Abstract
Immunity generated by in vivo inoculation of plasmid DNA is a straightforward and potentially valuable new approach to immunization. Little is known about the type of cells involved, the various immunological aspects, and the destiny of the transgene. In this report, we describe a system in which immunity is the result of in vivo targeting of B lymphocytes. This was accomplished using plasmid DNA encoding an immunoglobulin heavy-chain gene under the control of immunoglobulin promoter and enhancer elements. We show persistence of the transgene in splenic B lymphocytes for at least 3 months, i.e., the average life span of long-lived B lymphocytes in the mouse. The transgene could not be detected in any other lymphoid or nonlymphoid organs over a period of 6 months. We also established that the transgene is integrated in the host DNA. These studies bring new understanding to the events underlying the in vivo use of plasmid DNA. Moreover, the characteristics of this new approach make somatic transgene immunization a model system to study the immunogenicity of endogenous antigens in adult animals.
Since the discovery that inoculation of plasmid DNA induces specific immunity in vivo (1), reports have appeared to demonstrate the use of such an approach to elicit immunity against viruses (2–5), bacteria (6, 7), parasites (8–10), tumor antigens (11), self-antigens (12, 13), and allergens (14, 15). This has raised hopes for the development of simpler and more cost-effective methods of vaccination. From a practical standpoint, immunization via DNA inoculation relies on in vivo transduction, production and possibly secretion of the transgene product, and antigen presentation by specialized cells. However, in most studies neither the in vivo transduced cells nor the antigen-presenting cells involved in this process have been identified. Most experiments to date were based on expression of foreign DNA under the control of viral promoters (1–9) that have limited tissue specificity. Therefore, no control of expression is possible other than at the site of DNA inoculation.
B lymphocytes are generated in the bone marrow and then localize in secondary lymphoid organs and in the blood throughout life (16–18). Upon activation by antigen, a B cell can produce between 1 × 103 and 8 × 104 molecules of Ig per cell per second (19, 20). Consequently, B cells are formidable minifactories of proteins in mammals. B lymphocytes can also present antigen to T lymphocytes: (i) antigens internalized via their membrane Ig receptor (21), and (ii) peptides of secretory proteins including their own Ig (22, 23). Because of these properties, B lymphocytes constitute an ideal substrate for strategies of gene targeting and immunization with plasmid DNA.
Recently, we showed that direct intraspleen inoculation of plasmid DNA carrying a full-length Ig H chain gene under the control of Ig promoter and enhancer elements results in expression of the transgene in the spleen, secretion of transgene Ig in the serum in amounts ranging between 15 and 30 ng/ml, and induction of a specific immune response against epitopes expressed in the transgene product (ref. 24 and Gerloni, M., Bellou, W. R., Billetta, R., and Zanetti, M., unpublished data). We termed this process somatic transgene immunization to signify that a transgene is targeted and incorporated into somatic cells, and elicitation of immunity is the outcome of this event. Early in vitro studies (25–28) and analysis of Ig transgenic mice in vivo (29–31) showed that the Ig promoter and enhancer elements selectively direct gene expression in B cells. Therefore, we hypothesized that B lymphocytes might be the target of somatic transgene immunization. Here we study the fate of the transgene after inoculation and its tissue distribution and genetic stability, and we provide formal evidence that splenic B lymphocytes are in fact the cells involved in this process.
METHODS
Plasmid DNAs.
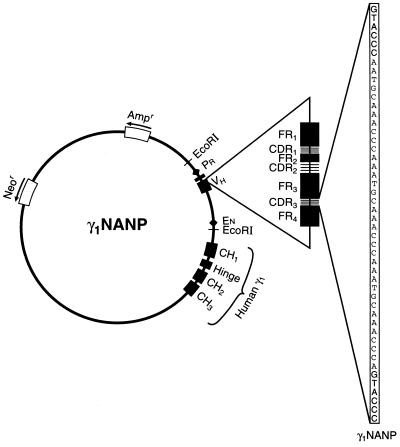
Plasmid γ1NANP (32) carries a chimeric H chain gene in which a productively rearranged murine V region gene is joined to a human γ1 C region gene. The V region of this H chain gene was modified in the third complementarity-determining region (CDR3) by introduction of the nucleotide sequence coding for three Asn-Ala-Asn-Pro repeats (32). The promoter and enhancer elements in this plasmid are those constitutively existing in Ig H chain genes and have been described previously (33). Plasmid pSVneo is the original plasmid vector that lacks the murine V region and the human γ1 C region genes (34).
DNA Inoculations.
The plasmid DNA was prepared from transformed DH5α Escherichia coli according to standard procedures (35) and analyzed for purity using the following equation: %N = (11.1R−6.32)/(2.16−R) where R = 260 nm/280 nm, %N = percent of nucleic acid (36). For DNA inoculation, 8 to 10-wk-old C57BL/6 (H-2b) female mice (The Jackson Laboratory) were inoculated with plasmid DNA (100 μg in 30 μl of sterile saline solution) directly into the spleen (24). Briefly, mice were anesthetized with a mixture of ketamine, xylazine, and acetopromazine and shaved locally, and a small incision (3–4 mm) was made with a scalpel. By pulling gently, the spleen was exposed, and the DNA was injected using a 28-gauge needle. The abdomen was immediately sutured using 4-0 sterile, nonabsorbable surgical suture thread. Mice were maintained in the animal facility of the University of California, San Diego (UCSD), and were handled according to UCSD and National Institutes of Health regulations.
Immunochemical Techniques.
The presence of transgene H chain Ig in the serum was detected using a capture ELISA (37). Briefly, a 1:10 dilution of individual mouse sera in 1% BSA in PBS (pH 7.3) containing 0.05% Tween 20 PBS albumin (PBSA) was incubated on 96-well plates (Dynatech) coated with a goat antibody to human gamma globulin (10 μg/ml). The bound antibodies were revealed using goat antibody to human gamma globulins conjugated with horseradish peroxidase absorbed with murine Ig (Sigma). The bound peroxidase activity was revealed by adding o-phenylenediamine dihydrochloride and H2O2. Plates were read after 30 min in a microplate reader (Vmax, Molecular Devices) at 492 nm. The concentration was determined by comparing the serum OD values with those of known amounts of human gamma globulins. Tests were done in duplicate. Antibodies to γ1NANP were detected on 96-well polyvinyl microtiter plates coated with affinity-purified antibody γ1NANP (2.5 μg/ml). Sera were diluted in PBSA. The bound antibodies were revealed using a horseradish peroxidase conjugated goat antibody to mouse gamma globulins absorbed with human gamma globulins (Pierce). The bound peroxidase was revealed by adding o-phenylenediamine dihydrochloride and H2O2. Tests were done in duplicate.
PCR and Southern Blot Hybridization.
Ten milligrams of the tissue were digested in the presence of protease, and the cell lysates were loaded onto the QIAamp spin column (Qiagen, Chatsworth, CA). After washing twice by centrifugation, the DNA was eluted from the column with distilled water and quantitated on a 1% agarose gel. PCR was performed with a total of four sets of primers (pCL/pCD, pSE/pNAD, pNEL/pNED, and pβA1/pβA2). pCL (from −107 to −85 nt: 5′-TTATTGAGAATAGAGGACATCTG-3′) and pCD (from 459 to 439 nt: 5′-ATGCTCATAAAACTCCATAAC-3′) were used to amplify the whole variable diversity joining (VDJ) region of the transgene; pSE (from −32 to −11 nt: 5′-AACAGTATTCTTTCTTTGCAGC-3′) and pNAD (from 352 to 333 nt: 5′-GAGAGTAGGGTACTGGGTTT-3′) were specific for amplification of the genetic marker, (NANP)3 in CDR3. pNEL (from 169 to 189 nt: 5′-AGCACCTACTATCCAGACACT-3′) and pNED (from 366 to 346 nt: 5′-GTAGTCCATACCATGAGAGTA-3′) were the inner primers for nested PCR. pβA1 (from 184 to 201 nt: 5′-TGGGCCGCCCTAGTCACC-3′) and pβA2 (from 427 to 408 nt: 5′-CGTTTGGCCTTAGGGTTCAG-3′) were designed to amplify the murine β-actin gene according to the sequence indicated in ref. 38. The PCR consisted of 30 cycles at 94°C for 45 sec, 58°C for 45 sec, and 72°C for 45 sec; 0.3 μM each primer, 0.2 mM each deoxynucleotide, 1.5 mM MgCl2 in 20 mM Tris⋅HCl (pH 8.4)/50 mM KCl, and 1 unit of Taq DNA polymerase (GIBCO/BRL). PCR products for Southern blot analysis were resolved in 1% (wt/vol) agarose gel and blotted onto Hybond-N nylon membrane (Amersham). The membranes were hybridized with the oligonucleotide pNAD and labeled using T4 polynucleotide kinase forward reaction in the presence of [γ-32P]ATP.
Integration Study.
Integration of the transgene in the host genomic DNA was studied by digestion–self-ligation–PCR approach. Briefly, the genomic DNA extracted from spleen was digested with XbaI and subsequently self-ligated (circularization) with T4 DNA ligase at a lower DNA concentration. The circularized DNA was used as the template for further PCR amplification with primers: p62L (from −177 to −199 nt: 5′-AGTGCTGATCACTGAATGTGAGG-3′) and p62U (from 441 to 460 nt: 5′-GTTATGGAGTTTTCTGAGCATT-3′). The PCR was performed with Taq polymerase and Elongase mix (GIBCO/BRL) in a wide range of MgCl2 concentrations. A total of 35 cycles were run under the following thermal cycling conditions: 94°C for 45 sec, 60 °C for 45 sec, and 68 °C for 10 min.
DNA Sequencing.
A 566-bp DNA fragment containing the whole VDJ coding region was amplified from splenic genomic DNA using two primers (pCL and pCD) specific for the rearranged murine VH (33). This fragment was subcloned into the pGEM-T vector (Promega). The plasmid DNA was extracted from transformed DH5α E. coli and sequenced by a dideoxy termination method with a Sequenase 2.0 DNA sequencing kit (United States Biochemical) using two primers (pSE and pCD) annealing in front of the FR1 and at the end of FR4 from opposite directions (see Fig. 2B).
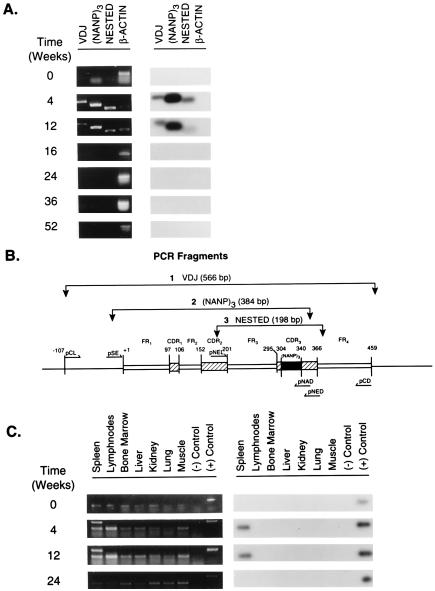
Figure 2.
PCR amplification and Southern blot hybridization to detect the presence of the transgene H chain in vivo. (A) Presence of the transgene in splenic genomic DNA at various times after DNA inoculation. Spleens were harvested 4, 12, 16, 24, 36, and 52 wk after DNA inoculation. The spleen of a naive mouse served as a negative control and is referred to as time 0. Detection of the transgene in the genomic DNA was performed by PCR amplification using three sets of primers (pCL/pCD, pSE/pNAD, and pNEL/pNED) specific for three different DNA fragments of plasmid DNA γ1NANP and confirmed by Southern blot hybridization using the 32P-labeled pNAD oligonucleotide. The location and size of the PCR fragments [VDJ, (NANP)3, and NESTED] are illustrated in Fig. 2B. A PCR fragment identified as β-actin (for the murine β-actin gene) served as an internal control. Left, the results of PCR amplification. Right, the results of Southern blot hybridization. B is a schematic representation of the VH gene contained in plasmid DNA γ1NANP. The annealing sites of the primers, the predicted amplification fragments and their molecular size, are identified: FR, framework region; VDJ refers to a fragment that is inclusive of the coding region for the rearranged VDJ gene segments; (NANP)3 refers to a 384-bp fragment containing in the CDR3 of the VH region the sequence coding for three repeats of the tetrapeptide Asn-Ala-Asn-Pro between nucleotides 304 and 340 (32); NESTED refers to a 198-bp fragment inclusive of the coding region for FR3 and the CDR3. +1 refers to the first nucleotide in the coding region of FR1. Any other position in the gene is numbered in reference to nucleotide +1. (C) Tissue distribution of the transgene in vivo. Genomic DNA was extracted from the tissues listed. Tissues were obtained at various times from DNA inoculation. Tissues from a naive mouse refers to time 0. Left, PCR amplification of the VDJ fragment of the transgene using the primers pCL/pCD. Right, results of Southern blot hybridization using the 32P-labeled pNAD oligonucleotide. {/TITL;;;center;stack}
Fluorescence-Activated Cell Sorting.
Spleen cells were prepared by grinding the spleen tissue harvested 15, 21, and 28 days after inoculation or from naive mice. The cell suspension was washed twice with 0.5% PBSA, and the red blood cells were removed by treatment with lysing buffer (Sigma). The lymphocytes were differentially stained with phycoerythrin-conjugated rat anti-mouse Ly-5 (B-220) Pan B cell (Caltag, South San Francisco, CA), fluorescein isothiocyanate-conjugated rat anti-mouse CD4 (Caltag), and fluorescein isothiocyanate-conjugated rat anti-mouse CD8 (Caltag) for 20 min at 4°C. The cell suspension was washed twice in 0.5% PBSA and resuspended at the concentration of 5 × 106 cells/ml in DMEM (Irvine Scientific). The cells were sorted on a FACStar (Becton Dickinson) at the Flow Cytometry core facility of the UCSD Cancer Center. Genomic DNA was extracted from 1 × 106 B or T lymphocytes using the QIAamp Blood kit (Qiagen). The DNA fragments were amplified by PCR and run on a 1% agarose gel. They were subsequently transferred to a nylon membrane for Southern blot hybridization using the 32P-labeled pNAD oligonucleotide.
RESULTS
In Vivo Production of Transgene H Chain Ig and the Humoral Response.
Plasmid DNA γ1NANP (Fig. 1) coding for an engineered murine variable region (39) joined with a human γ1 constant region gene was inoculated into the spleen of adult C57BL/6 mice. Following a single intraspleen inoculation, transgene H chain Igs were detected (∼12 ng/ml) in the serum of all mice inoculated with plasmid DNA γ1NANP but not in mice inoculated with the plasmid control pSVneo lacking the coding region for the H chain (Table 1). In all mice, together with detection of the transgene product antibodies against the γ1NANP, protein was also detected starting from week 2 (Table 1). This is in agreement with previous experiments (24).
Figure 1.
Schematic representation of plasmid DNA γ1NANP. This plasmid carries a chimeric H chain gene in which a productively rearranged murine variable region gene is joined to a human γ1 constant region gene. The variable region gene was modified in the CDR3 by introduction of the nucleotide sequence coding for three Asn-Ala-Asn-Pro repeats to serve as a specific molecular marker (32). In this plasmid, the promoter and enhancer elements are those constitutively existing in Ig H chain genes. Neor, neomycin resistance gene; Ampr, ampicillin resistance gene; PR, promoter; EN, enhancer; CH, heavy chain C region; VH, heavy chain variable region; FR, framework region.
Table 1.
Detection of transgene H chain immunoglobulins and antibodies to γ1NANP after intraspleen inoculation of plasmid DNA
| Inoculum (plasmid) | No. of mice | Transgene H-chain Ig* (ng/ml) | Antibodies to γ1NANP**
|
|||
|---|---|---|---|---|---|---|
| 0 wk | 2 wk | 4 wk | 6 wk | |||
| γ1NANP | 10 | 11.9 ± 5.4 | ≤2.3 | 2.8 ± 0.3 | 2.9 ± 0.4 | 3.0 ± 0.3 |
| pSVneo | 4 | 0 | ≤2.3 | ≤2.3 | ≤2.3 | 2.4 ± 0.2 |
Presence of transgene H chain immunoglobulins was determined at 2 wk after a single DNA inoculation. Detection was performed using a capture ELISA.
Antibodies are expressed as a Log titer. Titer determined as the last serum dilution giving an OD reading >0.200 (A492).
Kinetics and Tissue Distribution of the Transgene in vivo.
To monitor the kinetics of detection of the transgene in vivo, genomic DNA extracted from the spleen of inoculated mice was analyzed by PCR and Southern blot hybridization at various times after inoculation. As shown in Fig. 2A (Left) amplification of the transgene VDJ region was visible up to 12 wk after a single DNA inoculation. No amplification was seen in the subsequent time points (16, 24, 36, and 52 wk). To control for specificity and increase the sensitivity of the reaction, two additional PCR assays were performed using primers designed to anneal to sites within the VDJ region. One set of primers (pSE/pNAD) specifically amplified the (Asn-Ala-Asn-Pro)3 coding sequence, and another (inner primers: pNEL/pNED) served for nested PCR (see legend of Fig. 2B). The results confirmed those obtained with VDJ amplification. Southern blot analysis using a probe specific for the NANP-coding region further confirmed the PCR results (Fig. 2A Right). Thus, the transgene H chain persisted in vivo for a period of 3 months. To determine tissue distribution of the transgene in vivo, genomic DNA was extracted from various lymphoid (i.e., spleen, lymphnodes, and bone marrow) and nonlymphoid (i.e., liver, kidney, lung, and muscle) tissues explanted at different times and analyzed for specific amplification of the transgene VDJ by PCR. Whereas an amplification product was readily visible in splenic genomic DNA, no specific amplification occurred in any of the other tissues. This did not vary at any of the time points analyzed (Fig. 2C Left). Southern blot analysis confirmed the PCR results (Fig. 2C Right).
Detection of the Transgene in Splenic Lymphocytes.
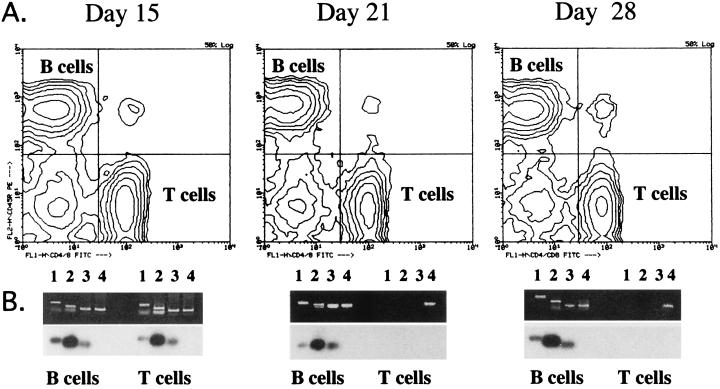
To formally demonstrate that B lymphocytes were in fact the target cell population in vivo for the transgene, the following experiment was performed. Starting from the second wk after plasmid DNA inoculation, splenic B and T lymphocytes were isolated to a high degree of purity (97–99%) by fluorescence activated cell sorting (Fig. 3). The genomic DNA was extracted from the two cell populations and amplified by PCR using the same sets of primers as in Fig. 2. At the 15 day time point, distinct amplification products were readily detectable in both B and T lymphocytes (Fig. 3 Left). However, at both the 21 and 28 day time points, specific amplification was observed only in B cells (Fig. 3 Middle and Right). Southern blot hybridization confirmed the specificity of the amplification products. Based on this analysis it is suggested that B lymphocytes in the spleen are the target cell population in which the transgene persists for a long time. Together with the fact that the transgene could not be amplified from peripheral blood lymphocytes (data not shown), these results indicate that in all likelihood the destiny of spleen B lymphocytes harboring the transgene is to remain localized in the tissue in which they were transduced. Uptake of plasmid DNA by B cells may have occurred either through surface Ig specific for DNA or through a non-Ig DNA receptor (40–42). As to the transient presence of the transgene in T lymphocytes, further studies are needed to understand whether these cells play any role. Experiments are also in progress to assess the participation of dendritic cells in somatic transgene immunization.
Figure 3.
Isolation of splenic B and T lymphocytes and detection of the transgene H chain in the purified lymphocyte populations. B and T lymphocytes from the spleen of DNA-inoculated mice were sorted and purified on a fluorescence-activated cell sorter at the times indicated. Lane 1, fragment amplified with the primers pCL/pCD (VDJ); lane 2, fragment amplified with the primers pSE/pNAD [(NANP)3]; lane 3, fragment amplified with the primers pNEL/pNED (NESTED); lane 4, fragment amplified with the primers pβA1/pβA2 (β-actin).
Integration of the Transgene in the Host DNA.
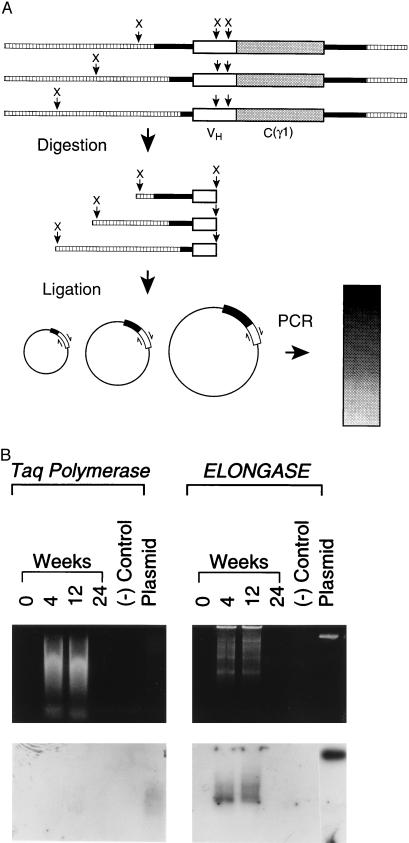
Upon activation via membrane Ig or mitogens, B lymphocytes go into a phase of high replicative activity (43). Because foreign plasmid DNA has greater propensity to integrate in cells that are actively replicating (44), persistence of the transgene in B lymphocytes for 3 months prompted us to investigate the possibility of integration. To this end, we used a PCR approach in which genomic DNA was first digested with XbaI and subsequently ligated and amplified. We reasoned that a pattern of multiple molecular size products (smear) would suggest integration of the transgene, whereas a single band of 15kb would suggest that the transgene persists in episomal form. The rationale of the experiment is illustrated in Fig. 4A. Both genomic DNA extracted from the spleen 4 and 12 wk after plasmid DNA inoculation gave rise to amplified products of multiple molecular sizes (a smear). No such a pattern was observed in the genomic DNA extracted 36 wk after inoculation (Fig. 4B Top Left) consistent with the kinetics of transgene detection shown in Fig. 2. To ascertain the presence of a nonintegrated (episomal) form of the transgene in addition to the integrated one, another PCR amplification was performed in which an Elongase mix was used to ensure amplification of large (up to 20 kb) DNA fragments. We reasoned that if plasmid DNA exists in episomal form, a sharp band of molecular size corresponding to the reference plasmid DNA would be seen. As shown in Fig. 4B (Top Right) no sharp band corresponding to the reference plasmid DNA was observed. This suggests that at the two time points examined only the integrated form is present. A hybridization pattern (smear) similar to the amplified PCR products was seen by Southern blot analysis (Fig. 4B Bottom). Further experiments are needed to determine the number of copies of the transgene/cell.
Figure 4.
Demonstration of integration of the transgene H chain into chromosomal DNA. (A) Schematic representation of the rationale to the experiment. Genomic DNA is digested with XbaI restriction enzyme that will cut (X) at multiple sites in the chromosomal DNA and in two sites in the transgene H chain. The digested DNA fragments are then recircularized with T4 DNA ligase and subsequently amplified by PCR using a set of primers (p62L/p62U) designed to anneal and extend in opposite directions (see ⇌). If genomic DNA contains the transgene H chain (integration), PCR amplification will give rise to a multitude of DNA products within an extended range of molecular sizes. This will be reflected in a pattern of diffuse gel migration (smear). Symbols are as follows: (□), splenic genomic DNA; (▪), backbone of plasmid DNA γ1NANP; (X), XbaI site; VH, variable region of the transgene H chain; C(γ1), constant region of the IgG1 subclass of the transgene H chain. (B) Results of PCR amplification and Southern blot hybridization. Genomic DNA was extracted from the spleen of mice inoculated with plasmid DNA γ1NANP at the times indicated. Time 0 refers to a naive mouse. DNA was digested with XbaI restriction enzyme, diluted in water, and self-ligated with T4 DNA ligase. PCR amplification (Top) was performed using Taq polymerase or Elongase mix, respectively (20). The amplified DNA products were subsequently transferred from the gels to a nylon membrane for Southern blot hybridization (Bottom) using the 32P-labeled pNAD oligonucleotide as a probe.
Sequencing the Transgene from Genomic DNA.
The immunogenic potential of a transgene-encoded product rely on the fact that no sense somatic mutation will affect the nucleotide sequence of the transgene while harbored in vivo. Hypermutation frequently occurs in the VDJ region of Ig, and in particular in the CDRs, a fact in agreement with the notion that hypermutation takes place during antigen selection and affinity maturation of the antibody response (45). Although the transgene used in this study lacks a transmembrane domain, rendering cell-surface anchoring unlikely, experiments were nevertheless performed to assess the accumulation of mutations as a result of protracted in vivo persistence in integrated form. The transgene VDJ region was amplified from splenic genomic DNA and subcloned and sequenced by the dideoxy termination method. No evidence of hypermutation was found in the VDJ region of the transgene even after 3 months in vivo (Table 2).
Table 2.
Lack of transgene mutations in PCR-generated clones from splenic genomic DNA
| Time (wk) | No. of clones sequenced | No. of clones mutated | No. of nucleotides mutated | Rate of mutation* (%) |
|---|---|---|---|---|
| 2 | 6 | 1/6 | 1** | 2.9 × 10−4 |
| 4 | 3 | 0/3 | 0 | |
| 12 | 3 | 0/3 | 0 |
Number of mutations per total number of base pairs sequenced.
A silent (C → T) mutation in FR3.
DISCUSSION
The experiments presented here are the first example of in vivo gene targeting to a specific cell type, B lymphocytes, to initiate an immune response. During somatic transgene immunization, two important prerequisites to immunogenicity, specific recruitment of antigen-producing cells and antigen-presenting cells, are met and linked operationally within the same cell in vivo. Our work indicates that targeting a tissue of high replicative activity with a gene possessing tissue-specific regulatory elements may result in integration. Possibly, integration also reflects the structural homology existing between the transgene and the host chromosomal DNA. Our finding contrasts, however, with reports in which plasmid DNA under the control of viral promoters injected into tissues with low replicative activity remains episomal (46). As to the persistence of the transgene in vivo, our experiments are in agreement with the observation that in vitro transduced B cells adoptively transferred into severe combined immunodeficient mice also maintained expression of the transgene for 3 months (47). Since resting long lived B lymphocytes in the mouse have an estimated average life span of 6 wk or more (48–50), it is suggested that the transgene persists in vivo in these cells until apoptosis occurs. Interestingly, effects on the immune response such as immunologic memory against transgene Ig extended long after (21 months) the detection of the transgene in vivo (unpublished data).
The characteristics of in vivo B cell targeting give a new perspective to the in vivo use of plasmid DNA. Targeting and delivery of DNA to B lymphocytes can be used to study the quantitative and qualitative aspects that render B lymphocytes and their secreted product immunogenic in vivo in a way that mimics a physiological mechanism, e.g., secretion of threshold amounts and no interference by immunological adjuvants or mitogens. Likewise, this novel approach can be exploited to identify cell types and dissect cellular networks during the immune response in vivo. Because of the low amounts of transgene product secreted and the apparent paucity of B cells involved (24), somatic transgenic mice can be used as an alternative to conventional (germ line) Ig transgenic mice (48–52). In the latter, the transgene is introduced in the zygote and is present from embryonic life through adult life in the vast majority of B cells. In contrast, our approach allows one to specifically target B lymphocytes and express an Ig transgene at any time during the adult life involving only a discrete number of B cells. This may lead to new possibilities for studying immunogenicity versus tolerance in adult immunocompetent animals.
Perhaps, if one can rule out untoward effects due to integration of the Ig transgene in B cells, our work could lead to new strategies for the induction of systemic immunity and immunologic memory. For instance, somatic transgene immunization can be used to induce immunity in selected circumstances against: (i) self idiotypes in patients with nonsecreting chronic lymphocytic leukemia (53); (ii) Ig genes chimerized with self-receptors, cytokines, or growth factors (54–57); and (iii) self or foreign antigen peptides coded in the CDRs of the transgene (58). It cannot be excluded that a similar approach may become useful in gene therapy and replacement to achieve good levels of secretion for a variety of proteins of biological or pharmacological interest. Before these practical approaches can be considered, noninvasive methods of gene delivery to target B lymphocytes need to be explored.
Acknowledgments
We thank Dennis Young for his expert collaboration in the sorting experiments and Drs. Martin Haas, Hyam Leffert, Maria-Grazia Roncarolo, and Jan De Vries for their critical reading of the manuscript. M.Z. was supported by National Institutes of Health grant AI36467 and is faculty member of the Biomedical Sciences Graduate Program. S.X. is on leave of absence from Shanghai Medical University of the People’s Republic of China and is the recipient of a scholarship from the same University. M.G. is recipient of a scholarship from Istituto Superiore di Sanitá, Roma, Italy.
ABBREVIATIONS
- CDR3
third complementarity-determining region
- PBSA
PBS albumin
References
- 1.Tang D, DeVit M, Johnston S A. Nature (London) 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 2.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, Dewitt C M, Friedman A, Hawe L A, Leander K R, Martinez D, Perry H C, Shiver J W, Montgomery D L, Liu M A. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 3.Davis H L, Whalen R G, Demeneix B A. Hum Gene Ther. 1993;4:151–159. doi: 10.1089/hum.1993.4.2-151. [DOI] [PubMed] [Google Scholar]
- 4.Raz E, Carson D A, Parker S E, Parr T B, Abai A M, Aichinger G, Gromkowski S H, Singh M, Lew D, Yankauckas M A, Baird S M, Rhodes G H. Proc Natl Acad Sci USA. 1994;91:9519–9523. doi: 10.1073/pnas.91.20.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang B, Ugen K, Srikantan V, Agadjanyan M, Dang K, Refaeli Y, Sato A, Boyer J, Williams W, Weiner D. Proc Natl Acad Sci USA. 1993;90:4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D’Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J-P, Liu M A, Ulmer J B. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 7.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 8.Sedegah M, Hedstrom R, Hobart P, Hoffman S L. Proc Natl Acad Sci USA. 1994;91:9866–9870. doi: 10.1073/pnas.91.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doolan D L, Sedegah M, Hedstrom R C, Hobart P, Charoenvit Y, Hoffman S L. J Exp Med. 1996;183:1739–1746. doi: 10.1084/jem.183.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu D, Liew F Y. Immunology. 1995;84:173–176. [PMC free article] [PubMed] [Google Scholar]
- 11.Conry R M, LoBuglio A F, Kantor J, Schlom J, Loechel F, Moore S E, Sumerel L A, Barlow D L, Abrams S, Curiel D T. Cancer Res. 1994;54:1164–1168. [PubMed] [Google Scholar]
- 12.Gilkeson G S, Ruiz P, Pippen A M, Alexander A L, Lefkowith J B, Pisetsky D S. J Exp Med. 1996;183:1389–97. doi: 10.1084/jem.183.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waisman A, Ruiz P J, Hirschberg D L, Gelman A, Oksenberg J R, Brocke S, Mor F, Cohen I R, Steinman L. Nat Med. 1996;2:899–905. doi: 10.1038/nm0896-899. [DOI] [PubMed] [Google Scholar]
- 14.Raz E, Tighe H, Sato Y, Corr M, Dudler J A, Roman M, Swain S L, Spiegelberg H L, Carson D A. Proc Natl Acad Sci USA. 1996;93:5141–5145. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu C H, Chua K Y, Tao M H, Lai Y L, Wu H D, Huang S K, Hsieh K H. Nat Med. 1996;2:540–544. doi: 10.1038/nm0596-540. [DOI] [PubMed] [Google Scholar]
- 16.Osmond D G. Immunol Rev. 1986;93:103–124. doi: 10.1111/j.1600-065x.1986.tb01504.x. [DOI] [PubMed] [Google Scholar]
- 17.Rajewsky K. Curr Opin Immunol. 1992;4:171–176. doi: 10.1016/0952-7915(92)90008-3. [DOI] [PubMed] [Google Scholar]
- 18.Rolink A, Melchers F. Curr Opin Immunol. 1993;5:207–217. doi: 10.1016/0952-7915(93)90006-e. [DOI] [PubMed] [Google Scholar]
- 19.Jerne N K. Immunol Rev. 1984;79:5–24. doi: 10.1111/j.1600-065x.1984.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 20.Langman R E, Cohn M. Mol Immunol. 1987;24:675–697. doi: 10.1016/0161-5890(87)90050-2. [DOI] [PubMed] [Google Scholar]
- 21.Lanzavecchia A. Nature (London) 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 22.Weiss S, Bogen B. Proc Natl Acad Sci USA. 1989;86:282–286. doi: 10.1073/pnas.86.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Billetta R, Filaci G, Zanetti M. Eur J Immunol. 1995;25:776–783. doi: 10.1002/eji.1830250323. [DOI] [PubMed] [Google Scholar]
- 24.Gerloni M, Billetta R, Xiong S, Zanetti M. DNA and Cell Biol. 1997;16:611–625. doi: 10.1089/dna.1997.16.611. [DOI] [PubMed] [Google Scholar]
- 25.Gillies S D, Morrison S L, Oi V T, Tonegawa S. Cell. 1983;33:717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- 26.Banerji J, Olson L, Schaffner W. Cell. 1983;33:729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- 27.Grosschedl R, Baltimore D. Cell. 1985;41:885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- 28.Mason J O, Williams G T, Neuberger M S. Cell. 1985;41:479–487. doi: 10.1016/s0092-8674(85)80021-0. [DOI] [PubMed] [Google Scholar]
- 29.Grosschedl R, Weaver D, Baltimore D, Costantini F. Cell. 1984;38:647–658. doi: 10.1016/0092-8674(84)90259-9. [DOI] [PubMed] [Google Scholar]
- 30.Storb U, Pinkert C, Arp B, Engler P, Gollahon K, Manz J, Brady W, Brinster R L. J Exp Med. 1986;164:627–641. doi: 10.1084/jem.164.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamura K, Kudo A, Ebihara T, Kamino K, Araki K, Kumahara Y, Watanabe T. Proc Natl Acad Sci USA. 1986;83:2152–2156. doi: 10.1073/pnas.83.7.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sollazzo M, Billetta R, Zanetti M. Protein Eng. 1990;4:215–220. doi: 10.1093/protein/4.2.215. [DOI] [PubMed] [Google Scholar]
- 33.Sollazzo M, Hasemann C A, Meek K D, Glotz D, Capra J D, Zanetti M. Eur J Immunol. 1989;19:453–457. doi: 10.1002/eji.1830190307. [DOI] [PubMed] [Google Scholar]
- 34.Mulligan R C, Berg P. Proc Natl Acad Sci USA. 1981;78:2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 36.Glasel J A. BioTechniques. 1995;18:62–63. [PubMed] [Google Scholar]
- 37.Billetta R, Zanetti M. ImmunoMethods. 1992;1:41–51. [Google Scholar]
- 38.Harris D E, Warshaw D M, Periasamy M. Gene. 1992;112:265–266. doi: 10.1016/0378-1119(92)90388-6. [DOI] [PubMed] [Google Scholar]
- 39.Sollazzo M, Castiglia D, Billetta R, Tramontano A, Zanetti M. Protein Eng. 1990;3:531–539. doi: 10.1093/protein/3.6.531. [DOI] [PubMed] [Google Scholar]
- 40.Holmberg D, Freitas A A, Portnoi D, Jacquemart F, Avrameas S, Coutinho A. Immunol Rev. 1986;93:147–169. doi: 10.1111/j.1600-065x.1986.tb01506.x. [DOI] [PubMed] [Google Scholar]
- 41.Glotz D, Sollazzo M, Riley S, Zanetti M. J Immunol. 1988;141:383–390. [PubMed] [Google Scholar]
- 42.Bennett R M, Gabor G T, Merritt M M. J Clin Invest. 1985;76:2182–2190. doi: 10.1172/JCI112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melchers F. In: Immunoglobulin Genes. Honjo T, Alt F T, editors. Vol. 2. London: Academic; 1995. pp. 33–56. [Google Scholar]
- 44.Richter A, Ozer H L, DesGroseillers L, Jolicoeur P. Mol Cell Biol. 1984;4:151–159. doi: 10.1128/mcb.4.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griffiths G M, Berek C, Kaartinen M, Milstein C. Nature (London) 1984;312:271–275. doi: 10.1038/312271a0. [DOI] [PubMed] [Google Scholar]
- 46.Wolff J A, Ludtke J J, Acsadi G, Williams P, Jani A. Hum Mol Genet. 1992;1:363–369. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 47.Sutkowski N, Kuo M L, Varela E A, Dougherty J P, Ron Y. Proc Natl Acad Sci USA. 1994;91:8875–8879. doi: 10.1073/pnas.91.19.8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forster I, Rajewsky K. Proc Natl Acad Sci USA. 1990;87:4781–4784. doi: 10.1073/pnas.87.12.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajewsky K, Schittek B. Nature (London) 1990;346:749–751. doi: 10.1038/346749a0. [DOI] [PubMed] [Google Scholar]
- 50.Osmond D G. Curr Op Immunol. 1991;3:179–185. doi: 10.1016/0952-7915(91)90047-5. [DOI] [PubMed] [Google Scholar]
- 51.Goodnow C C. Annu Rev Immunol. 1992;10:489–518. doi: 10.1146/annurev.iy.10.040192.002421. [DOI] [PubMed] [Google Scholar]
- 52.Goodnow C C. Proc Natl Acad Sci USA. 1996;93:2264–2271. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsu F J, Kwak L, Campbell M, Liles T, Czerwinski D, Hart S, Syrengelas A, Miller R, Levy R. Ann NY Acad Sci. 1993;690:385–387. doi: 10.1111/j.1749-6632.1993.tb44039.x. [DOI] [PubMed] [Google Scholar]
- 54.Gascoigne N R, Goodnow C C, Dudzik K I, Oi V T, Davis M M. Proc Natl Acad Sci USA. 1987;84:2936–2940. doi: 10.1073/pnas.84.9.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Capon D J, Chamow S M, Mordenti J, Marsters S A, Gregory T, Mitsuya H, Byrn R A, Lucas C, Wurm F M, Groopman J E. Nature (London) 1989;337:525–531. doi: 10.1038/337525a0. [DOI] [PubMed] [Google Scholar]
- 56.Tao M H, Levy R. Nature (London) 1993;362:755–758. doi: 10.1038/362755a0. [DOI] [PubMed] [Google Scholar]
- 57.Shin S U, Friden P, Moran M, Olson T, Kang Y S, Pardridge W M, Morrison S L. Proc Natl Acad Sci USA. 1995;92:2820–2824. doi: 10.1073/pnas.92.7.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zanetti M, Rossi F, Lanza P, Filaci G, Lee R H, Billetta R. Immunol Rev. 1992;130:125–150. doi: 10.1111/j.1600-065x.1992.tb01524.x. [DOI] [PubMed] [Google Scholar]