Abstract
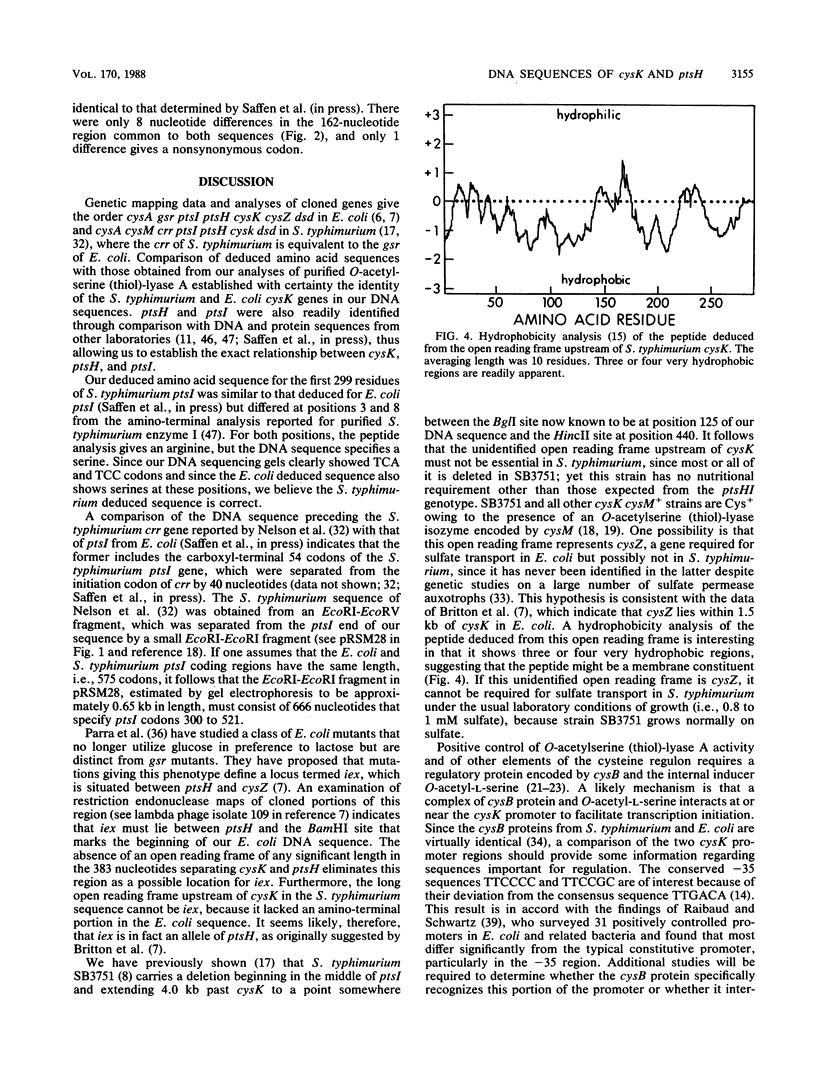
Nucleotide sequences of the cysK regions of Salmonella typhimurium and Escherichia coli have been determined. A total of 3,812 and 2,595 nucleotides were sequenced from S. typhimurium and E. coli, respectively. Open reading frames of 323 codons were found in both species and were identified as those of cysK by comparison of deduced amino acid sequences with amino- and carboxyl-terminal amino acid analyses of the S. typhimurium cysK gene product O-acetylserine (thiol)-lyase A. The two cysK DNA sequences were 85% identical, and the deduced amino acid sequences were 96% identical. The major transcription initiation sites for cysK were found to be virtually identical in the two organisms, by using primer extension and S1 nuclease protection techniques. The -35 region corresponding to the major transcription start site was TTCCCC in S. typhimurium and TTCCGC in E. coli. The deviation of these sequences from the consensus sequence TTGACA may reflect the fact that cysK is subject to positive control and requires the cysB regulatory protein for expression. Sequences downstream of cysK were found to include ptsH and a portion of ptsI, thus establishing the exact relationship of cysK with these two genes. A 290-codon open reading frame, which may represent the cysZ gene, was identified upstream of cysK.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M. A., Kredich N. M., Tomkins G. M. The purification and characterization of O-acetylserine sulfhydrylase-A from Salmonella typhimurium. J Biol Chem. 1969 May 10;244(9):2418–2427. [PubMed] [Google Scholar]
- Becker M. A., Tomkins G. M. Pleiotrophy in a cysteine-requiring mutant of Samonella typhimurium resulting from altered protein-protein interaction. J Biol Chem. 1969 Nov 10;244(21):6023–6030. [PubMed] [Google Scholar]
- Boronat A., Britton P., Jones-Mortimer M. C., Kornberg H. L., Lee L. G., Murfitt D., Parra F. Location on the Escherichia coli genome of a gene specifying O-acetylserine (thiol)-lyase. J Gen Microbiol. 1984 Mar;130(3):673–685. doi: 10.1099/00221287-130-3-673. [DOI] [PubMed] [Google Scholar]
- Britton P., Boronat A., Hartley D. A., Jones-Mortimer M. C., Kornberg H. L., Parra F. Phosphotransferase-mediated regulation of carbohydrate utilization in Escherichia coli K12: location of the gsr (tgs) and iex (crr) genes by specialized transduction. J Gen Microbiol. 1983 Feb;129(2):349–356. doi: 10.1099/00221287-129-2-349. [DOI] [PubMed] [Google Scholar]
- Cordaro J. C., Roseman S. Deletion mapping of the genes coding for HPr and enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system in Salmonella typhimurium. J Bacteriol. 1972 Oct;112(1):17–29. doi: 10.1128/jb.112.1.17-29.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- De Reuse H., Roy A., Danchin A. Analysis of the ptsH-ptsI-crr region in Escherichia coli K-12: nucleotide sequence of the ptsH gene. Gene. 1985;35(1-2):199–207. doi: 10.1016/0378-1119(85)90172-6. [DOI] [PubMed] [Google Scholar]
- Filutowicz M., Wiater A., Hulanicka D. Delayed inducibility of sulphite reductase in cysM mutants of Salmonella typhimurium under anaerobic conditions. J Gen Microbiol. 1982 Aug;128(8):1791–1794. doi: 10.1099/00221287-128-8-1791. [DOI] [PubMed] [Google Scholar]
- Fimmel A. L., Loughlin R. E. Isolation and characterization of cysK mutants of Escherichia coli K12. J Gen Microbiol. 1977 Nov;103(1):37–43. doi: 10.1099/00221287-103-1-37. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson G. S., Davidson B. E. Nucleotide sequence and transcription of the phenylalanine and tyrosine operons of Escherichia coli K12. J Mol Biol. 1984 Dec 25;180(4):1023–1051. doi: 10.1016/0022-2836(84)90269-9. [DOI] [PubMed] [Google Scholar]
- Hulanicka M. D., Garrett C., Jagura-Burdzy G., Kredich N. M. Cloning and characterization of the cysAMK region of Salmonella typhimurium. J Bacteriol. 1986 Oct;168(1):322–327. doi: 10.1128/jb.168.1.322-327.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulanicka M. D., Hallquist S. G., Kredich N. M., Mojica-A T. Regulation of O-acetylserine sulfhydrylase B by L-cysteine in Salmonella typhimurium. J Bacteriol. 1979 Oct;140(1):141–146. doi: 10.1128/jb.140.1.141-146.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulanicka M. D., Kredich N. M., Treiman D. M. The structural gene for O-acetylserine sulfhydrylase A in Salmonella typhimurium. Identity with the trzA locus. J Biol Chem. 1974 Feb 10;249(3):867–872. [PubMed] [Google Scholar]
- Hunkapiller M. W., Hewick R. M., Dreyer W. J., Hood L. E. High-sensitivity sequencing with a gas-phase sequenator. Methods Enzymol. 1983;91:399–413. doi: 10.1016/s0076-6879(83)91038-8. [DOI] [PubMed] [Google Scholar]
- Jones-Mortimer M. C. Positive control of sulphate reduction in Escherichia coli. The nature of the pleiotropic cysteineless mutants of E. coli K12. Biochem J. 1968 Dec;110(3):597–602. doi: 10.1042/bj1100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Mortimer M. C., Wheldrake J. F., Pasternak C. A. The control of sulphate reduction in Escherichia coli by O-acetyl-L-serine. Biochem J. 1968 Mar;107(1):51–53. doi: 10.1042/bj1070051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M. Regulation of L-cysteine biosynthesis in Salmonella typhimurium. I. Effects of growth of varying sulfur sources and O-acetyl-L-serine on gene expression. J Biol Chem. 1971 Jun 10;246(11):3474–3484. [PubMed] [Google Scholar]
- Kredich N. M., Tomkins G. M. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem. 1966 Nov 10;241(21):4955–4965. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller B. E., Kredich N. M. Purification of the cysB protein from Salmonella typhimurium. J Biol Chem. 1987 May 5;262(13):6006–6009. [PubMed] [Google Scholar]
- Monroe R. S., Kredich N. M. Isolation of Salmonella typhimurium cys genes by transduction with a library of recombinant plasmids packaged in bacteriophage P22HT capsids. J Bacteriol. 1988 Jan;170(1):42–47. doi: 10.1128/jb.170.1.42-47.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Iwahashi H., Eguchi Y. Enzymatic proof for the identity of the S-sulfocysteine synthase and cysteine synthase B of Salmonella typhimurium. J Bacteriol. 1984 Jun;158(3):1122–1127. doi: 10.1128/jb.158.3.1122-1127.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Kon Y., Iwahashi H., Eguchi Y. Evidence that thiosulfate assimilation by Salmonella typhimurium is catalyzed by cysteine synthase B. J Bacteriol. 1983 Nov;156(2):656–662. doi: 10.1128/jb.156.2.656-662.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. O., Schuitema A. R., Benne R., van der Ploeg L. H., Plijter J. S., Aan F., Postma P. W. Molecular cloning, sequencing, and expression of the crr gene: the structural gene for IIIGlc of the bacterial PEP:glucose phosphotransferase system. EMBO J. 1984 Jul;3(7):1587–1593. doi: 10.1002/j.1460-2075.1984.tb02015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski J., Jagura-Burdzy G., Kredich N. M. DNA sequences of the cysB regions of Salmonella typhimurium and Escherichia coli. J Biol Chem. 1987 May 5;262(13):5999–6005. [PubMed] [Google Scholar]
- Ota N., Galsworthy P. R., Pardee A. B. Genetics of sulfate transport by Salmonella typhimurium. J Bacteriol. 1971 Mar;105(3):1053–1062. doi: 10.1128/jb.105.3.1053-1062.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASTERNAK C. A. Sulphate activation and its control in Escherichia coli and Bacillus subtilis. Biochem J. 1962 Oct;85:44–49. doi: 10.1042/bj0850044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra F., Britton P., Castle C., Jones-Mortimer M. C., Kornberg H. L. Two separate genes involved in sulphate transport in Escherichia coli K12. J Gen Microbiol. 1983 Feb;129(2):357–358. doi: 10.1099/00221287-129-2-357. [DOI] [PubMed] [Google Scholar]
- Parra F., Jones-Mortimer M. C., Kornberg H. L. Phosphotransferase-mediated regulation of carbohydrate utilization in Escherichia coli K12: the nature of the iex (crr) and gsr (tgs) mutations. J Gen Microbiol. 1983 Feb;129(2):337–348. doi: 10.1099/00221287-129-2-337. [DOI] [PubMed] [Google Scholar]
- Powers D. A., Roseman S. The primary structure of Salmonella typhimurium HPr, a phosphocarrier protein of the phosphoenolpyruvate:glycose phosphotransferase system. A correction. J Biol Chem. 1984 Dec 25;259(24):15212–15214. [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Weigel N., Powers D. A., Roseman S. Sugar transport by the bacterial phosphotransferase system. Primary structure and active site of a general phosphocarrier protein (HPr) from Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14499–14509. [PubMed] [Google Scholar]
- Weigel N., Waygood E. B., Kukuruzinska M. A., Nakazawa A., Roseman S. Sugar transport by the bacterial phosphotransferase system. Isolation and characterization of enzyme I from Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14461–14469. [PubMed] [Google Scholar]



