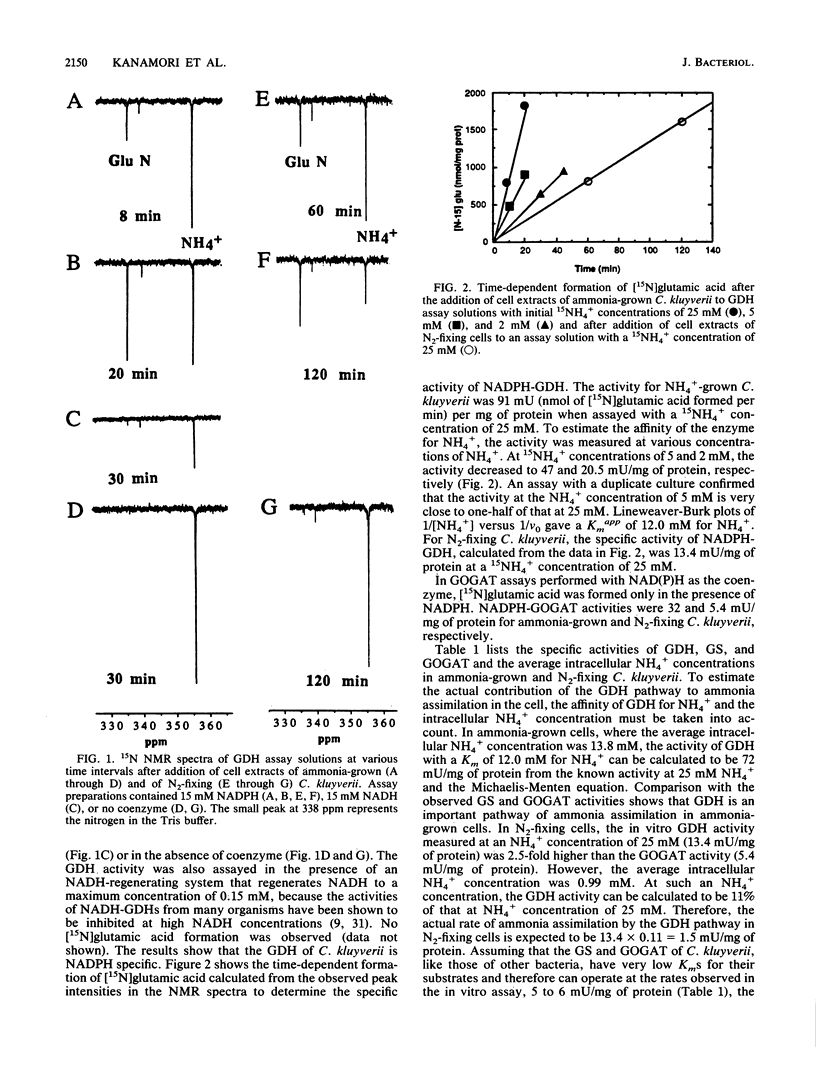
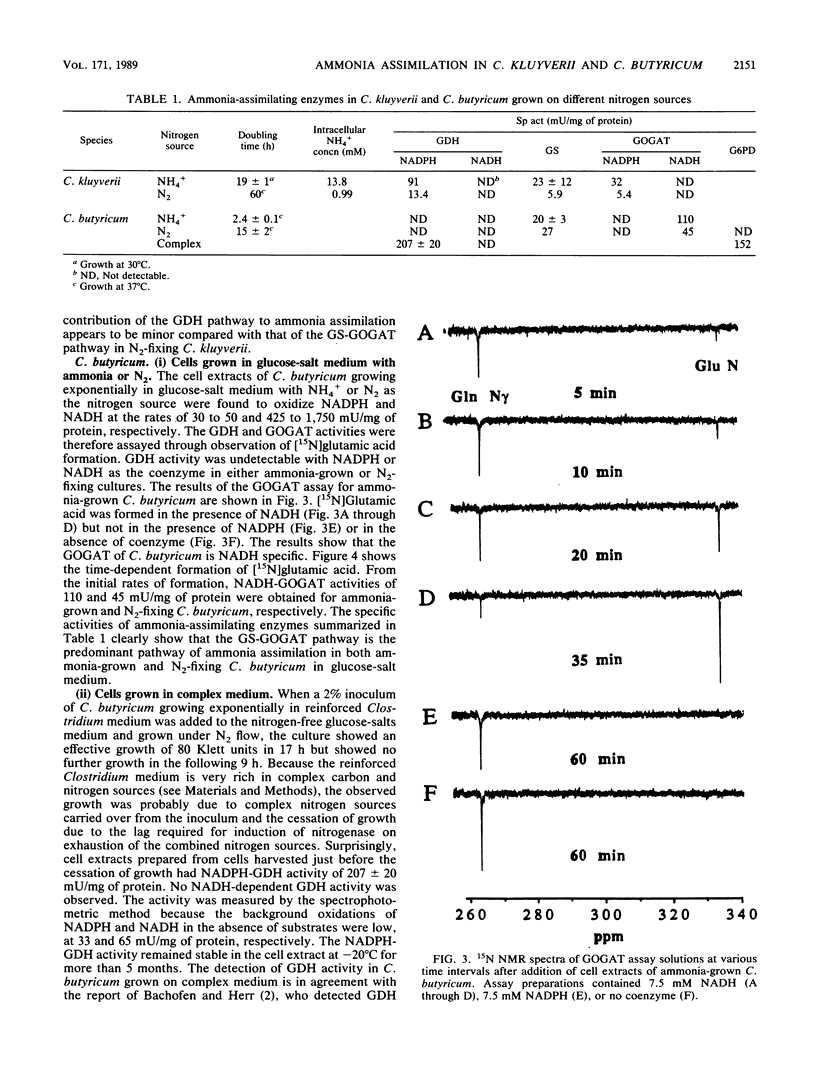
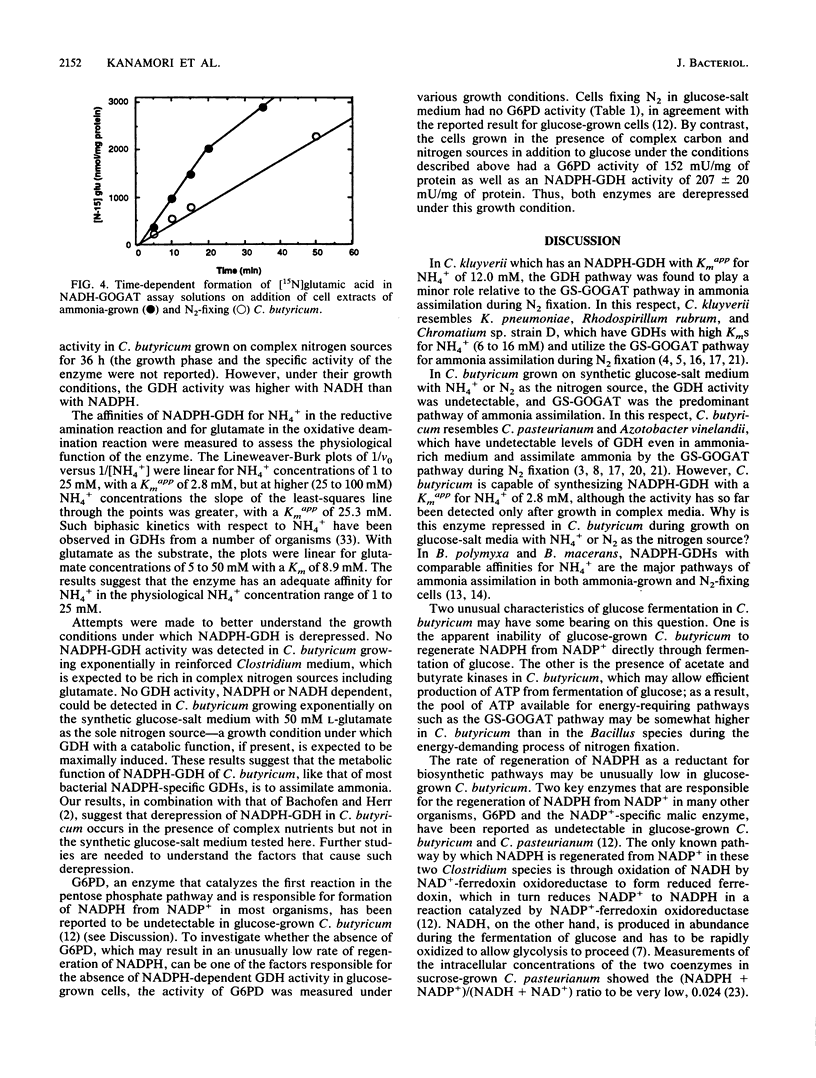
Abstract
Pathways of ammonia assimilation into glutamic acid were investigated in ammonia-grown and N2-fixing Clostridium kluyverii and Clostridium butyricum by measuring the specific activities of glutamate dehydrogenase, glutamine synthetase, and glutamate synthase. C. kluyverii had NADPH-glutamate dehydrogenase with a Km of 12.0 mM for NH4+. The glutamate dehydrogenase pathway played an important role in ammonia assimilation in ammonia-grown cells but was found to play a minor role relative to that of the glutamine synthetase/NADPH-glutamate synthase pathway in nitrogen-fixing cells when the intracellular NH4+ concentration and the low affinity of the enzyme for NH4+ were taken into account. In C. butyricum grown on glucose-salt medium with ammonia or N2 as the nitrogen source, glutamate dehydrogenase activity was undetectable, and the glutamine synthetase/NADH-glutamate synthase pathway was the predominant pathway of ammonia assimilation. Under these growth conditions, C. butyricum also lacked the activity of glucose-6-phosphate dehydrogenase, which catalyzes the regeneration of NADPH from NADP+. However, high activities of glucose-6-phosphate dehydrogenase as well as of NADPH-glutamate dehydrogenase with a Km of 2.8 mM for NH4+ were present in C. butyricum after growth on complex nitrogen and carbon sources. The ammonia-assimilating pathway of N2-fixing C. butyricum, which differs from that of the previously studied Bacillus polymyxa and Bacillus macerans, is discussed in relation to possible effects of the availability of ATP and of NADPH on ammonia-assimilating pathways.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachofen R., Heer E. Uber die Synthese von Alanin und Aspartat aus Acetat und CO2 bei Clostridium butyricum. Pathol Microbiol (Basel) 1966;29(5):747–755. [PubMed] [Google Scholar]
- CARNAHAN J. E., CASTLE J. E. Some requirements of biological nitrogen fixation. J Bacteriol. 1958 Feb;75(2):121–124. doi: 10.1128/jb.75.2.121-124.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbendam P. M., Neijssel O. M., Tempest D. W. Metabolic and energetic aspects of the growth of Clostridium butyricum on glucose in chemostat culture. Arch Microbiol. 1985 Sep;142(4):375–382. doi: 10.1007/BF00491907. [DOI] [PubMed] [Google Scholar]
- Dainty R. H. Glutamate biosynthesis in Clostridium pasteurianum and its significance in nitrogen metabolism. Biochem J. 1972 Feb;126(4):1055–1056. doi: 10.1042/bj1261055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher H. F. Glutamate dehydrogenase--ligand complexes and their relationship to the mechanism of the reaction. Adv Enzymol Relat Areas Mol Biol. 1973;39:369–417. doi: 10.1002/9780470122846.ch6. [DOI] [PubMed] [Google Scholar]
- Gottschalk G., Barker H. A. Synthesis of glutamate and citrate by Clostridium kluyveri. A new type of citrate synthase. Biochemistry. 1966 Apr;5(4):1125–1133. doi: 10.1021/bi00868a003. [DOI] [PubMed] [Google Scholar]
- Jungermann K., Thauer R. K., Leimenstoll G., Decker K. Function of reduced pyridine nucleotide-ferredoxin oxidoreductases in saccharolytic Clostridia. Biochim Biophys Acta. 1973 May 30;305(2):268–280. doi: 10.1016/0005-2728(73)90175-8. [DOI] [PubMed] [Google Scholar]
- Kanamori K., Weiss R. L., Roberts J. D. Ammonia assimilation in Bacillus polymyxa. 15N NMR and enzymatic studies. J Biol Chem. 1987 Aug 15;262(23):11038–11045. [PubMed] [Google Scholar]
- Kanamori K., Weiss R. L., Roberts J. D. Glutamate biosynthesis in Bacillus azotofixans. 15N NMR and enzymatic studies. J Biol Chem. 1988 Feb 25;263(6):2817–2823. [PubMed] [Google Scholar]
- Kanamori K., Weiss R. L., Roberts J. D. Role of glutamate dehydrogenase in ammonia assimilation in nitrogen-fixing Bacillus macerans. J Bacteriol. 1987 Oct;169(10):4692–4695. doi: 10.1128/jb.169.10.4692-4695.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner D. Ammonium uptake and metabolism by mitrogen fixing bacteria. II. Klebsiella pneumoniae. Arch Microbiol. 1976 Dec 1;111(1-2):85–91. doi: 10.1007/BF00446553. [DOI] [PubMed] [Google Scholar]
- Krishnan I. S., Singhal R. K., Dua R. D. Purification and characterization of glutamine synthetase from Clostridium pasteurianum. Biochemistry. 1986 Apr 8;25(7):1589–1599. doi: 10.1021/bi00355a021. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mortenson L. E. Regulation of nitrogen fixation. Curr Top Cell Regul. 1978;13:179–232. doi: 10.1016/b978-0-12-152813-3.50010-0. [DOI] [PubMed] [Google Scholar]
- Nagatani H., Shimizu M., Valentine R. C. The mechanism of ammonia assimilation in nitrogen fixing Bacteria. Arch Mikrobiol. 1971;79(2):164–175. doi: 10.1007/BF00424923. [DOI] [PubMed] [Google Scholar]
- Opheim D., Bernlohr R. W. Purification and regulation of glucose-6-phosphate dehydrogenase from Bacillus licheniformis. J Bacteriol. 1973 Dec;116(3):1150–1159. doi: 10.1128/jb.116.3.1150-1159.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitdemange H., Lambert D., Gay R. Détermination des nucléotides pyridiniques oxydés et réduits chez Clostridium pastorianum. C R Seances Soc Biol Fil. 1973;167(1):111–115. [PubMed] [Google Scholar]
- Prusiner S., Milner L. A rapid radioactive assay for glutamine synthetase, glutaminase, asparagine synthetase, and asparaginase. Anal Biochem. 1970 Oct;37(2):429–438. doi: 10.1016/0003-2697(70)90069-2. [DOI] [PubMed] [Google Scholar]
- Rosenblum E. D., Wilson P. W. FIXATION OF ISOTOPIC NITROGEN BY CLOSTRIDIUM. J Bacteriol. 1949 Apr;57(4):413–414. [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujita S., Kimura K. Glucose-6-phosphate dehydrogenase, vegetative and spore Bacillus subtilis. Methods Enzymol. 1982;89(Pt 500):258–261. doi: 10.1016/s0076-6879(82)89046-0. [DOI] [PubMed] [Google Scholar]
- Upchurch R. G., Mortenson L. E. In vivo energetics and control of nitrogen fixation: changes in the adenylate energy charge and adenosine 5'-diphosphate/adenosine 5'-triphosphate ratio of cells during growth on dinitrogen versus growth on ammonia. J Bacteriol. 1980 Jul;143(1):274–284. doi: 10.1128/jb.143.1.274-284.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnacker E. L., Barker H. A. Purification and properties of a NAD-dependent glutamate dehydrogenase from Clostridium SB4. Biochim Biophys Acta. 1970 Aug 15;212(2):225–242. doi: 10.1016/0005-2744(70)90203-2. [DOI] [PubMed] [Google Scholar]
- Wootton J. C. Re-assessment of ammonium-ion affinities of NADP-specific glutamate dehydrogenases. Activation of the Neurospora crassa enzyme by ammonium and rubidium ions. Biochem J. 1983 Feb 1;209(2):527–531. doi: 10.1042/bj2090527. [DOI] [PMC free article] [PubMed] [Google Scholar]


