Abstract
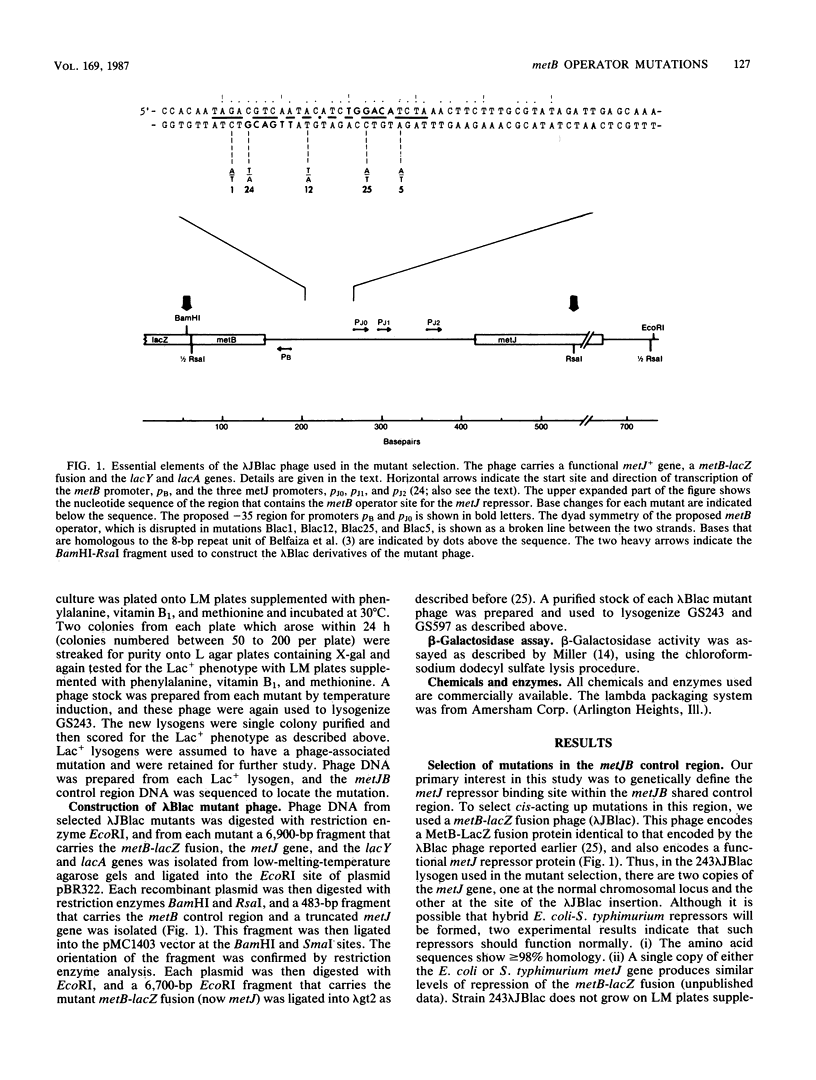
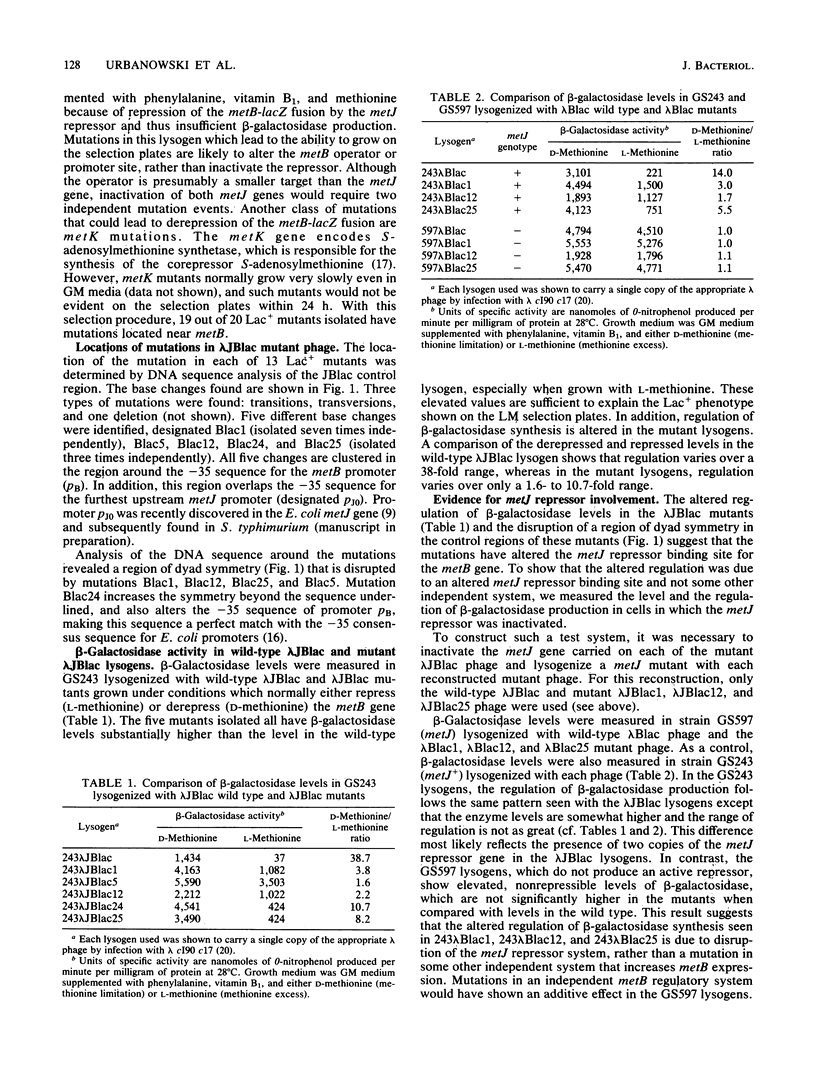
We isolated and characterized cis-acting mutations that affect the regulation of the metB gene of Salmonella typhimurium LT2. The mutations were isolated in an Escherichia coli lac deletion strain lysogenized with lambda bacteriophage carrying a metB-lacZ gene fusion (lambda JBlac) in which beta-galactosidase production is dependent upon metB gene expression. The mutant lysogens show elevated, poorly regulated beta-galactosidase production. The altered regulation is a result of disruption of the methionine control system mediated by the metJ repressor. The mutations are located in a region of dyad symmetry centered near the -35 sequence of the metB promoter. We propose that these mutations alter the repressor binding site and define the metB operator sequence. In addition, we discuss a highly conserved, nonsymmetric DNA sequence of unknown function which occurs in the control regions of the metA, metC, metE, metF, metG, and metJB genes of both S. typhimurium and E. coli.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. G., Ebel J. P., Jakes R., Bruton C. J. Methionyl-tRNA synthetase from Escherichia coli. Primary structure of the active crystallised tryptic fragment. Eur J Biochem. 1982 Oct;127(3):449–457. [PubMed] [Google Scholar]
- Belfaiza J., Parsot C., Martel A., de la Tour C. B., Margarita D., Cohen G. N., Saint-Girons I. Evolution in biosynthetic pathways: two enzymes catalyzing consecutive steps in methionine biosynthesis originate from a common ancestor and possess a similar regulatory region. Proc Natl Acad Sci U S A. 1986 Feb;83(4):867–871. doi: 10.1073/pnas.83.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchange N., Zakin M. M., Ferrara P., Saint-Girons I., Park I., Tran S. V., Py M. C., Cohen G. N. Structure of the metJBLF cluster in Escherichia coli K12. Sequence of the metB structural gene and of the 5'- and 3'-flanking regions of the metBL operon. J Biol Chem. 1983 Dec 25;258(24):14868–14871. [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii T., Ogawa T., Ogawa H. Nucleotide sequence of the lexA gene of E. coli. Cell. 1981 Mar;23(3):689–697. doi: 10.1016/0092-8674(81)90432-3. [DOI] [PubMed] [Google Scholar]
- Kirby T. W., Hindenach B. R., Greene R. C. Regulation of in vivo transcription of the Escherichia coli K-12 metJBLF gene cluster. J Bacteriol. 1986 Mar;165(3):671–677. doi: 10.1128/jb.165.3.671-677.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham G. D., DeParasis J., Gatmaitan J. The sequence of metK, the structural gene for S-adenosylmethionine synthetase in Escherichia coli. J Biol Chem. 1984 Dec 10;259(23):14505–14507. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Michaeli S., Mevarech M., Ron E. Z. Regulatory region of the metA gene of Escherichia coli K-12. J Bacteriol. 1984 Dec;160(3):1158–1162. doi: 10.1128/jb.160.3.1158-1162.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panasenko S. M., Cameron J. R., Davis R. W., Lehman I. R. Five hundredfold overproduction of DNA ligase after induction of a hybrid lambda lysogen constructed in vitro. Science. 1977 Apr 8;196(4286):188–189. doi: 10.1126/science.322281. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Saint-Girons I., Duchange N., Zakin M. M., Park I., Margarita D., Ferrara P., Cohen G. N. Nucleotide sequence of metF, the E. coli structural gene for 5-10 methylene tetrahydrofolate reductase and of its control region. Nucleic Acids Res. 1983 Oct 11;11(19):6723–6732. doi: 10.1093/nar/11.19.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J Mol Biol. 1972 Feb 14;63(3):483–503. doi: 10.1016/0022-2836(72)90443-3. [DOI] [PubMed] [Google Scholar]
- Smith A. A., Greene R. C., Kirby T. W., Hindenach B. R. Isolation and characterization of the product of the methionine-regulatory gene metJ of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6104–6108. doi: 10.1073/pnas.82.18.6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer G. V., Plamann M. D., Stauffer L. T. Construction and expression of hybrid plasmids containing the Escherichia coli glyA genes. Gene. 1981 Jun-Jul;14(1-2):63–72. doi: 10.1016/0378-1119(81)90148-7. [DOI] [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer G. V. Autoregulation by tandem promoters of the Salmonella typhimurium LT2 metJ gene. J Bacteriol. 1986 Mar;165(3):740–745. doi: 10.1128/jb.165.3.740-745.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer G. V. Cloning and initial characterization of the metJ and metB genes from Salmonella typhimurium LT2. Gene. 1985;35(1-2):187–197. doi: 10.1016/0378-1119(85)90171-4. [DOI] [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer G. V. Nucleotide sequence and biochemical characterization of the metJ gene from Salmonella typhimurium LT2. Nucleic Acids Res. 1985 Feb 11;13(3):673–685. doi: 10.1093/nar/13.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youderian P., Bouvier S., Susskind M. M. Sequence determinants of promoter activity. Cell. 1982 Oct;30(3):843–853. doi: 10.1016/0092-8674(82)90289-6. [DOI] [PubMed] [Google Scholar]


