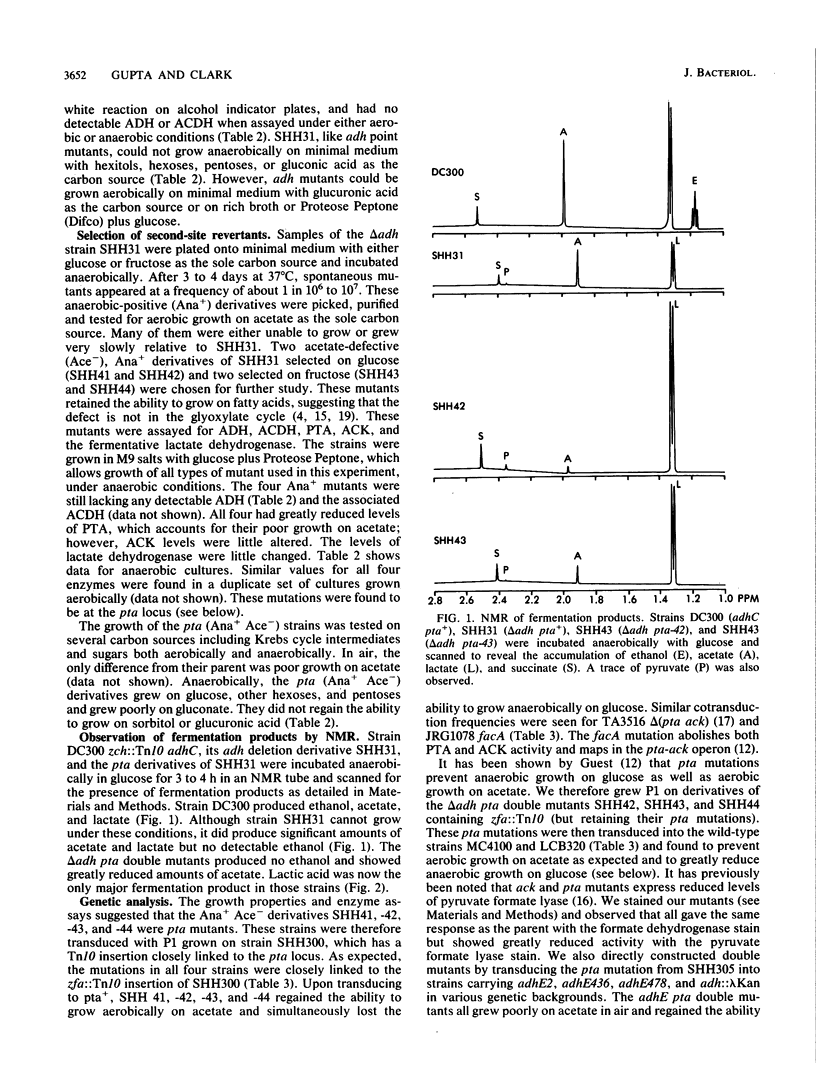
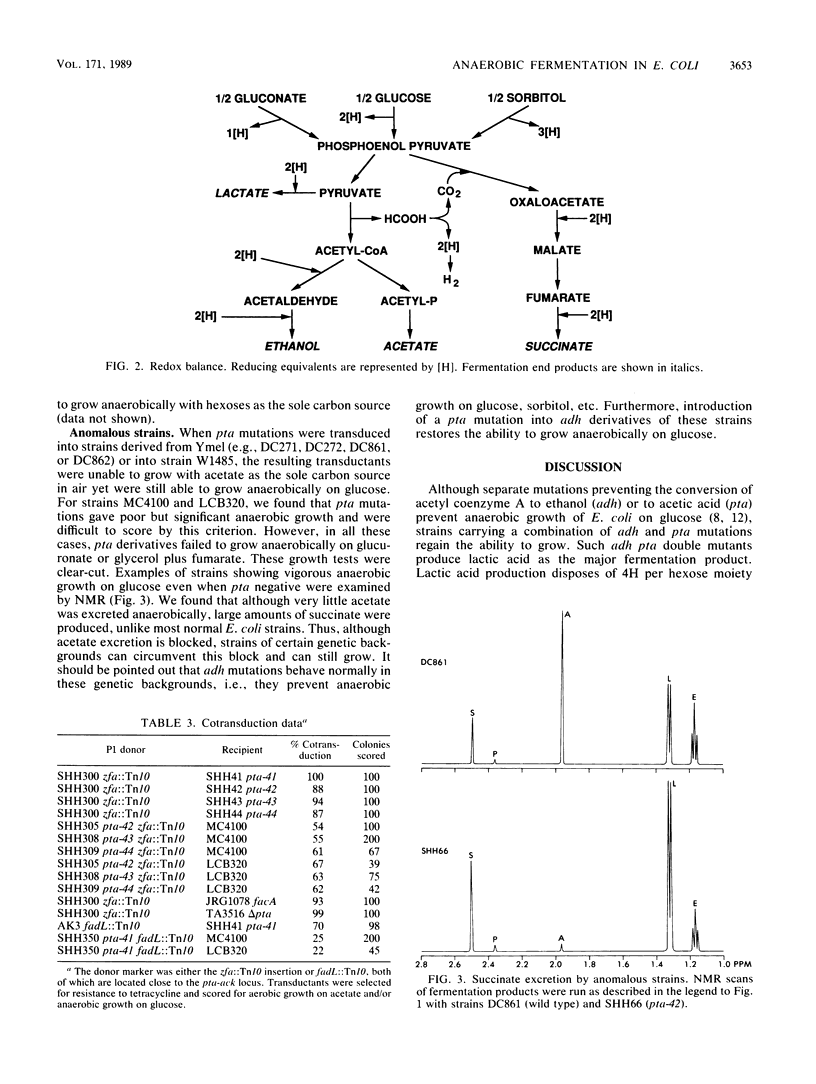
Abstract
Escherichia coli mutants lacking alcohol dehydrogenase (adh mutants) cannot synthesize the fermentation product ethanol and are unable to grow anaerobically on glucose and other hexoses. Similarly, phosphotransacetylase-negative mutants (pta mutants) neither excrete acetate nor grow anaerobically. However, when a strain carrying an adh deletion was selected for anaerobic growth on glucose, spontaneous pta mutants were isolated. Strains carrying both adh and pta mutations were observed by in vivo nuclear magnetic resonance and shown to produce lactic acid as the major fermentation product. Various combinations of adh pta double mutants regained the ability to grow anaerobically on hexoses, by what amounts to a homolactic fermentation. Unlike wild-type strains, such adh pta double mutants were unable to grow anaerobically on sorbitol or on glucuronic acid. The growth properties of strains carrying various mutations affecting the enzymes of fermentation are discussed in terms of redox balance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Savageau M. A. Generalized indicator plate for genetic, metabolic, and taxonomic studies with microorganisms. Appl Environ Microbiol. 1977 Feb;33(2):434–444. doi: 10.1128/aem.33.2.434-444.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brice C. B., Kornberg H. L. Genetic control of isocitrate lyase activity in Escherichia coli. J Bacteriol. 1968 Dec;96(6):2185–2186. doi: 10.1128/jb.96.6.2185-2186.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. D., Jones-Mortimer M. C., Kornberg H. L. The enzymic interconversion of acetate and acetyl-coenzyme A in Escherichia coli. J Gen Microbiol. 1977 Oct;102(2):327–336. doi: 10.1099/00221287-102-2-327. [DOI] [PubMed] [Google Scholar]
- Clark D. P., Cunningham P. R., Reams S. G., Mat-Jan F., Mohammedkhani R., Williams C. R. Mutants of Escherichia coli defective in acid fermentation. Appl Biochem Biotechnol. 1988 Apr;17:163–173. doi: 10.1007/BF02779155. [DOI] [PubMed] [Google Scholar]
- Clark D., Cronan J. E., Jr Escherichia coli mutants with altered control of alcohol dehydrogenase and nitrate reductase. J Bacteriol. 1980 Jan;141(1):177–183. doi: 10.1128/jb.141.1.177-183.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham P. R., Clark D. P. The use of suicide substrates to select mutants of Escherichia coli lacking enzymes of alcohol fermentation. Mol Gen Genet. 1986 Dec;205(3):487–493. doi: 10.1007/BF00338087. [DOI] [PubMed] [Google Scholar]
- DAWES E. A., FOSTER S. M. The formation of ethanol in Escherichia coli. Biochim Biophys Acta. 1956 Nov;22(2):253–265. doi: 10.1016/0006-3002(56)90148-2. [DOI] [PubMed] [Google Scholar]
- Fox D. K., Meadow N. D., Roseman S. Phosphate transfer between acetate kinase and enzyme I of the bacterial phosphotransferase system. J Biol Chem. 1986 Oct 15;261(29):13498–13503. [PubMed] [Google Scholar]
- Guest J. R. Anaerobic growth of Escherichia coli K12 with fumarate as terminal electron acceptor. Genetic studies with menaquinone and fluoroacetate-resistant mutants. J Gen Microbiol. 1979 Dec;115(2):259–271. doi: 10.1099/00221287-115-2-259. [DOI] [PubMed] [Google Scholar]
- Ingledew W. J., Poole R. K. The respiratory chains of Escherichia coli. Microbiol Rev. 1984 Sep;48(3):222–271. doi: 10.1128/mr.48.3.222-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. M., Gunsalus R. P. Transcription of the Escherichia coli fumarate reductase genes (frdABCD) and their coordinate regulation by oxygen, nitrate, and fumarate. J Bacteriol. 1985 Dec;164(3):1100–1109. doi: 10.1128/jb.164.3.1100-1109.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan H. S., Chui H. W., Wong K. K. ack::Mu d1-8 (Apr lac) operon fusions of Salmonella typhimurium LT2. Mol Gen Genet. 1988 Jan;211(1):183–185. doi: 10.1007/BF00338411. [DOI] [PubMed] [Google Scholar]
- LeVine S. M., Ardeshir F., Ames G. F. Isolation and Characterization of acetate kinase and phosphotransacetylase mutants of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1980 Aug;143(2):1081–1085. doi: 10.1128/jb.143.2.1081-1085.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorowitz W., Clark D. Escherichia coli mutants with a temperature-sensitive alcohol dehydrogenase. J Bacteriol. 1982 Nov;152(2):935–938. doi: 10.1128/jb.152.2.935-938.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Genetic regulation of the glyoxylate shunt in Escherichia coli K-12. J Bacteriol. 1982 Jan;149(1):173–180. doi: 10.1128/jb.149.1.173-180.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason T. G., Richardson G. Escherichia coli and the human gut: some ecological considerations. J Appl Bacteriol. 1981 Aug;51(1):1–16. doi: 10.1111/j.1365-2672.1981.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Mat-Jan F., Alam K. Y., Clark D. P. Mutants of Escherichia coli deficient in the fermentative lactate dehydrogenase. J Bacteriol. 1989 Jan;171(1):342–348. doi: 10.1128/jb.171.1.342-348.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino T., Arata Y., Fujiwara S. Proton correlation nuclear magnetic resonance study of metabolic regulations and pyruvate transport in anaerobic Escherichia coli cells. Biochemistry. 1980 Aug 5;19(16):3684–3691. doi: 10.1021/bi00557a008. [DOI] [PubMed] [Google Scholar]
- Ogino T., Arata Y., Fujiwara S., Shoun H., Beppu T. Proton correlation nuclear magnetic resonance study of anaerobic metabolism of Escherichia coli. Biochemistry. 1978 Oct 31;17(22):4742–4745. doi: 10.1021/bi00615a022. [DOI] [PubMed] [Google Scholar]
- Pascal M. C., Chippaux M., Abou-Jaoudé A., Blaschkowski H. P., Knappe J. Mutants of Escherichia coli K12 with defects in anaerobic pyruvate metabolism. J Gen Microbiol. 1981 May;124(1):35–42. doi: 10.1099/00221287-124-1-35. [DOI] [PubMed] [Google Scholar]
- STADTMAN E. R., NOVELLI G. D., LIPMANN F. Coenzyme A function in and acetyl transfer by the phosphotransacetylase system. J Biol Chem. 1951 Jul;191(1):365–376. [PubMed] [Google Scholar]
- Stewart V. Nitrate respiration in relation to facultative metabolism in enterobacteria. Microbiol Rev. 1988 Jun;52(2):190–232. doi: 10.1128/mr.52.2.190-232.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarmy E. M., Kaplan N. O. Chemical characterization of D-lactate dehydrogenase from Escherichia coli B. J Biol Chem. 1968 May 25;243(10):2579–2586. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Winkelman J. W., Clark D. P. Anaerobically induced genes of Escherichia coli. J Bacteriol. 1986 Jul;167(1):362–367. doi: 10.1128/jb.167.1.362-367.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


