Abstract
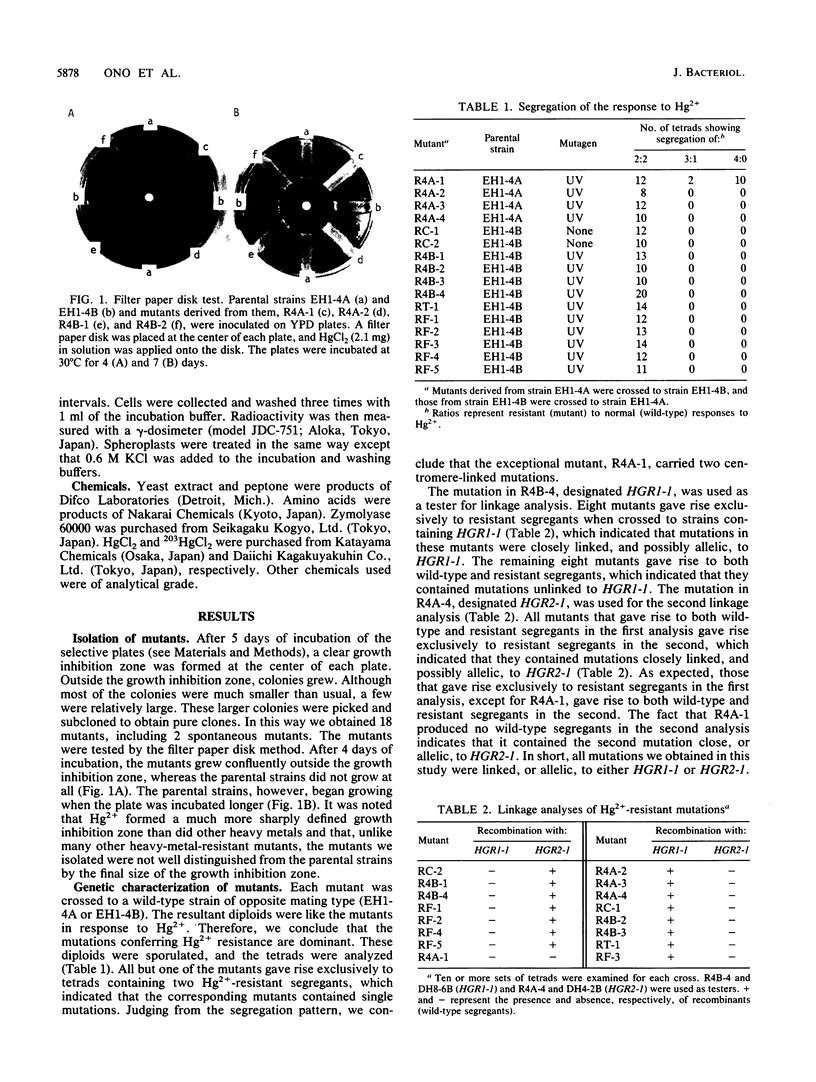
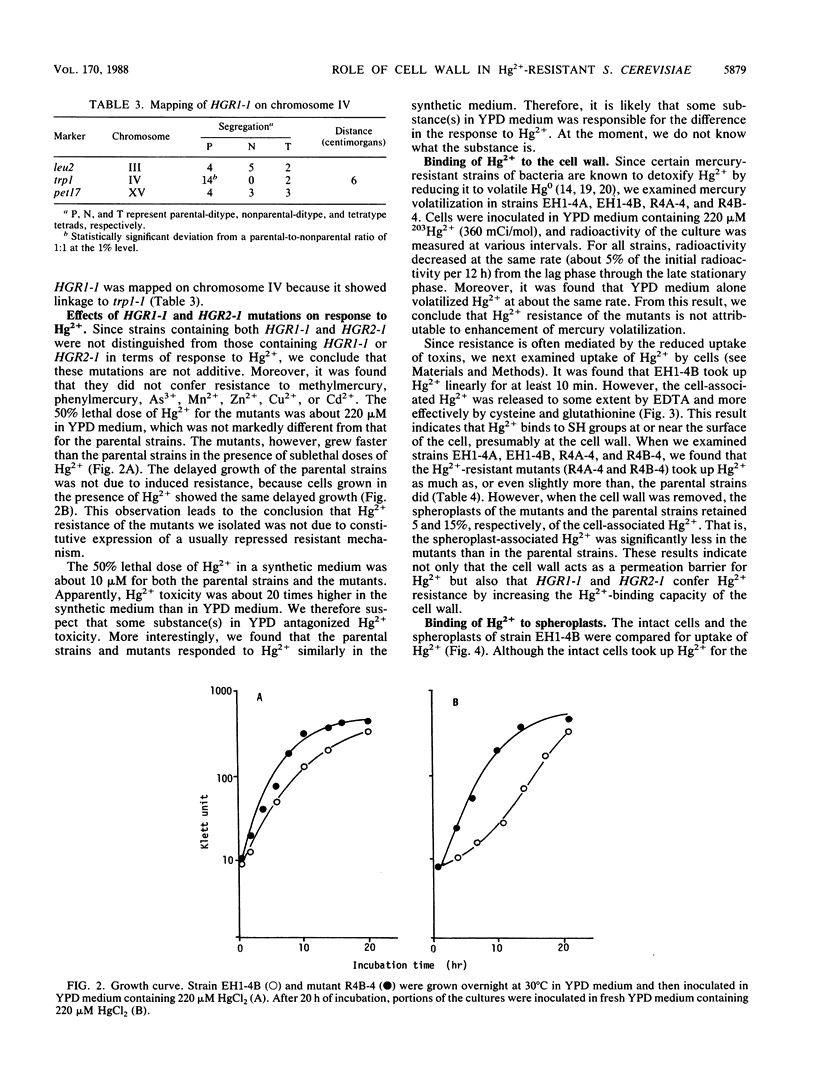
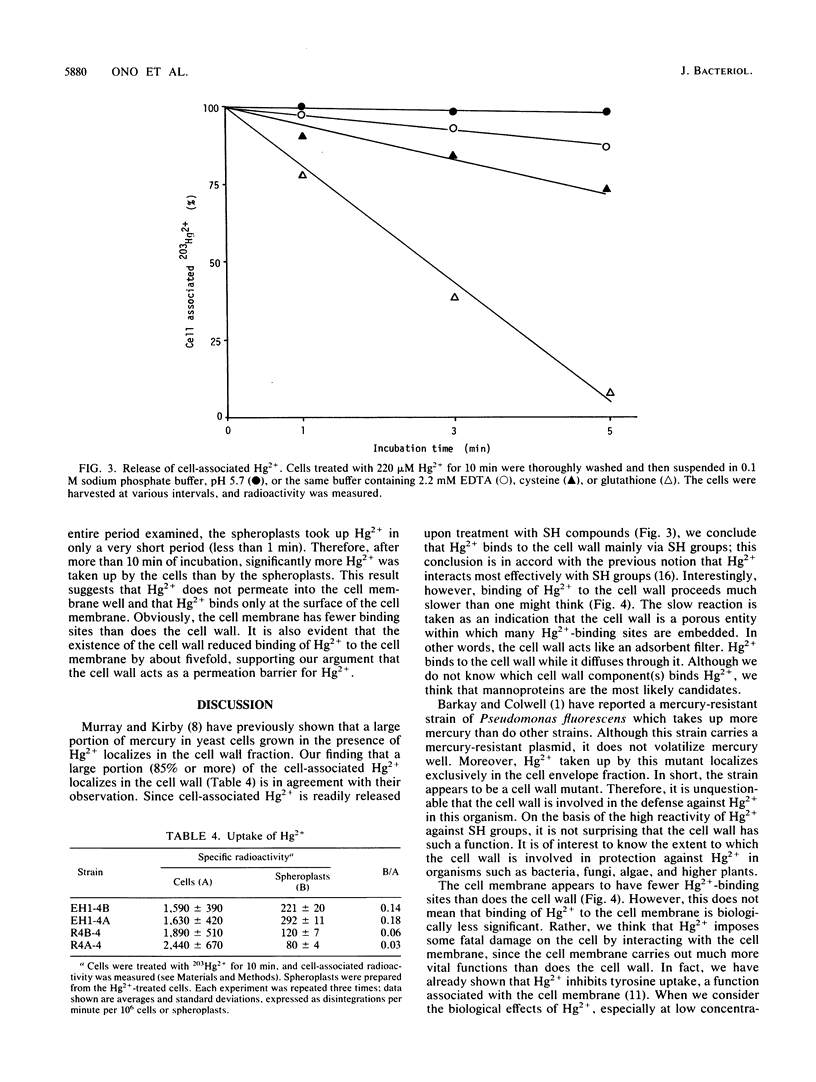
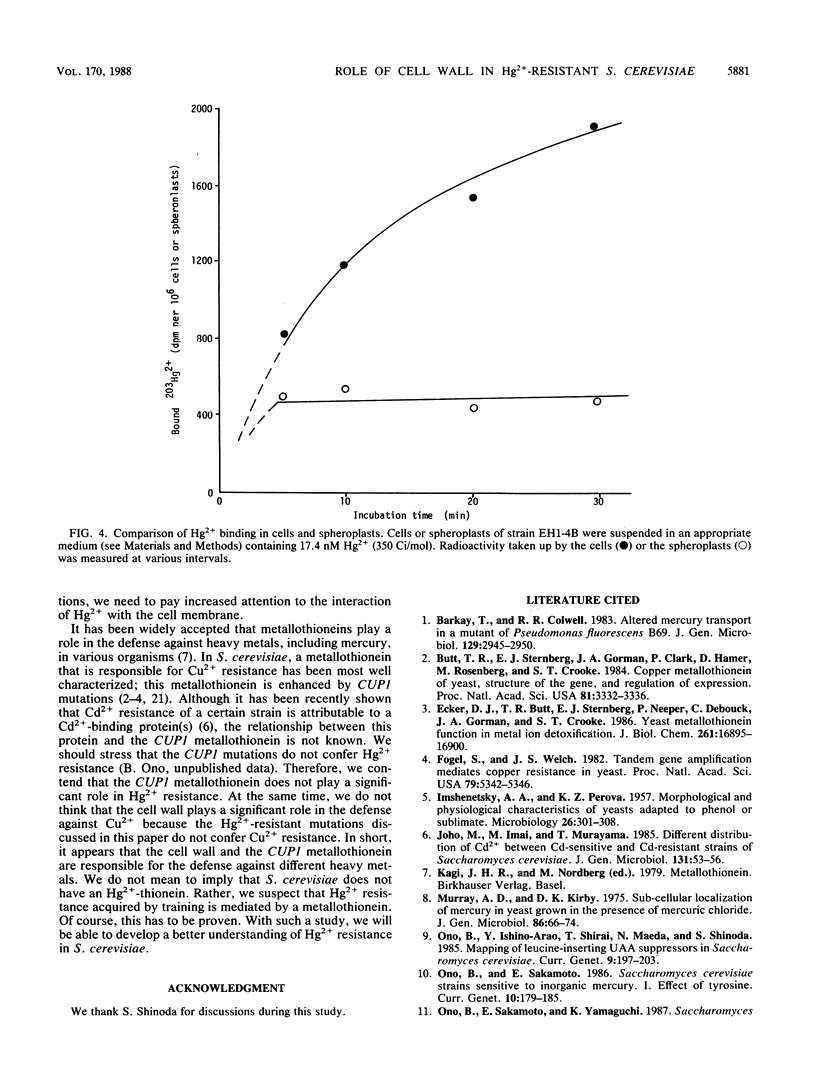
Hg2+-resistant mutants were isolated from Saccharomyces cerevisiae. Although they were very much like the parental strains in terms of colony-forming ability, they grew faster than the parental strains in the presence of sublethal doses of Hg2+. The Hg2+-resistant mutations were dominant. They were centromere linked and were divided into two groups by means of recombination; one of the mutations, designated HGR1-1, was mapped on chromosome IV because of its linkage to the TRP1 locus. The Hg2+-resistant mutants took up Hg2+ as much as, or slightly more than, the parental strains did. The mutants and parental strains retained only about 5 and 15%, respectively, of the cell-associated Hg2+ after removal of the cell wall; therefore, the mutants had less spheroplast-associated Hg2+ than did the parental strains. These results indicate that the cell wall plays an important role in protection against Hg2+ by acting as an adsorption filter and that the mutations described confer Hg2+ resistance by increasing the Hg2+-binding capacity of the cell wall.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butt T. R., Sternberg E. J., Gorman J. A., Clark P., Hamer D., Rosenberg M., Crooke S. T. Copper metallothionein of yeast, structure of the gene, and regulation of expression. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3332–3336. doi: 10.1073/pnas.81.11.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker D. J., Butt T. R., Sternberg E. J., Neeper M. P., Debouck C., Gorman J. A., Crooke S. T. Yeast metallothionein function in metal ion detoxification. J Biol Chem. 1986 Dec 25;261(36):16895–16900. [PubMed] [Google Scholar]
- Fogel S., Welch J. W. Tandem gene amplification mediates copper resistance in yeast. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5342–5346. doi: 10.1073/pnas.79.17.5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. D., Kidby D. K. Sub-cellular location of mercury in yeast grown in the presence of mercuric chloride. J Gen Microbiol. 1975 Jan;86(1):66–74. doi: 10.1099/00221287-86-1-66. [DOI] [PubMed] [Google Scholar]
- Ono B., Sakamoto E. Saccharomyces cerevisiae strains sensitive to inorganic mercury. I. Effect of tyrosine. Curr Genet. 1985;10(3):179–185. doi: 10.1007/BF00798747. [DOI] [PubMed] [Google Scholar]
- Perkins D. D. Biochemical Mutants in the Smut Fungus Ustilago Maydis. Genetics. 1949 Sep;34(5):607–626. doi: 10.1093/genetics/34.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto E., Urata H., Ono B. Saccharomyces cerevisiae strains sensitive to inorganic mercury. II. Effect of glucose. Curr Genet. 1985;10(3):187–195. doi: 10.1007/BF00798748. [DOI] [PubMed] [Google Scholar]
- Schottel J. L. The mercuric and organomercurial detoxifying enzymes from a plasmid-bearing strain of Escherichia coli. J Biol Chem. 1978 Jun 25;253(12):4341–4349. [PubMed] [Google Scholar]
- Singh A., Sherman F. Association of methionine requirement with methyl mercury resistant mutants of yeast. Nature. 1974 Jan 25;247(5438):227–229. doi: 10.1038/247227a0. [DOI] [PubMed] [Google Scholar]
- Singh A., Sherman F. Characteristics and relationships of mercury-resistant mutants and methionine auxotrophs of yeast. J Bacteriol. 1974 Jun;118(3):911–918. doi: 10.1128/jb.118.3.911-918.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Lewis E. Volatilization of mercuric chloride by mercury-resistant plasmid-bearing strains of Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa. J Bacteriol. 1973 Feb;113(2):1070–1072. doi: 10.1128/jb.113.2.1070-1072.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonomura K., Kanzaki F. The reductive decomposition of organic mercurials by cell-free extract of a mercury-resistant pseudomonad. Biochim Biophys Acta. 1969 Jun 17;184(1):227–229. doi: 10.1016/0304-4165(69)90124-x. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Nielson K. B., Gray W. R., Hamer D. H. Yeast metallothionein. Sequence and metal-binding properties. J Biol Chem. 1985 Nov 25;260(27):14464–14470. [PubMed] [Google Scholar]



