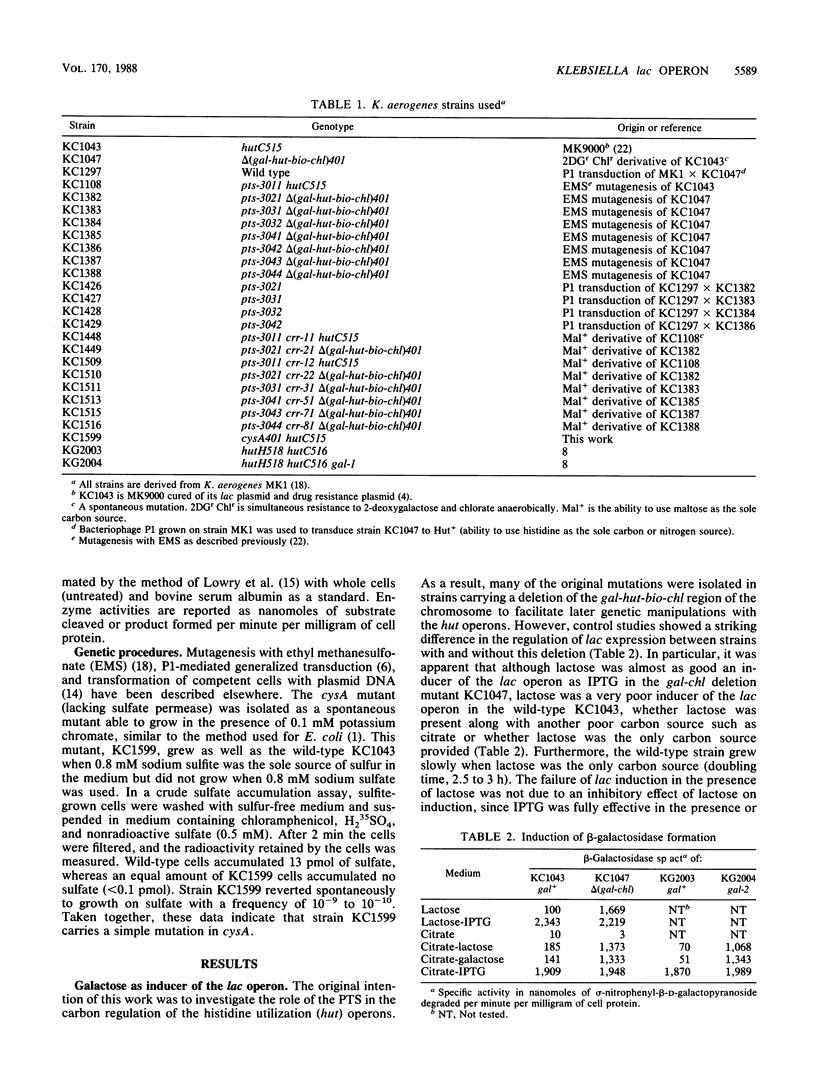
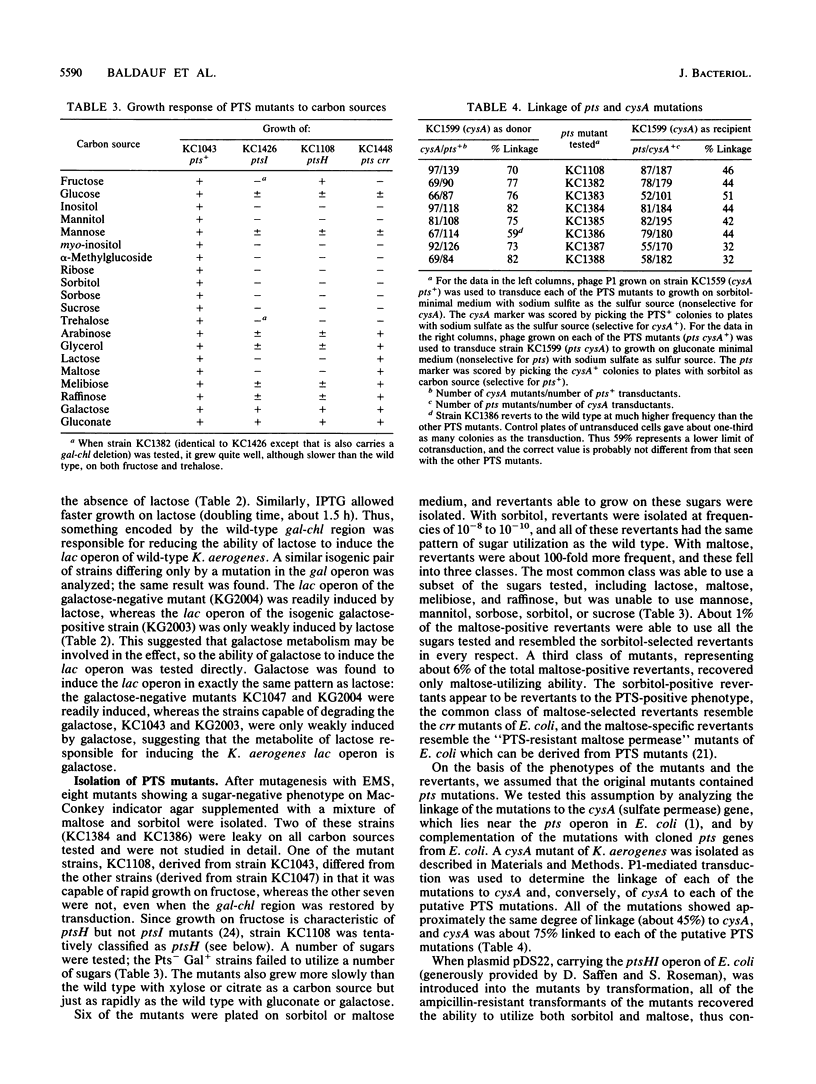
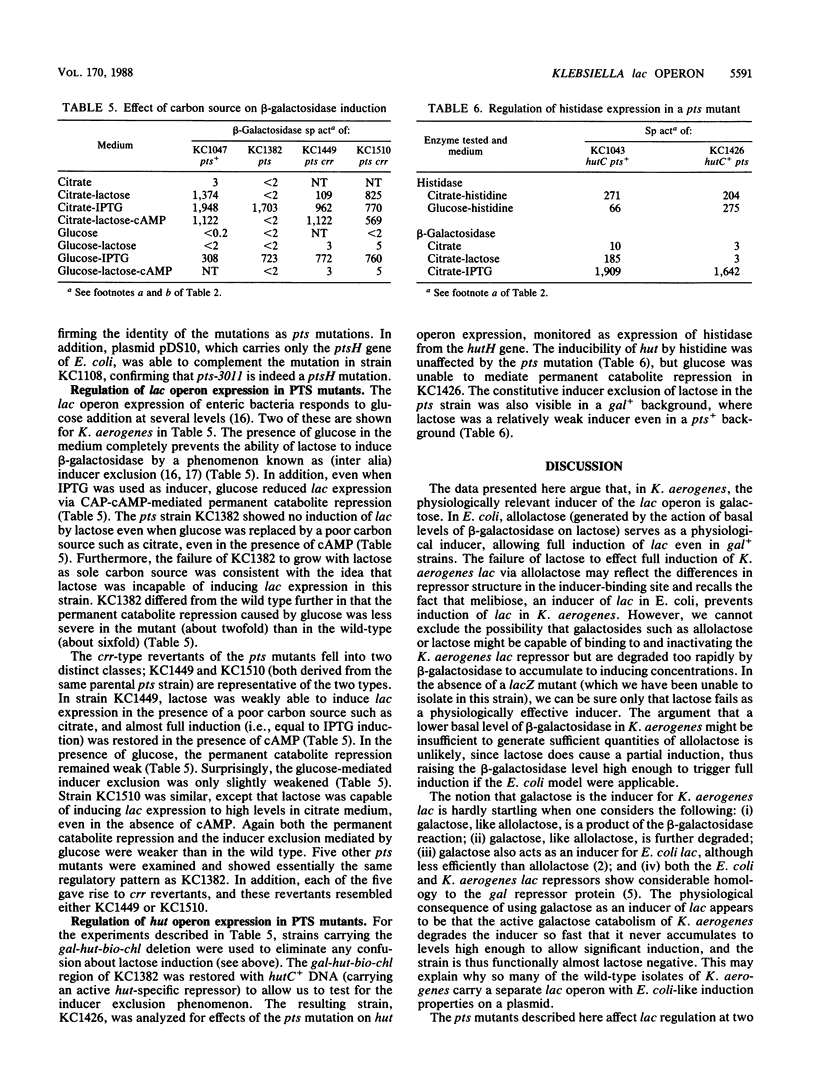
Abstract
Galactose appears to be the physiological inducer of the chromosomal lac operon in Klebsiella aerogenes. Both lactose and galactose are poor inducers in strains having a functional galactose catabolism (gal) operon, but both are excellent inducers in gal mutants. Thus the slow growth of K. aerogenes on lactose reflects the rapid degradation of the inducer. Several pts mutations were characterized and shown to affect both inducer exclusion and permanent catabolite repression. The beta-galactosidase of pts mutants cannot be induced at all by lactose, and pts mutants appear to have a permanent and constitutive inducer exclusion phenotype. In addition, pts mutants show a reduced rate of glucose metabolism, leading to slower growth on glucose and a reduced degree of glucose-mediated permanent catabolite repression. The crr-type pseudorevertants of pts mutations relieve the constitutive inducer exclusion for lac but do not restore the full level of glucose-mediated permanent catabolite repression and only slightly weaken the glucose-mediated inducer exclusion. Except for weakening the glucose-mediated permanent catabolite repression, pts and crr mutations have no effect on expression of the histidine utilization (hut) operons.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R. A., Janssen K. A., Resnick A. D., Blumenberg M., Foor F., Magasanik B. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J Bacteriol. 1977 Feb;129(2):1001–1009. doi: 10.1128/jb.129.2.1001-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley J. E., Magasanik B. Klebsiella aerogenes strain carrying drug-resistance determinants and a lac plasmid. J Bacteriol. 1972 Oct;112(1):200–205. doi: 10.1128/jb.112.1.200-205.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buvinger W. E., Riley M. Nucleotide sequence of Klebsiella pneumoniae lac genes. J Bacteriol. 1985 Sep;163(3):850–857. doi: 10.1128/jb.163.3.850-857.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R. B., Bender R. A., Streicher S. L. Direct selection for P1-sensitive mutants of enteric bacteria. J Bacteriol. 1974 Jun;118(3):810–814. doi: 10.1128/jb.118.3.810-814.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R. B., Bloom F. R., Magasanik B. Regulation of histidase synthesis in intergeneric hybrids of enteric bacteria. J Bacteriol. 1976 Jul;127(1):114–119. doi: 10.1128/jb.127.1.114-119.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R. B., Magasanik B. Gene order of the histidine utilization (hut) operons in Klebsiella aerogenes. J Bacteriol. 1975 Jun;122(3):1025–1031. doi: 10.1128/jb.122.3.1025-1031.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G., Reeve E. C. A third beta-galactosidase in a strain of Klebsiella that possesses two lac genes. J Bacteriol. 1977 Oct;132(1):219–223. doi: 10.1128/jb.132.1.219-223.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIDHARDT F. C. Mutant of Aerobacter aerogenes lacking glucose repression. J Bacteriol. 1960 Oct;80:536–543. doi: 10.1128/jb.80.4.536-543.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neijssel O. M., Hardy G. P., Lansbergen J. C., Tempest D. W., O'Brien R. W. Influence of growth environment on the phosphoenolpyruvate: glucose phosphotransferase activities of Escherichia coli and Klebsiella aerogenes: a comparative study. Arch Microbiol. 1980 Mar;125(1-2):175–179. doi: 10.1007/BF00403216. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prival M. J., Magasanik B. Resistance to catabolite repression of histidase and proline oxidase during nitrogen-limited growth of Klebsiella aerogenes. J Biol Chem. 1971 Oct 25;246(20):6288–6296. [PubMed] [Google Scholar]
- Reeve E. C., Braithwaite J. A. Lac-plus plasmids are responsible for the strong lactose-positive phenotype found in many strains of Klebsiella species. Genet Res. 1973 Dec;22(3):329–333. doi: 10.1017/s0016672300013124. [DOI] [PubMed] [Google Scholar]
- Reeve E. C., Braithwaite J. A. The lactose system in Klebsiella aerogenes V9A. 4. A comparison of the lac operons of Klebsiella and Escherichia coli. Genet Res. 1974 Dec;24(3):323–331. doi: 10.1017/s0016672300015329. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Straud H., Massman L. S., Judice J. J., Newman M. J., Feucht B. U. Permease-specific mutations in Salmonella typhimurium and Escherichia coli that release the glycerol, maltose, melibiose, and lactose transport systems from regulation by the phosphoenolpyruvate:sugar phosphotransferase system. J Bacteriol. 1978 Mar;133(3):1358–1367. doi: 10.1128/jb.133.3.1358-1367.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher S. L., Bender R. A., Magasanik B. Genetic control of glutamine synthetase in Klebiella aerogenes. J Bacteriol. 1975 Jan;121(1):320–331. doi: 10.1128/jb.121.1.320-331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Lin E. C. Two classes of pleiotropic mutants of Aerobacter aerogenes lacking components of a phosphoenolpyruvate-dependent phosphotransferase system. Proc Natl Acad Sci U S A. 1967 Apr;57(4):913–919. doi: 10.1073/pnas.57.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter R. W., Jr, Anderson R. L. Evidence that the inducible phosphoenolpyruvate:D-fructose 1-phosphotransferase system of Aerobacter aerogenes does not require "HPr". Biochem Biophys Res Commun. 1973 May 1;52(1):93–97. doi: 10.1016/0006-291x(73)90958-3. [DOI] [PubMed] [Google Scholar]


