Abstract
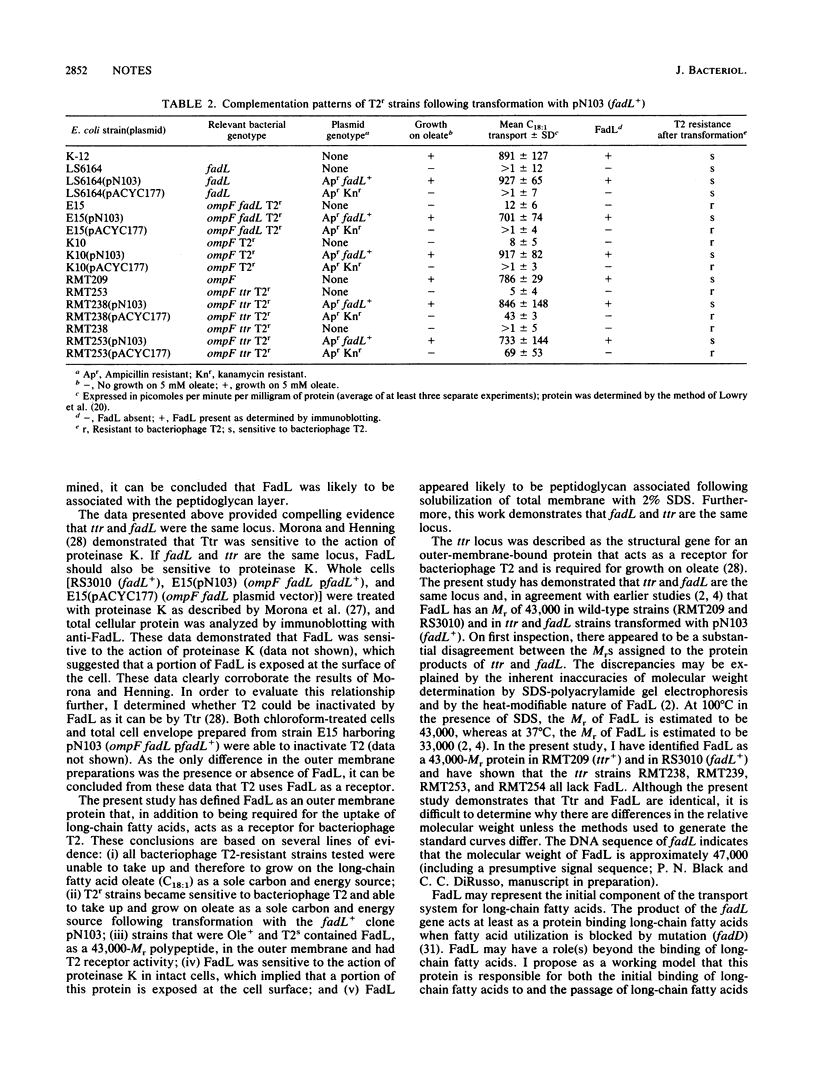
The fadL+ gene of Escherichia coli encodes an outer membrane protein (FadL) essential for the uptake of long-chain fatty acids (C12 to C18). The present study shows that in addition to being required for uptake of and growth on the long-chain fatty acid oleate (C18:1), FadL acts as a receptor of bacteriophage T2. Bacteriophage T2-resistant (T2r) strains lacked FadL and were unable to take up and grow on long-chain fatty acids. Upon transformation with the fadL+ clone pN103, T2r strains became sensitive to bacteriophage T2 (T2s), became able to take up long-chain fatty acids at wild-type levels, and contained FadL in the outer membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black P. N., Kianian S. F., DiRusso C. C., Nunn W. D. Long-chain fatty acid transport in Escherichia coli. Cloning, mapping, and expression of the fadL gene. J Biol Chem. 1985 Feb 10;260(3):1780–1789. [PubMed] [Google Scholar]
- Black P. N., Landers M. H., Happ G. M. Cytodifferentiation in the accessory glands of Tenebrio molitor. VIII. Crossed immunoelectrophoretic analysis of terminal differentiation in the postecdysial tubular accessory glands. Dev Biol. 1982 Nov;94(1):106–115. doi: 10.1016/0012-1606(82)90073-2. [DOI] [PubMed] [Google Scholar]
- Black P. N., Said B., Ghosn C. R., Beach J. V., Nunn W. D. Purification and characterization of an outer membrane-bound protein involved in long-chain fatty acid transport in Escherichia coli. J Biol Chem. 1987 Jan 25;262(3):1412–1419. [PubMed] [Google Scholar]
- Boehler-Kohler B. A., Boos W., Dieterle R., Benz R. Receptor for bacteriophage lambda of Escherichia coli forms larger pores in black lipid membranes than the matrix protein (porin). J Bacteriol. 1979 Apr;138(1):33–39. doi: 10.1128/jb.138.1.33-39.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Crowlesmith I., Gamon K., Henning U. Precursor proteins are intermediates in vivo in the synthesis of two major outer membrane proteins, the OmpA and OmpF proteins, of Escherichia coli K12. Eur J Biochem. 1981 Jan;113(2):375–380. doi: 10.1111/j.1432-1033.1981.tb05076.x. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Ginsburgh C. L., Black P. N., Nunn W. D. Transport of long chain fatty acids in Escherichia coli. Identification of a membrane protein associated with the fadL gene. J Biol Chem. 1984 Jul 10;259(13):8437–8443. [PubMed] [Google Scholar]
- Hall M. N., Schwartz M., Silhavy T. J. Sequence information within the lamB genes in required for proper routing of the bacteriophage lambda receptor protein to the outer membrane of Escherichia coli K-12. J Mol Biol. 1982 Mar 25;156(1):93–112. doi: 10.1016/0022-2836(82)90461-2. [DOI] [PubMed] [Google Scholar]
- Hantke K. Major outer membrane proteins of E. coli K12 serve as receptors for the phages T2 (protein Ia) and 434 (protein Ib). Mol Gen Genet. 1978 Aug 17;164(2):131–135. doi: 10.1007/BF00267377. [DOI] [PubMed] [Google Scholar]
- Hantke K. Phage T6--colicin K receptor and nucleoside transport in Escherichia coli. FEBS Lett. 1976 Nov;70(1):109–112. doi: 10.1016/0014-5793(76)80737-5. [DOI] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Transfer of fatty acids from the 1-position of phosphatidylethanolamine to the major outer membrane lipoprotein of Escherichia coli. J Biol Chem. 1986 Aug 25;261(24):11328–11333. [PubMed] [Google Scholar]
- Klein K., Steinberg R., Fiethen B., Overath P. Fatty acid degradation in Escherichia coli. An inducible system for the uptake of fatty acids and further characterization of old mutants. Eur J Biochem. 1971 Apr;19(3):442–450. doi: 10.1111/j.1432-1033.1971.tb01334.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenski R. E. Two-step resistance by Escherichia coli B to bacteriophage T2. Genetics. 1984 May;107(1):1–7. doi: 10.1093/genetics/107.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Specificity of diffusion channels produced by lambda phage receptor protein of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):167–171. doi: 10.1073/pnas.77.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Wayne R., Neilands J. B. In vitro competition between ferrichrome and phage for the outer membrane T5 receptor complex of Escherichia coli. Biochem Biophys Res Commun. 1975 May 19;64(2):687–693. doi: 10.1016/0006-291x(75)90375-7. [DOI] [PubMed] [Google Scholar]
- Maloy S. R., Ginsburgh C. L., Simons R. W., Nunn W. D. Transport of long and medium chain fatty acids by Escherichia coli K12. J Biol Chem. 1981 Apr 25;256(8):3735–3742. [PubMed] [Google Scholar]
- Menichi B., Buu A. Peptidoglycan association of bacteriophage T5 receptor in Escherichia coli K-12. J Bacteriol. 1986 Jun;166(3):1137–1140. doi: 10.1128/jb.166.3.1137-1140.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona R., Henning U. New locus (ttr) in Escherichia coli K-12 affecting sensitivity to bacteriophage T2 and growth on oleate as the sole carbon source. J Bacteriol. 1986 Nov;168(2):534–540. doi: 10.1128/jb.168.2.534-540.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona R., Tommassen J., Henning U. Demonstration of a bacteriophage receptor site on the Escherichia coli K12 outer-membrane protein OmpC by the use of a protease. Eur J Biochem. 1985 Jul 1;150(1):161–169. doi: 10.1111/j.1432-1033.1985.tb09002.x. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn W. D., Colburn R. W., Black P. N. Transport of long-chain fatty acids in Escherichia coli. Evidence for role of fadL gene product as long-chain fatty acid receptor. J Biol Chem. 1986 Jan 5;261(1):167–171. [PubMed] [Google Scholar]
- Nunn W. D., Simons R. W., Egan P. A., Maloy S. R. Kinetics of the utilization of medium and long chain fatty acids by mutant of Escherichia coli defective in the fadL gene. J Biol Chem. 1979 Sep 25;254(18):9130–9134. [PubMed] [Google Scholar]
- Nunn W. D., Simons R. W. Transport of long-chain fatty acids by Escherichia coli: mapping and characterization of mutants in the fadL gene. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3377–3381. doi: 10.1073/pnas.75.7.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Raufuss E. M. The induction of the enzymes of fatty acid degradation in Escherichia coli. Biochem Biophys Res Commun. 1967 Oct 11;29(1):28–33. doi: 10.1016/0006-291x(67)90535-9. [DOI] [PubMed] [Google Scholar]
- Rock C. O., Jackowski S. Pathways for the incorporation of exogenous fatty acids into phosphatidylethanolamine in Escherichia coli. J Biol Chem. 1985 Oct 15;260(23):12720–12724. [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Sallus L., Haselbeck R. J., Nunn W. D. Regulation of fatty acid transport in Escherichia coli: analysis by operon fusion. J Bacteriol. 1983 Sep;155(3):1450–1454. doi: 10.1128/jb.155.3.1450-1454.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Egan P. A., Chute H. T., Nunn W. D. Regulation of fatty acid degradation in Escherichia coli: isolation and characterization of strains bearing insertion and temperature-sensitive mutations in gene fadR. J Bacteriol. 1980 May;142(2):621–632. doi: 10.1128/jb.142.2.621-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]