Abstract
Oncolytic adenoviruses as a treatment for cancer have demonstrated limited clinical activity. Contributing to this may be the relevance of preclinical animal models used to study these agents. Syngeneic mouse tumor models are generally non-permissive for adenoviral replication, where as human tumor xenograft models exhibit attenuated immune responses to the vector. The cotton rat (Sigmodon hispidus) is susceptible to human adenovirus infection, permissive for viral replication and exhibits similar inflammatory pathology to humans with adenovirus replicating in the lungs, respiratory passages and cornea. We evaluated three transplantable tumorigenic cotton rat cell lines, CCRT, LCRT and VCRT as models for the study of oncolytic adenoviruses. All three cells lines were readily infected with adenovirus type-5-based vectors and exhibited high levels of transgene expression. The cell lines supported viral replication demonstrated by the induction of cytopathogenic effect (CPE) in tissue culture, increase in virus particle numbers and assembly of virions seen on transmission electron microscopy. In vivo, LCRT and VCRT tumors demonstrated delayed growth after injection with replicating adenovirus. No in vivo antitumor activity was seen in CCRT tumors despite in vitro oncolysis. Adenovirus was also rapidly cleared from the CCRT tumors compared to LCRT and VCRT tumors. The effect observed with the different cotton rat tumor cell lines mimics the variable results of human clinical trials highlighting the potential relevance of this model for assessing the activity and toxicity of oncolytic adenoviruses.
Keywords: Replicating adenovirus, cotton rat, oncolysis, virotherapy, cancer
INTRODUCTION
The concept of using replicating lytic viruses as a treatment for cancer is not new. A study of wild type adenovirus as a potential treatment for carcinoma of the uterine cervix was reported in 1956, three years after the initial discovery of the virus (Smith et al. 1956). Intratumoral injections of adenovirus resulted in necrosis and cavity formation in 65% of treated tumors and live adenovirus was recovered from two-thirds of these patients suggesting that viral replication was occurring in vivo. In contrast, no virus was isolated from the tumors of patients treated with heat-inactivated virus. A second clinical trial targeting a variety of tumor using adenovirus administered by various routes was less successful, with only 2 of 14 patients showing transient tumor regression and virus recovered from only a single subject (Southam, et al. 1956). Shortly thereafter, advances in chemotherapy coupled with low viral yields due to inefficient production techniques, and concerns over the risk of viral dissemination and illness lead to the abandonment of virotherapy.
Over the last decade, new understanding and developments in molecular biology, virology and genetic engineering have lead to the reemergence of virotherapy as a potential treatment for cancer. A number of replicating oncolytic adenovirus-based vectors have entered into clinical trials. These include the conditionally replicating ONYX-015 (dl1520), CV706, CG7870, Ad5-CD/TKrep and the Ad-OC-E1a vectors (Benjamin et al. 2001, Bischoff et al. 1996, DeWeese et al. 2001, Freytag et al. 2002, Small et al. 2006). Despite promising preclinical studies, much of the initial excitement surrounding oncolytic adenoviruses has dampened with reports of a lack of tumor specificity and few observed clinical responses when used as single agents (Kirn 2001).
The animal models used to evaluate these vectors may be a significant factor in this discordance. Human adenoviruses are unable to generate productive infections in most non-human tissues limiting the usefulness of syngeneic immunocompetent animal tumor models for the pre-clinical evaluation of these agents. To date, the model of choice to study these agents has been human tumor xenografts grown immunodeficient mice. While immunodeficient mouse models are permissive to adenoviral replication occurring in the xenograft, they do not adequately assess the effect of viral dissemination and replication on other tissues, and the host immune responses to the virus and related toxicity. The inflammatory responses induced by adenovirus are attenuated in these animals (Zhang et al. 2001). In order to better assess factors important to the efficacy of oncolytic adenovirus vectors, a preclinical model that utilizes a syngeneic transplantable tumor in an immunocompetent host would be advantageous.
Cotton rats (Sigmodon hispidus) are susceptible to infection with a number of human respiratory viruses including Group C adenoviruses (Niewiesk & Prince 2002). Cotton rats exhibit similar inflammatory pathology to humans with the virus actively replicating in the lungs, tracheobronchial tree, nasal passages and cornea (Pacini, et al. 1984, Prince et al. 1993, Tsai et al. 1992). This has resulted in their use to assess the in vivo spread and toxicity of adenoviral-based vectors prior to their use in human gene therapy trials (Rojas-Martinez et al. 1998). Until recently syngeneic transplantable cotton rat tumor cell lines were unavailable. We studied three recently isolated tumorigenic cell lines derived from spontaneous tumors of cotton rats, CCRT, LCRT (Toth et al. 2005) and VCRT. Subcutaneous implantation of these cell lines lead to the formation of tumors in immunocompetent cotton rats. We evaluated each of the tumor cell lines for permissiveness for adenoviral infection and replication, and assessed their efficacy as an in vivo model for human adenovirus virotherapy for cancer.
RESULTS
CCRT, LCRT and VCRT cotton rat tumor cells are readily infected with adenovirus type-5 and express encoded transgenes
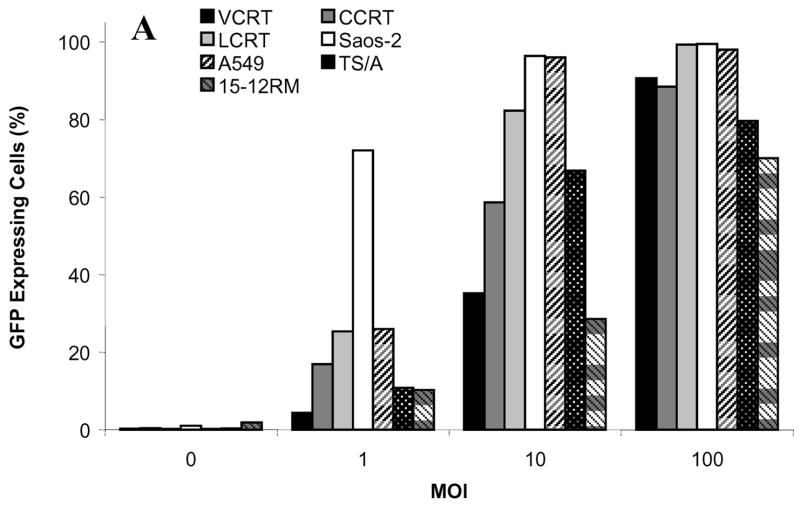
The in vitro ability of the cotton rat tumor cell lines to be infected with adenovirus was examined using Ad.GFP, an E1-deleted replication-deficient vector expressing green fluorescent protein. The cotton rat tumor cells were compared to mouse 15-12RM sarcoma cells and TS/A mammary carcinoma cells, and human Saos-2 osteosarcoma and A549 lung cancer cells (Fig. 1). LCRT cells were the most readily infected of the cotton rat tumors lines, with 99.3% of the cells expressing GFP at an MOI of 100 PFU/cell as detected by flow cytometry. This was similar to the human cell lines examined, with 99.5% of Saos-2 cells and 98% of A549 cells expressing GFP. At MOI 100 PFU/cell, VCRT and CCRT cells also showed high levels of expression with 90.6% and 88.5% cells expressing GFP, respectively. At MOI 100 PFU/cell, 79.6% of TS/A cells and 70% 15-12RM cells were positive for GFP.
FIG 1.
Transgene expression in cotton rat tumor cells infected with a non-replicating adenovirus expressing GFP. CCRT, LCRT and VCRT cotton rat tumor cells, the A549 and Saos-2 human cancer cell lines and TS/A and 15-12RM murine cancer cells were infected with Ad.GFP at MOI 1, 10 and 100 and the percentage of cells expressing GFP was determined at 48 h by flow cytometry.
Induction of cytopathogenic effect (CPE) in cotton rat tumor cells by replicating adenovirus
Cotton rat tumor cell lines were examined for induction of CPE after infection with the E1+ replicating Ad.OW94 expressing GFP. This was compared to cells infected with Ad.GFP. Using fluorescence microscopy, GFP expression was detected in each of the cotton rat tumor lines infected with Ad.GFP; however, no CPE was observed at 7 days in any of the cell lines infected Ad.GFP up to an MOI of 100 PFU/cell (Fig. 2). Expression of GFP was observed in the cotton rat cell lines infected with Ad.OW94 with marked CPE noted on microscopic evaluation and staining with crystal violet. CPE was observed in CCRT and LCRT cells infected with Ad.OW94 at an MOI as low as 10 PFU/cell. In contrast, only limited CPE was noted in VCRT cells infected with Ad.OW94 at this MOI; however, at an MOI of 100 PFU/cell, high levels of GFP expression were noted with significant CPE observed in all three cotton rat cell lines 7 days after infection. As expected, HEK-293 cells exhibited high levels of GFP expression and were almost completely lysed 72 h after infection with either Ad.GFP or Ad.OW94.
FIG 2.
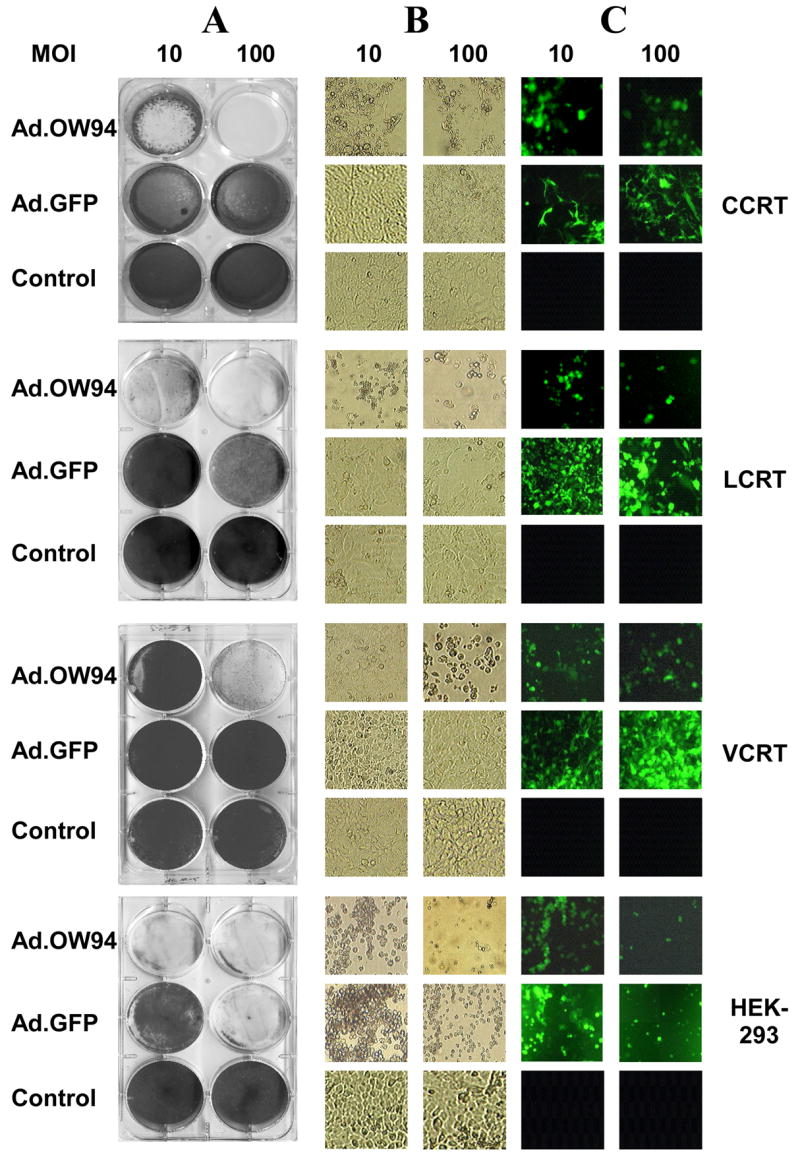
Lytic activity of Ad.OW94 (E1+) or Ad.GFP (E1−) on CCRT, LCRT, VCRT, and HEK-293 cells. Each of the cell lines were seeded at 2 × 105 cells per well onto 6 well plates and infected with Ad.GFP or Ad.OW94 at MOI of 10 or 100 PFU per cell, or treated with a PBS control. After 7 days, cells were: (A) stained with 1% crystal violet, (B) examined microscopically for CPE, and (C) examined by fluorescence microscopy for GFP expression.
Productive replication of adenovirus in cotton rat tumor cells
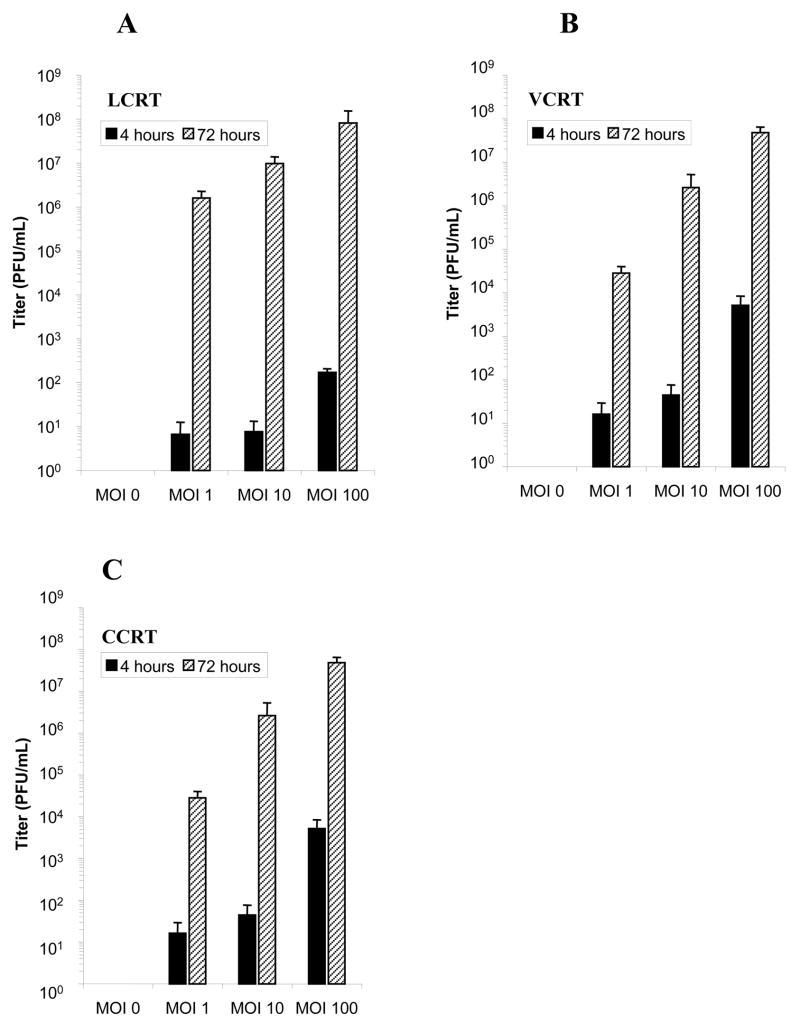
Viral burst assays were performed to determine the titer of infective adenovirus produced by the cotton rat cells at a given viral input. Following infection with Ad5.wt at MOI 0, 1, 10, or 100 PFU/cell, the quantity of adenovirus present in the cells was assessed at 4 h and 72 h after initial infection by plaque titer. Lysates of LCRT, VCRT and CCRT cells showed significantly higher titers of adenovirus at 72 h than at 4 h after infection indicating replication of Ad5.wt was occurring in the cell lines (Fig. 3). At an MOI of 1 PFU/cell, LCRT cells produced significantly more adenovirus (57-fold) than either the CCRT or VCRT cell lines (P<0.05). At an MOI of 1 PFU/cell there was no difference in production of adenovirus between the CCRT and VCRT cells. At 100 PFU/cell there was no significant difference between the amounts of adenovirus detected at 72 h in CCRT or LCRT cells; however, VCRT cells consistently produced lower viral titers.
FIG 3.
Viral burst assays to determine the quantity of adenovirus released by (A) LCRT, (B) VCRT and (C) CCRT cells infected with Ad5.wt. The titer of infectious adenovirus was assessed at 4 h and 72 h following infection with Ad5.wt at MOI 0, 1, 10, or 100. Error bars represent SD of the mean.
Detection of mature adenoviral particles in cotton rat tumor cells in a time dependent manner
CCRT, LCRT and VCRT cells infected with Ad5.wt at MOI of 1 PFU/cell were examined at 4 h and 72 h post-infection by transmission electron microscopy. No virions were detected when each of the cell lines were examined 4 h after infection (Fig. 4A, 4C & 4E). Infecting adenovirus would have uncoated at this time point and the viral DNA released. In contrast, mature appearing adenoviral particles were visible in the nucleus of cotton rat tumor lines at 72 h post-infection (Fig. 4B, 4D & 4F).
FIG 4.
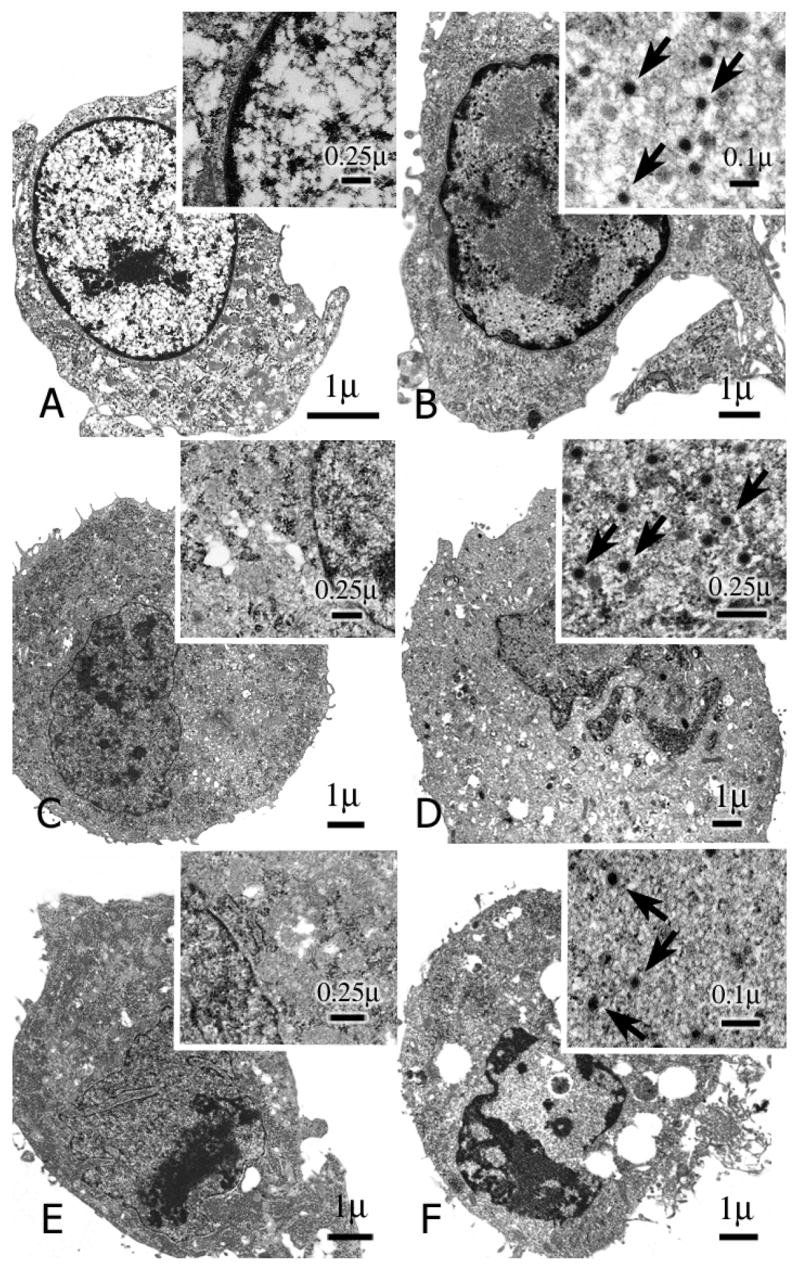
Transmission electron micrographs of CCRT, LCRT and VCRT cells performed 4 h and 72 h after infection with adenovirus. (A & B), CCRT (C & D) LCRT and (E & F) VCRT cotton rat cell lines were infected with Ad5.wt at MOI 1 and examined by electron microscopy 4 h (A, C & E) and 72 h (B, D & F) after infection. Arrows indicate adenoviral particles.
Time-dependent increase in functional vector
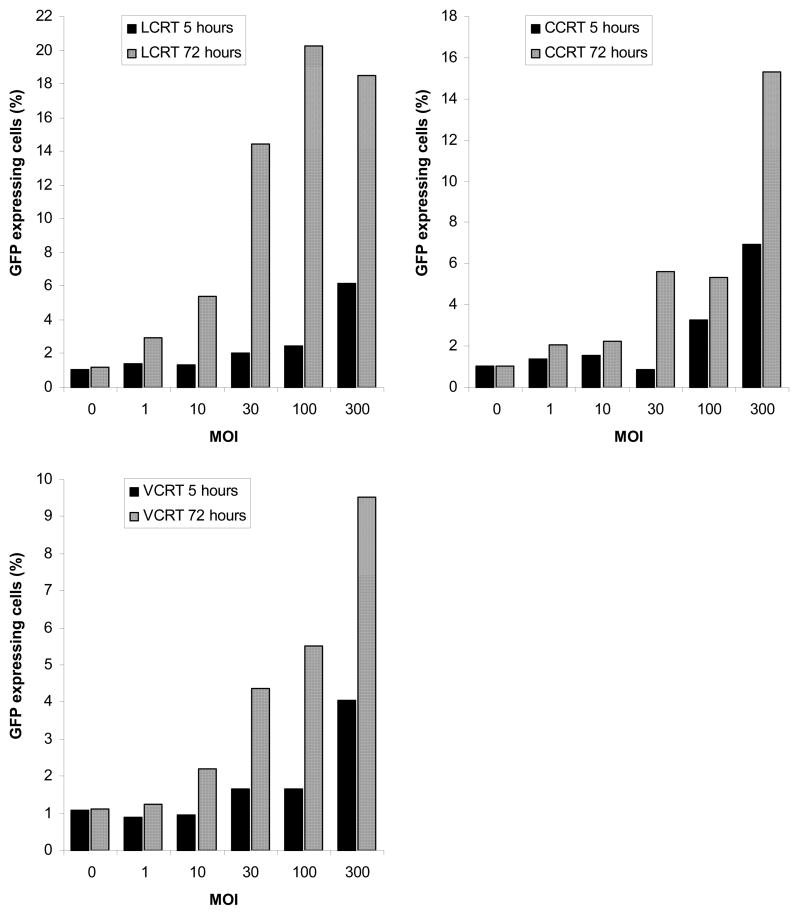
We examined the functionality of the virus produced from Ad.OW94 infected CCRT, LCRT and VCRT cells by transducing HeLa cell monolayers with cell-free supernatants obtained from the cotton rat cell lines 5 h and 72 h after initial infection. HeLa cells were examined 48 h later for expression of GFP. The level of GFP expression in the HeLa cells indicated the presence of higher titers of functional vector at 72 h than at 5 h, indicating the production of functioning vector (Fig. 5). This finding was consistent at all MOI’s examined. The 72 h supernatants from LCRT cells infected at an MOI of 100 PFU/cell, resulted in the greatest number of GFP-expressing HeLa cells (20.2%). This represented a 10-fold increase in the number of GFP expressing cells compared to 5 h. Supernatants removed at 72 h from CCRT cells infected at an MOI of 300 PFU/cell, resulted in 15.3% GFP expression by the HeLa cells. This was a 2.2-fold increase in expression compared to cells transduced with the 5 h supernatant. Supernatants from VCRT cells resulted in the lowest number of GFP expressing HeLa cells (9.5%) even an initial MOI of 300 PFU/cell, indicating less effective virus production by VCRT cells. This was still 2.3-fold more than observed with the 5 h supernatant.
FIG 5.
Time-dependent increase in functional viral titer after infection with replicating adenovirus. LCRT, VCRT and CCRT cells were infected with Ad.OW94. At 5 h and 72 h cell-free media supernatants were transferred to HeLa cell monolayers. HeLa cells were analyzed 24 h later for GFP expression.
Replication of adenovirus in cotton rat tumors in vivo
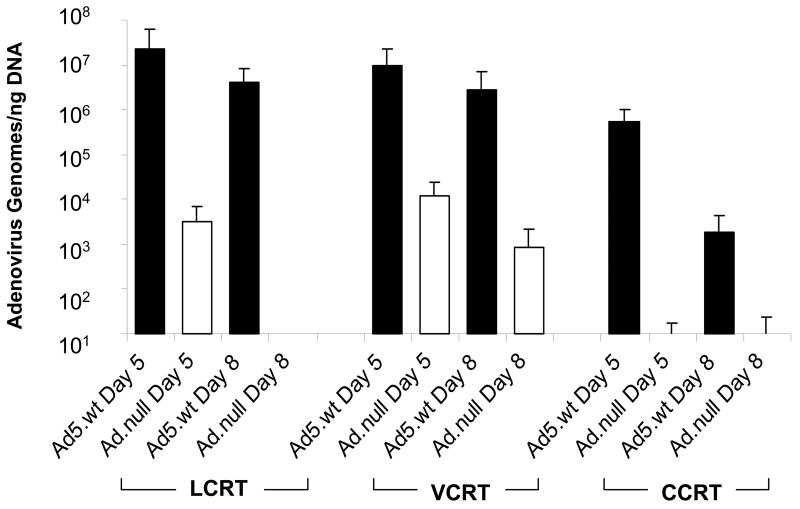
Subcutaneous CCRT, LCRT and VCRT tumors grown in cotton rats and injected with Ad5.wt or non-replicating Ad.null were excised on days 5 or 8 following the last injection and were submitted for quantitative real-time PCR for detection of adenoviral DNA. Viral genomes were detected in the CCRT, LCRT and VCRT tumors at day 5; however, significantly greater numbers of viral genomes were present in the tumors injected with replicating Ad5.wt compared to the tumors injected with non-replicating Ad.null (Fig. 6). No significant differences in the number of Ad5.wt genomes were detected between any of the cotton rat cell lines at this time point. Adenovirus was detected in tumors injected with Ad5.wt in CCRT, LCRT and VCRT tumors excised 8 days after infection. LCRT and VCRT tumors had means of 4 × 106 and 3 × 106 adenoviral genomes per ng of DNA at 8 days, respectively. These levels were not significantly different compared to that detected at day 5. CCRT tumors contained 2 × 103 viral genomes per ng DNA at 8 days. This was decreased compared to the numbers of viral genomes detected at 5 days (P<0.05). Negligible levels of adenovirus were detected in the LCRT and CCRT tumors treated with Ad.null at 8 days. VCRT tumors injected with Ad.null contained 8 × 102 genomes/ng DNA which was significantly less than that detected in the Ad5.wt injected VCRT tumors (P<0.05).
FIG 6.
Quantification of adenoviral genomes in LCRT, VCRT and CCRT tumors in vivo. CCRT, LCRT and VCRT tumors were established in cotton rats and infected with Ad5.wt or Ad.null. Five and eight days after the initial injection, tumors were removed from the animals from both groups, total DNA was extracted and the number of adenovirus genomes measured using real-time PCR. The amount of virus was determined against a standard curve of known adenovirus. Error bars represent the SD of the mean for 8 tumor samples.
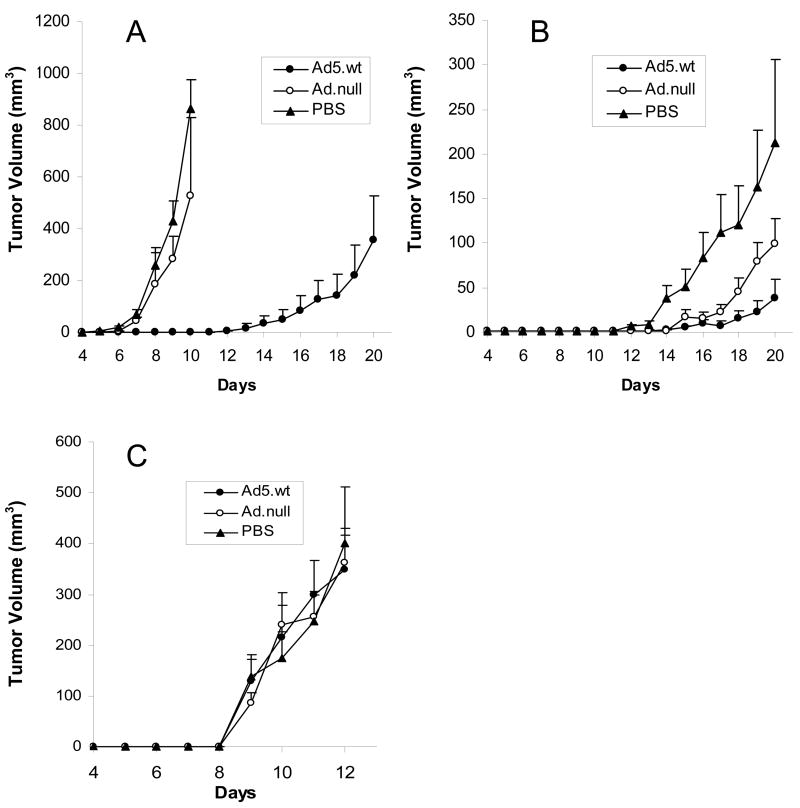
Growth of LCRT and VCRT tumors were inhibited by replicating adenovirus
To assess the activity of an oncolytic adenovirus on CCRT, LCRT and VCRT tumors in vivo, each cotton rat tumor cell line was infected ex vivo at their respective ID50 with Ad5.wt or Ad.null, and subcutaneously injected into the shoulder regions of cotton rats. Animals injected with the respective uninfected cotton rat tumor cell lines served as controls. Animals receiving uninfected LCRT cells developed palpable tumors 6 days after injection. At day 10, the mean tumor volume for LCRT cells was 863 mm3 (Fig. 7A). Cotton rats injected with LCRT cells infected with Ad.null also developed tumors by day 6, and by day 10 the tumors had a mean volume of 526 mm3. Cotton rats injected with the LCRT cells infected with Ad5.wt exhibited delayed growth with the tumors becoming palpable at 16.5 days post-implantation. On day 20, these tumors had a mean volume of 354 mm3. The animals injected with unmodified LCRT cells or LCRT cells infected with Ad.null, were euthanized before day 20 due to rapid growth of tumor.
FIG 7.
Growth curves for (A) LCRT, (B) VCRT and (C) CCRT tumors in vivo following infection with Ad.null (open circle), Ad5.wt (closed circle) or PBS (triangle). Cotton rats were implanted subcutaneously with 1 × 106 infected tumor cells. Tumor size was measured daily with calipers and the tumor volume expressed the area of a rotational ellipse. Error bars represent the SEM.
VCRT cells infected with Ad5.wt also exhibited growth delay compared to untreated VCRT cells or VCRT cells infected with Ad.null. Untreated VCRT tumors were palpable at a mean of 11.3 days, while the Ad5.wt treated tumors became palpable at 16.4 days (Fig. 7B). By day 20, the untreated tumors achieved a mean volume of 213 mm3, while the Ad5.wt treated tumors averaged 38 mm3. The Ad.null treated VCRT tumors appeared at 14.3 days and grew to a mean volume of 99 mm3 by day 20.
CCRT tumors showed rapid growth regardless of any treatment (Fig. 7C). Tumors were palpable in all treatment groups at 9 days post-implantation. There was no significant difference between the growth rates of the CCRT tumors infected with Ad5.wt, Ad.null, or the untreated CCRT cells at any time point.
DISCUSSION
Oncolytic adenoviruses for the treatment of cancer have shown promise in pre-clinical studies; however, their transition from the bench to success at the bedside has been inconsistent (Neumaniatis et al. 2001; Habib et al. 2002; Freytag et al. 2007). One possibility for this difference may be the limitations of the preclinical animal models that are used to evaluate these agents. Human adenoviruses do not effectively replicate in non-human tissues and cell lines. To overcome this problem, these vectors are commonly evaluated in immunodeficient mice bearing human tumor xenografts. While these systems give valuable information, they are limited because viral replication is restricted to the tumor xenograft. Recently, a number of reports have demonstrated variable levels of adenovirus replication in certain mouse cell lines (Hallden et al. 2003), primary swine cells (Jogler et al. 2006), some canine cell lines (Terovoi et al. 2005) and in Syrian hamsters (Thomas et al. 2006).
In addition, adenoviruses elicit strong immune responses that can impact on their efficacy as well as result in serious adverse effects on the host (Yang et al. 1994; Grinsberg et al. 1990; Everett et al. 2003). The attenuated inflammatory responses in immunodeficient rodent models limit the ability to assess these effects. The lungs, tracheobronchial tree, nasal passages and cornea of cotton rats have been shown to be permissive for replication of Group C adenoviruses and exhibit similar pathology to that found in man (Pacini, et al. 1984). As a result, many currently consider the cotton rat as the model of choice for assessing the safety and toxicity of replicating adenoviruses (Shine et al. 1997).
The development of transplantable cotton rat tumor lines represents an advance over immunodeficient rodent models to assess the activity and potential toxicity of oncolytic adenoviruses. We examined three tumorigenic cotton rat cell lines to assess the levels of viral replication supported by these cells, and the in vitro and in vivo oncolytic activity of adenovirus on these tumors. Jogler et al. 2006 compared the replication of adenovirus in primary cotton rat lung cells and found that they showed comparable infectivity to human A549 cells, but were superior to that of murine primary kidney cells. Similarly, we found that all three cell lines studied were readily infected with adenovirus. LCRT cells were comparable to human A549 cells and more readily infected than the two murine cell lines that we examined. The major determinant of permissiveness to adenovirus infection in human cells is the expression of the coxsackie-adenovirus receptor (CAR) (Bergelson et al. 1997). Cells that lack or express low levels of the receptor show resistance to infection with adenovirus (van’t Hof & Crystal 2001). Western blot using an antibody directed against human CAR protein detected a strong band in LCRT cells that was not found in CCRT or VCRT cells (data not shown). Whether the detected protein plays a role in the uptake of adenovirus is unknown, but it is intriguing that LCRT cells also showed the greatest ability to be of infected with adenovirus.
The ability of adenoviruses to replicate, induce cell lysis, and undergo secondary rounds of infection forms the basis of oncolytic virotherapy. CCRT, LCRT and VCRT cells should possess these characteristics to be useful as model system for cancer virotherapy. Over 72 h, each of the cell lines showed a 3 to 5 log increase in viral titers indicating active replication. These results were confirmed by transmission electron microscopy demonstrating the presence of virions. Four hours after infection no intracellular viral particles could be demonstrated. The absence of adenoviral particles at 4 h was expected as the virions have entered the cells and unencapsidated (Leopold et al. 1998). The appearance of viral particles in the nuclei at the late time point is indicative of productive replication and assembly of mature virions.
The cotton rat cells underwent viral induced cell lysis when infected with replicating adenovirus. Cells infected with the replicating GFP-expressing virus, Ad.OW94 showed that each of the cotton rat cell lines was readily infected and underwent lysis. In contrast, cotton rat cells infected with non-replicating E1-deleted Ad.GFP showed high levels of infection as evidenced by expression of GFP, but showed no CPE and cell lysis.
We also showed that fully functional adenovirus was released from the infected cotton rat cell lines that could then undergo secondary rounds of infection when transferred to HeLa cells. Significantly greater levels of GFP expression were seen in the HeLa cells exposed to the supernatants collected at 72 h compared to those exposed to supernatants collected at 5 h, indicating increased amounts of functional adenovirus was released from the cotton rat cell lines over time. As expected, no increase in the level of fluorescence over baseline was noted in the HeLa cells exposed to supernatants from the cotton rat cells infected with non-replicating Ad.GFP. In vitro, CCRT, LCRT and VCRT cells fulfilled the requirements of a virotherapy model allowing adenovirus to replicate, induce cell lysis, and undergo secondary rounds of infection.
In vivo, significantly more adenovirus was detectable in tumors injected with replication-competent Ad5.wt compared to tumors treated with equal numbers of non-replicating Ad.null. This was consistent for all of the cotton rat cell lines suggesting that viral replication is occurring in vivo. The amount of virus detected in the LCRT and VCRT tumors treated with the Ad5.wt remained elevated out to day 8; however, CCRT tumors showed a dramatic decrease in the number of viral genomes between 5 and 8 days. The conditions that mediate the rapid in vivo clearance of adenovirus from CCRT tumors are unknown. Elucidation of these factors may shed light as to why trials of replication competent adenoviruses have had limited success despite showing significant antitumor activity in preclinical xenograft models (McCormick 2003).
In vivo, VCRT and LCRT cotton rat tumors treated with Ad.5wt demonstrated growth delay compared to tumors treated with either Ad.null or with PBS. LCRT tumors maintained higher numbers of viral genomes over a longer period of time and showed the greatest inhibition of growth. These results are consistent with our in vitro findings that LCRT cells are more readily infected by adenovirus, and supported higher levels of viral replication and oncolysis. Toth and colleges reported that they were able to prevent or delay LCRT tumor formation and growth using VRX-007, a replicating adenovirus that over-expresses the adenoviral death protein (Toth et al. 2005). They had previously reported that VRX-007 induces greater cell lysis and viral spread compared to Ad5.wt (Doronin et al. 2003). This may account for the prevention of tumor formation in their study whereas our results using Ad5.wt showed that while the virus was able to delay in tumor formation, it was unable to completely prevent the formation of tumors. These differences also highlight the potential of this animal model to study improvements or modifications of adenoviral vectors that my enhance their oncolytic activity, such as over expression of “death” proteins, suicide genes or immune stimulating genes, designed to increase the therapeutic potential of virotherapy.
In vivo, VCRT tumors also maintained a significant viral load out to 8 days suggesting viral replication; however, the oncolytic sensitivity of VCRT tumors to Ad5wt was less than that of LCRT tumors. This was consistent with the in vitro findings in which VCRT cells were less readily infected and produced lower viral titers compared to LCRT cells. CCRT tumors treated with Ad5.wt did not demonstrate any inhibition of growth when compared to controls in two separate experiments. In vitro, CCRT cells readily infect with adenovirus and produced significant viral titers; however, adenovirus is rapidly cleared from CCRT tumors in vivo. The in vitro as compared to in vivo disparity of the oncolytic effect on CCRT cells, highlights the need for permissive immunocompetent animal tumor models to assess the effects of in vivo tumor dynamics and a functional immune system on replicating viruses.
Replicating adenoviruses efficiently infect, productively replicate and induced oncolysis in three transplantable tumorigenic cotton rat cell lines. We demonstrated that despite the permissiveness of the cotton rat tumor lines to adenovirus replication in vitro, a variable antitumor effect was observed in vivo. These results corresponds with the variable and limited tumor responses reported in most human clinical trials highlighting the relevance of the cotton rat tumor model for assessing replication competent adenovirus vector for oncolytic therapy of tumors.
MATERIALS AND METHODS
Cells
Three transplantable tumorigenic cell lines, CCRT, LCRT and VCRT were isolated from spontaneous tumors occurring in different animals from an inbred colony of cotton rats (Sigmodon hispidus). CCRT cells represent an osteosarcoma, LCRT cells were derived from a mammary fibrosarcoma, and VCRT cells were isolated from a spindle cell sarcoma of the mandible. These tumor cell lines were maintained in high-glucose Dulbecco’s modified Eagle’s medium (DMEM; Biosource, Rockville, MD) with 10% fetal bovine serum (FBS; Gemini, Woodland, CA) and gentamicin (Biosource). Human embryonic kidney (HEK)-293 cells, human A549 lung cancer cells, human Saos-2 osteosarcoma cells and HeLa cells were obtained from American Type Culture Collection (ATCC; Manassas, VA) and were grown in high-glucose DMEM with 10% FBS and gentamicin. The murine fibrosarcoma cell line 15-12RM (Matsui et al. 1999) and the murine breast cancer cell line TS/A (Nanni et al. 1983) were gifts of Jay A. Berzofsky (NCI, Bethesda, MD) and Patrizia Nanni (University of Bologna, Italy), respectively and were grown in high-glucose DMEM with 10% FBS and gentamicin. All cell cultures were maintained at 37°C and 5% CO2.
Adenoviral vectors
Wild-type adenovirus type-5 was obtained from ATCC (VR-5) (Rowe et al. 1953); Ad.GFP, an E1-deleted adenovirus expressing enhanced green fluorescent protein under the control of the cytomegalovirus IE (CMV-IE) promoter was purchased from Quantum Biotechnologies (Quebec, Canada). Ad.null, an “empty” control vector, containing deletions in E1 and E3 and the CMV-IE promoter with no transgene, was generated by homologous recombination using the AdMax system (Microbix, Toronto, Canada; Sakai et al. 2004). Ad.OW94 contains in the E1 region a GFP-IRES-Ad5 E1 expression cassette driven by the human CMV-IE promoter flanked upstream by the Ad5 packaging sequence and downstream by the Ad5 pIX. Ad.OW94 was generated by homologous recombination of pAd.CMV-GFP and pBHG10 in 293 cells, as described previously (Jogler et al. 2006). All viruses were expanded on HEK-293 cells, purified by two-step CsCl density-gradient ultracentrifugation, dialyzed, titered by serial dilution, quantified as plaque forming units (PFU)/mL on HEK-293 cells and stored at −70°C (Graham & Prevec 1995).
Animals
All studies were carried out on an Animal Care and Use Committee approved protocol (Virion Systems, Inc., Rockville, MD). Inbred cotton rats weighing 150–200 g were maintained under clean, light and temperature controlled conditions, housed two per cage with access to food and water ad libitum.
Adenoviral infections
To determine the efficiency of adenoviral infection and transgene expression in vitro, the cotton rat tumor cell lines were compared to human A549 and Saos-2 cells, and murine 15-12RM and TS/A cells using Ad.GFP as a reporter. Briefly, each cell line was seeded onto 6-well plates at 1 × 106 cells/well and infected with Ad.GFP at multiplicities of infection (MOI) of 0, 1, 10, 30, 100, or 300 PFU/cell. After 6 h, the cells were washed with phosphate buffered saline (PBS; Biosource) and the media replaced. The cells were incubated for an additional 36 h, trypsinized, washed and analyzed for expression of GFP using a FACSCalibur flow cytometer (Becton-Dickinson Immunocytometry Systems; Mansfield, MA).
Determination of cytopathogenic effect (CPE)
CCRT, LCRT, VCRT and HEK-293 cells were seeded onto 6 well plates at 2 × 105 cells/well and infected with Ad.GFP or Ad.OW94 at MOI 0, 10, or 100 PFU/cell. After 4 h the cells were washed three times with PBS, the media replaced and the cells incubated at 37°C. Seven days later, the cells were examined microscopically for lysis and stained with 1% crystal violet in 10% formaldehyde and 20% ethanol for 15 min. The plates were washed with ddH2O, air-dried and examined.
Viral burst assay
CCRT, LCRT and VCRT cells were seeded in duplicate onto 6 well plates at 3 × 105 cells/well and infected with Ad5.wt at MOI 0, 1, 10, or 100 PFU/cell. The cells were incubated at 37°C for 4 h, at which point the media was removed, the cells washed 3 times with PBS and fresh media added. Adenovirus was harvested from one duplicate set of plates by scraping cells, freezing and thawing three times and passing the lysate through a 0.22 μm filter (Millipore, Billerica, MA). The lysate was stored at −70°C. The other duplicate set of plates was similarly harvested and stored at 72 h. The amount of virus at each time point (4 h and 72 h) was quantified by plaque titer on HEK-293 cells.
Time-dependent viral release assay
CCRT, LCRT and VCRT cells were seeded onto 6-well plates at 3 × 105 cells/well in duplicate plates and infected with either Ad.GFP or Ad.OW94 at MOI 0, 1, 10, 30, 100, or 300 PFU/cell. The cells were incubated at 37°C for 4 h, at which point the media was removed, the cells were washed 5 times with PBS and 2 mL fresh media was added back. The cells were incubated for an additional 1 h. The media was removed, passed though a 0.22 μM filter and stored at −70°C. Two mL of media was added back to the cells and they were incubated for an additional 48 h. The media was then removed, filtered and stored at −70°C. One mL of these filtrates was added to wells in 6-well plates containing 3 × 105 HeLa cells/well. At 48 h, the HeLa cells were trypsinized, washed and assayed for GFP expression by flow cytometry.
Transmission electron microscopy
CCRT, LCRT and VCRT cells were seeded at 1 × 107 cells onto 75 cm2 tissue culture flasks in duplicate and infected with Ad5.wt at 1 PFU/cell. Following a 4 h incubation at 37°C, the media was removed and the cells were washed 3 times. In one set of flasks, the cells were fixed with 4% glutaraldehyde and incubated at 4°C for 1 h. The cells were dislodged using a scraper, pelleted and resuspended in 500 μL 4% glutaraldehyde and stored at 4°C. The other set of flasks was refreshed with media and the cells incubated for an additional 72 h, at which point the cells were similarly fixed and harvested. Cells from both sets of flasks were then double-fixed in PBS-buffered glutaraldehyde (2.5%) and osmium tetroxide (0.5%), dehydrated, and embedded in Spurr’s epoxy resin. Ultrathin sections (90 nm) were made and double-stained with uranyl acetate and lead citrate, and viewed using a Philips CM10 transmission electron microscope (Electronic Instruments, Mahwah, NJ).
Establishment of subcutaneous tumors in cotton rats and intratumoral injection of adenovirus
CCRT, LCRT and VCRT tumors were established by subcutaneous injection of 1 × 106 tumor cells into the shoulder regions of cotton rats. When the tumors reached a volume of 100–200 mm3 (day 6), they were injected with Ad5.wt 2.5 × 108 PFU or Ad.null 2.5 × 108 PFU in 100 μL PBS, or 100 μL PBS daily for 4 days using a tuberculin syringe and a 27 gauge needle by injecting in a separate quadrant of the tumor each day. Four animals from each group were euthanized 24 h and 96 h after the last injection. The tumors were excised, dissected free of surrounding tissue and stored in liquid nitrogen until processing.
Quantitation of adenovirus by real-time PCR
Quantitative PCR was used to measure the amount of adenovirus in tissue samples (Wildner & Morris 2002). Total DNA was extracted from the CCRT, LCRT and VCRT tumors using a DNeasy tissue kit (Qiagen Inc., Valencia, CA) and quantified by spectrophotometer (A260). A quantitative Ad5 real-time PCR assay was performed using the following primers and probe: Ad5 33414 fwd- GTAATTCACCACCTCCCGGTA, Ad5 33562 rev-GGCTCTCCACTGTCATTGTTC, Ad5 probe-ACCTCTGATTAAACATGGCGCCATCC (FAM labeled 5′, TAMRA quencher 3′). DNA was amplified using 100 nM of each primer and 200 nM of the probe using the following cycling conditions: 2 min at 50°C, 10 min at 95°C, 45 cycles of 15 s at 95°C (denaturation) and 45 cycles of 1 min at 63°C (extension/annealing) in an ABI 7700 DNA sequencer (Applied Biosystems, Foster City, CA). A standard curve was generated using pre-titered adenovirus standards ranging from 101 to 107 pfu. PCR reactions for each sample were performed in triplicate using the same oligonucleotide primers and fluorescent probe, reaction conditions and cycling.
Effect of adenovirus on cotton rat tumors in vivo
CCRT, LCRT or VCRT cells were seeded at 1 × 107 cells/flask into 175 cm2 tissue culture flasks. The cells were infected with Ad5.wt or non-replicating Ad.null at the infectious dose-50% (ID50) and incubated for 6 h at 37°C. ID50 was determined from the tissue culture infectious dose-50 (TCID50) for each of the cotton rat cell lines infected with Ad5.wt. Briefly, 96-well plates were seeded with 1 × 104 cells/well. Serial dilutions of Ad5.wt were added to the wells in each column. The plates were then incubated at 37°C. Each well was examined daily for 14 days for cytopathogenic effect (CPE). The TCID50 value was calculated using the Reed-Muench formula (Reed & Muench 1938). The respective ID-50’s of the LCRT, CCRT and VCRT cells that were used to infect the cells lines were 4, 8 and 20 PFU/cell (results not shown). The cells were washed 3 times with PBS, trypsinized and counted. Cotton rats were subcutaneously injected in the shoulder regions with 1 × 106 tumor cells. The animals were examined daily for tumor growth. Tumors were measured with Vernier calipers and tumor size was expressed as the volume of a rotational ellipse (Dethlefsen, et al. 1968).
Statistics and software
Statistical analysis was preformed using Student’s t-test using SigmaStat™version 3.1 statistical software (Systat, Point Richmond, CA). In vitro data are presented as mean ± SD; in vivo data is represented as mean ± SEM.
Acknowledgments
This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benjamin R, Helman L, Meyers P, Reaman G. A phase III dose escalation and activity study of intravenous injections of OCaP1 for subjects with refractory osteosarcoma metastatic to lung. Hum Gene Ther. 2001;12:1591–1593. [PubMed] [Google Scholar]
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–3. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- Dethlefsen LA, Prewitt JM, Mendelsohn ML. Analysis of tumor growth curves. J Natl Cancer Inst. 1968;40:389–405. doi: 10.1093/jnci/40.2.389. [DOI] [PubMed] [Google Scholar]
- DeWeese TL, van der Poel H, Li S, Mikhak B, Drew R, Goemann M, Hamper U, DeJong R, Detorie N, Rodriguez R, Haulk T, DeMarzo AM, Piantadosi S, Yu DC, Chen Y, Henderson DR, Carducci MA, Nelson WG, Simons JW. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61:7464–7472. [PubMed] [Google Scholar]
- Everett RS, Hodges BL, Ding EY, Xu F, Serra D, Amalfitano A. Liver toxicities typically induced by first-generation adenovirsl vectors can be reduced by use of E1, E2b-delted adenoviral vectors. Hum Gene Ther. 2003;14:1715–1726. doi: 10.1089/104303403322611737. [DOI] [PubMed] [Google Scholar]
- Freytag SO, Khil M, Stricker H, Peabody J, Menon M, DePeralta-Venturina M, Nafziger D, Pegg J, Paielli D, Brown S, Barton K, Lu M, Aguilar-Cordova E, Kim JH. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer. Cancer Res. 2002;62:4968–4976. [PubMed] [Google Scholar]
- Freytag SO, Movsas B, Aref I, Stricker H, Peabody J, Pegg J, Zhang Y, Barton KN, Brown SL, Lu M, Savera A, Kim JH. Phase I trial of replication-competent adenovirus-mediated suicide gene therapy combined with IMRT for prostate cancer. Mol Ther. 2007;15:1016–1023. doi: 10.1038/mt.sj.6300120. [DOI] [PubMed] [Google Scholar]
- Graham FL, Prevec L. Methods for construction of adenovirus vectors. Mol Biotechnol. 1995;3:207–220. doi: 10.1007/BF02789331. [DOI] [PubMed] [Google Scholar]
- Ginsberg HS, Horswood RL, Chanock RM, Prince GA. Role of early genes in pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A. 1990;87:6191–5. doi: 10.1073/pnas.87.16.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib N, Salama H, Abd El Latif Abu Median A, Isac Anis I, Abd Al Aziz RA, Sarraf C, Mitry R, Havlik R, Seth P, Hartwigsen J, Bhushan R, Nicholls J, Jensen S. Clinical trial of E1B-deleted adenovirus (dl1520) gene therapy for hepatocellular carcinoma. Cancer Gene Ther. 2002;9:254–9. doi: 10.1038/sj.cgt.7700431. [DOI] [PubMed] [Google Scholar]
- Hallden G, Hill R, Wang Y, Anand A, Liu TC, Lemoine NR, Francis J, Hawkins L, Kirn D. Novel immunocompetent murine tumor models for the assessment of replication-competent oncolytic adenovirus efficacy. Mol Ther. 2003;8(3):412–24. doi: 10.1016/s1525-0016(03)00199-0. [DOI] [PubMed] [Google Scholar]
- Jogler C, Hoffmann D, Theegarten D, Grunwald T, Uberla K, Wildner O. Replication properties of human adenovirus in vivo and in cultures of primary cells from different animal species. J Virol. 2006;80(7):3549–58. doi: 10.1128/JVI.80.7.3549-3558.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn D. Oncolytic virotherapy for cancer with the adenovirus dl1520 (Onyx-015): results of phase I and II trials. Expert Opin Biol Ther. 2001;1:525–538. doi: 10.1517/14712598.1.3.525. [DOI] [PubMed] [Google Scholar]
- Leopold PL, Ferris B, Grinberg I, Worgall S, Hackett NR, Crystal RG. Fluorescent virions: dynamic tracking of the pathway of adenoviral gene transfer vectors in living cells. Hum Gene Ther. 1998;9(3):367–378. doi: 10.1089/hum.1998.9.3-367. [DOI] [PubMed] [Google Scholar]
- Matsui S, Ahlers JD, Vortmeyer AO, Terabe M, Tsukui T, Carbone DP, Liotta LA, Berzofsky JA. A model for CD8+ CTL tumor immunosurveillance and regulation of tumor escape by CD4 T cells through an effect on quality of CTL. J Immunol. 1999;163:184–93. [PubMed] [Google Scholar]
- McCormick F. Cancer-specific viruses and the development of ONYX-015. Cancer Biol Ther. 2003;2:S157–S160. [PubMed] [Google Scholar]
- Nanni P, de Giovanni C, Lollini PL, Nicoletti G, Prodi G. TS/A: a new metastasizing cell line from a BALB/c spontaneous mammary adenocarcinoma. Clin Exp Metastasis. 1983;1:373–80. doi: 10.1007/BF00121199. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J, Khuri F, Ganly I, Arseneau J, Posner M, Vokes E, Kuhn J, McCarty T, Landers S, Blackburn A, Romel L, Randlev B, Kaye S, Kirn D. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19:289–98. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- Niewiesk S, Prince G. Diversifying animal models: the use of hispid cotton rats (Sigmodon hispidus) in infectious diseases. Lab Anim. 2002;36:357–372. doi: 10.1258/002367702320389026. [DOI] [PubMed] [Google Scholar]
- Pacini DL, Dubovi EJ, Clyde WA., Jr A new animal model for human respiratory tract disease due to adenovirus. J Infect Dis. 1984;150:92–7. doi: 10.1093/infdis/150.1.92. [DOI] [PubMed] [Google Scholar]
- Prince GA, Porter DD, Jenson AB, Horswood RL, Chanock RM, Ginsberg HS. Pathogenesis of adenovirus type 5 pneumonia in cotton rats (Sigmodon hispidus) J Virol. 1993;67:101–111. doi: 10.1128/jvi.67.1.101-111.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench HA. Simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- Rojas-Martinez A, Wyde PR, Montgomery CA, Chen SH, Woo SLC, Aguilar-Cordova E. Distribution, persistency, toxicity, and lack of replication of an E1A-deficient adenoviral vector after intracardiac delivery in the cotton rat. Cancer Gene Ther. 1998;5(6):365–370. [PubMed] [Google Scholar]
- Rowe WP, Huebner RJ, Gilmore LK, Parrott RH, Ward TG. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med. 1953;84(3):570–3. doi: 10.3181/00379727-84-20714. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Morrison BJ, Burke JD, Park JM, Terabe M, Janik JE, Forni G, Berzofsky JA, Morris JC. Vaccination by genetically modified dendritic cells expressing a truncated neu oncogene prevents development of breast cancer in transgenic mice. Cancer Res. 2004;64:8022–8028. doi: 10.1158/0008-5472.CAN-03-3442. [DOI] [PubMed] [Google Scholar]
- Shine HD, Wyde PR, Aguilar-Cordova E, Chen SH, Woo SL, Grossman RG, Goodman JC. Neurotoxicity of intracerebral injection of a replication-defective adenoviral vector in a semipermissive species (cotton rat. Gene Ther. 1997;4(4):275–9. doi: 10.1038/sj.gt.3300397. [DOI] [PubMed] [Google Scholar]
- Small EJ, Carducci MA, Burke JM, Rodriguez R, Fong L, van Ummersen L, Yu DC, Aimi J, Ando D, Working P, Kirn D, Wilding G. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther. 2006;14(1):107–17. doi: 10.1016/j.ymthe.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Smith R, Huebner RJ, Rowe WP, Schatten WE, Thomas LB. Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer. 1956;9:1211–1218. doi: 10.1002/1097-0142(195611/12)9:6<1211::aid-cncr2820090624>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Southam CM, Hilleman MR, Werner JH. Pathogenicity and oncolytic capacity of RI virus strain RI-67 in man. J Lab Clin Med. 1956;47(4):573–82. [PubMed] [Google Scholar]
- Ternovoi VV, Le LP, Belousova N, Smith BF, Siegal GP, Curiel DT. Productive replication of human adenovirus type 5 in canine cells. J Virol. 2005;79(2):1308–11. doi: 10.1128/JVI.79.2.1308-1311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MA, Spencer JF, La Regina MC, Dhar D, Tollefson AE, Toth K, Wold WS. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006;66(3):1270–6. doi: 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- Toth K, Spencer JF, Tollefson AE, Kuppuswamy M, Doronin K, Lichtenstein DL, La Regina MC, Prince GA, Wold WS. Cotton Rat Tumor Model for the Evaluation of Oncolytic Adenoviruses. Hum Gene Ther. 2005;16:139–146. doi: 10.1089/hum.2005.16.139. [DOI] [PubMed] [Google Scholar]
- Tsai JC, Garlinghouse G, McDonnell PJ, Trousdale MD. An experimental animal model of adenovirus-induced ocular disease. The cotton rat. Arch Ophthalmol. 1992;110:1167–1170. doi: 10.1001/archopht.1992.01080200147043. [DOI] [PubMed] [Google Scholar]
- van’t Hof W, Crystal RG. Manipulation of the cytoplasmic and transmembrane domains alters cell surface levels of the coxsackie-adenovirus receptor and changes the efficiency of adenovirus infection. Hum Gene Ther. 2001;12(1):25–34. doi: 10.1089/104303401450933. [DOI] [PubMed] [Google Scholar]
- Wildner O, Morris JC. Subcutaneous administration of a replication-competent adenovirus expressing HSV-tk to cotton rats: dissemination, persistence, shedding, and pathogenicity. Hum Gene Ther. 2002;13:101–112. doi: 10.1089/10430340152712656. [DOI] [PubMed] [Google Scholar]
- Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci (USA) 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chirmule N, Gao G-P, Qian R, Croyle M, Joshi B, Tazelaar J, Wilson JM. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol Ther. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]