complex operon with several modes of transcription. J Bacteriol. 1988 Sep;170(9):3827–3837. doi: 10.1128/jb.170.9.3827-3837.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz G. W., Jr The hexose phosphate transport system of Escherichia coli. Adv Enzymol Relat Areas Mol Biol. 1976;44:237–259. doi: 10.1002/9780470122891.ch7. [DOI] [PubMed] [Google Scholar]
- Dunn T. M., Schleif R. Deletion analysis of the Escherichia coli ara PC and PBAD promoters. J Mol Biol. 1984 Nov 25;180(1):201–204. doi: 10.1016/0022-2836(84)90437-6. [DOI] [PubMed] [Google Scholar]
- Erni B., Zanolari B., Kocher H. P. The mannose permease of Escherichia coli consists of three different proteins. Amino acid sequence and function in sugar transport, sugar phosphorylation, and penetration of phage lambda DNA. J Biol Chem. 1987 Apr 15;262(11):5238–5247. [PubMed] [Google Scholar]
- Friedrich M. J., Kadner R. J. Nucleotide sequence of the uhp region of Escherichia coli. J Bacteriol. 1987 Aug;169(8):3556–3563. doi: 10.1128/jb.169.8.3556-3563.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez C., Chapon C., Schwartz M. Indirect effects of the 3'-5' cyclic adenosine monophosphate binding protein (CAP) on the transcription of the malPQ operon in Escherichia coli. Biochimie. 1985 Jan;67(1):145–148. doi: 10.1016/s0300-9084(85)80241-8. [DOI] [PubMed] [Google Scholar]
- Holmes R. P., Russell R. R. Mutations affecting amino sugar metabolism in Escherichia coli K-12. J Bacteriol. 1972 Jul;111(1):290–291. doi: 10.1128/jb.111.1.290-291.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Mortimer M. C., Kornberg H. L. Amino-sugar transport systems of Escherichia coli K12. J Gen Microbiol. 1980 Apr;117(2):369–376. doi: 10.1099/00221287-117-2-369. [DOI] [PubMed] [Google Scholar]
- Joseph E., Danchin A., Ullmann A. Regulation of galactose operon expression: glucose effects and role of cyclic adenosine 3',5'-monophosphate. J Bacteriol. 1981 Apr;146(1):149–154. doi: 10.1128/jb.146.1.149-154.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N. L., Gielow W. O., Wallace R. G. Mechanism of araC autoregulation and the domains of two overlapping promoters, Pc and PBAD, in the L-arabinose regulatory region of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Feb;78(2):752–756. doi: 10.1073/pnas.78.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichenstein H. S., Hamilton E. P., Lee N. Repression and catabolite gene activation in the araBAD operon. J Bacteriol. 1987 Feb;169(2):811–822. doi: 10.1128/jb.169.2.811-822.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Johnson H. N., Gartenberg M. R., Crothers D. M. The DNA binding domain and bending angle of E. coli CAP protein. Cell. 1986 Dec 26;47(6):995–1005. doi: 10.1016/0092-8674(86)90814-7. [DOI] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell W. J., Saffen D. W., Roseman S. Sugar transport by the bacterial phosphotransferase system. In vivo regulation of lactose transport in Escherichia coli by IIIGlc, a protein of the phosphoenolpyruvate:glycose phosphotransferase system. J Biol Chem. 1987 Nov 25;262(33):16254–16260. [PubMed] [Google Scholar]
- Miyada C. G., Stoltzfus L., Wilcox G. Regulation of the araC gene of Escherichia coli: catabolite repression, autoregulation, and effect on araBAD expression. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4120–4124. doi: 10.1073/pnas.81.13.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. O., Wright J. K., Postma P. W. The mechanism of inducer exclusion. Direct interaction between purified III of the phosphoenolpyruvate:sugar phosphotransferase system and the lactose carrier of Escherichia coli. EMBO J. 1983;2(5):715–720. doi: 10.1002/j.1460-2075.1983.tb01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny M. J., Frederickson W. L., Waygood E. B., Saier M. H., Jr Allosteric regulation of glycerol kinase by enzyme IIIglc of the phosphotransferase system in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1985 May;162(2):810–816. doi: 10.1128/jb.162.2.810-816.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden S., Haggerty D., Stoner C. M., Kolodrubetz D., Schleif R. The Escherichia coli L-arabinose operon: binding sites of the regulatory proteins and a mechanism of positive and negative regulation. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3346–3350. doi: 10.1073/pnas.77.6.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri K. G., Waygood E. B. Sequence of cloned enzyme IIN-acetylglucosamine of the phosphoenolpyruvate:N-acetylglucosamine phosphotransferase system of Escherichia coli. Biochemistry. 1988 Aug 9;27(16):6054–6061. doi: 10.1021/bi00416a034. [DOI] [PubMed] [Google Scholar]
- Plumbridge J. A. Sequence of the nagBACD operon in Escherichia coli K12 and pattern of transcription within the nag regulon. Mol Microbiol. 1989 Apr;3(4):505–515. doi: 10.1111/j.1365-2958.1989.tb00197.x. [DOI] [PubMed] [Google Scholar]
- Plumbridge J. Organisation of the Escherichia coli chromosome between genes glnS and glnU, V. Mol Gen Genet. 1987 Oct;209(3):618–620. doi: 10.1007/BF00331173. [DOI] [PubMed] [Google Scholar]
- Plumbridge J., Söll D. Characterization of cis-acting mutations which increase expression of a glnS-lacZ fusion in Escherichia coli. Mol Gen Genet. 1989 Mar;216(1):113–119. doi: 10.1007/BF00332238. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud O., Vidal-Ingigliardi D., Richet E. A complex nucleoprotein structure involved in activation of transcription of two divergent Escherichia coli promoters. J Mol Biol. 1989 Feb 5;205(3):471–485. doi: 10.1016/0022-2836(89)90218-0. [DOI] [PubMed] [Google Scholar]
- Rephaeli A. W., Saier M. H., Jr Regulation of genes coding for enzyme constituents of the bacterial phosphotransferase system. J Bacteriol. 1980 Feb;141(2):658–663. doi: 10.1128/jb.141.2.658-663.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M. J., Ohgi T., Plumbridge J., Söll D. Nucleotide sequences of the Escherichia coli nagE and nagB genes: the structural genes for the N-acetylglucosamine transport protein of the bacterial phosphoenolpyruvate: sugar phosphotransferase system and for glucosamine-6-phosphate deaminase. Gene. 1988;62(2):197–207. doi: 10.1016/0378-1119(88)90558-6. [DOI] [PubMed] [Google Scholar]
- Roy A., Haziza C., Danchin A. Regulation of adenylate cyclase synthesis in Escherichia coli: nucleotide sequence of the control region. EMBO J. 1983;2(5):791–797. doi: 10.1002/j.1460-2075.1983.tb01502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Novotny M. J., Comeau-Fuhrman D., Osumi T., Desai J. D. Cooperative binding of the sugar substrates and allosteric regulatory protein (enzyme IIIGlc of the phosphotransferase system) to the lactose and melibiose permeases in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1983 Sep;155(3):1351–1357. doi: 10.1128/jb.155.3.1351-1357.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr Protein phosphorylation and allosteric control of inducer exclusion and catabolite repression by the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Microbiol Rev. 1989 Mar;53(1):109–120. doi: 10.1128/mr.53.1.109-120.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck-Eidens D. M., Kadner R. J. Exogenous induction of the Escherichia coli hexose phosphate transport system defined by uhp-lac operon fusions. J Bacteriol. 1981 Oct;148(1):203–209. doi: 10.1128/jb.148.1.203-209.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus L., Wilcox G. Effect of mutations in the cyclic AMP receptor protein-binding site on araBAD and araC expression. J Bacteriol. 1989 Feb;171(2):1178–1184. doi: 10.1128/jb.171.2.1178-1184.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler A. P., Lengeler J. W. Analysis of the nag regulon from Escherichia coli K12 and Klebsiella pneumoniae and of its regulation. Mol Gen Genet. 1989 Oct;219(1-2):97–105. doi: 10.1007/BF00261163. [DOI] [PubMed] [Google Scholar]
- Warwicker J., Engelman B. P., Steitz T. A. Electrostatic calculations and model-building suggest that DNA bound to CAP is sharply bent. Proteins. 1987;2(4):283–289. doi: 10.1002/prot.340020404. [DOI] [PubMed] [Google Scholar]
- Weber I. T., Steitz T. A. Structure of a complex of catabolite gene activator protein and cyclic AMP refined at 2.5 A resolution. J Mol Biol. 1987 Nov 20;198(2):311–326. doi: 10.1016/0022-2836(87)90315-9. [DOI] [PubMed] [Google Scholar]
- Weston L. A., Kadner R. J. Identification of uhp polypeptides and evidence for their role in exogenous induction of the sugar phosphate transport system of Escherichia coli K-12. J Bacteriol. 1987 Aug;169(8):3546–3555. doi: 10.1128/jb.169.8.3546-3555.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J. Control of amino sugar metabolism in Escherichia coli and isolation of mutants unable to degrade amino sugars. Biochem J. 1968 Feb;106(4):847–858. doi: 10.1042/bj1060847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J. The role of the phosphoenolpyruvate phosphotransferase system in the transport of N-acetyl-D-glucosamine by Escherichia coli. Biochem J. 1970 Jun;118(1):89–92. doi: 10.1042/bj1180089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- Yamada M., Saier M. H., Jr Glucitol-specific enzymes of the phosphotransferase system in Escherichia coli. Nucleotide sequence of the gut operon. J Biol Chem. 1987 Apr 25;262(12):5455–5463. [PubMed] [Google Scholar]
- de Crombrugghe B., Busby S., Buc H. Cyclic AMP receptor protein: role in transcription activation. Science. 1984 May 25;224(4651):831–838. doi: 10.1126/science.6372090. [DOI] [PubMed] [Google Scholar]