Abstract
p53 is an important tumor suppressor, normally preventing cancer development via apoptosis. A genomic Arg72Pro substitution in the p53 protein has important influence on cell death via apoptosis, which could be beneficial. We therefore tested the hypotheses that this polymorphism influences longevity, survival after a cancer diagnosis, and risk of cancer in the general population. We examined a cohort of 9,219 participants ages 20–95 from the Danish general population with 100% follow-up. The overall 12-yr survival was increased in p53 Arg/Pro heterozygotes with 3% (P = 0.003) and in Pro/Pro homozygotes with 6% (P = 0.002) versus Arg/Arg homozygotes, corresponding to an increase in median survival of 3 yr for Pro/Pro versus Arg/Arg homozygotes. We also demonstrated an increased survival after the development of cancer, or even after the development of other life-threatening diseases, for Pro/Pro versus Arg/Arg homozygotes. The Arg72Pro substitution did not associate with decreased risk of cancer. In conclusion, in this large cohort from the general population, we show that a well-known functional single nucleotide polymorphism in the tumor suppressor p53 protein leads to increased longevity, but not to decreased risk of cancer. The increased longevity may be due to increased survival after a diagnosis of cancer or other life-threatening diseases.
In half of all human cancers, the tumor suppressor p53 protein is damaged by somatic mutation in tumor cells (1). Furthermore, germline alterations of the TP53 gene encoding the p53 protein have been observed in the majority of families with the Li-Fraumeni syndrome, a rare dominantly inherited disorder (2). This syndrome is characterized by early-onset cancer. The p53 protein is at the center of cell regulatory pathways, influencing transcription and activity of several replication and transcription factors. In case of UV radiation, protooncogene activation, or DNA damage, p53 is activated. Normally, this leads to activation of repair mechanisms, induction of cell cycle arrest, and the prevention of cancer growth via apoptosis; when p53 is damaged, cancer cells continue to multiply.
In genomic DNA in exon 4 of the TP53 gene, a polymorphism causing an Arg72Pro substitution has genotype frequencies in Europeans of ∼60, 30, and 10% for Arg/Arg, Arg/Pro, and Pro/Pro, respectively (3). This substitution is in the putative SH3 binding domain of p53, influencing binding capacity and thereby functional properties of p53 (4). The Arg allele increases the ability of p53 to locate to mitochondria and induce cellular death, whereas the Pro allele exhibits a lower apoptotic potential and an increased cellular arrest in G1 of the cell cycle (5–7). Thus, this is an important gain-of-function polymorphism at the cellular level. Increased levels of p53 activity protect against cancer at the cost of premature aging (8–10), indicating that the level of expression of p53 influences not only local cancer development but also degenerative processes in the whole organism.
Collectively, these observations suggest that the p53 Arg72Pro polymorphism may influence longevity, prognosis after a cancer diagnosis, and risk of cancer. These three hypotheses were tested in the present prospective study of >9,000 individuals from the Danish general population with 12, 12, and 55 yr of follow-ups, respectively.
RESULTS AND DISCUSSION
In this large cohort from the general population, we show that a well-known functional polymorphism in the tumor suppressor p53 protein leads to increased longevity and increased survival after a diagnosis of cancer or other life-threatening diseases, but not to decreased risk of cancer. Among 9,219 individuals from the Danish general population, we observed 2,264 deaths as well as 2,048 participants with a first cancer. For the Arg72Pro polymorphism, 54, 39, and 7% were Arg/Arg homozygotes, Arg/Pro heterozygotes, and Pro/Pro homozygotes, respectively (test for Hardy-Weinberg equilibrium: P = 0.93). Conventional risk factors for death, cancer, and other common multifactorial diseases were equally distributed between individuals with the three different genotypes (Table I.).
Table I.
Basic characteristics according to p53 Arg72Pro genotype in individuals in the general population
| Characteristic | Arg/Arg | Arg/Pro | Pro/Pro |
|---|---|---|---|
| Number (%) | 4,934 (54) | 3,623 (39) | 662 (7) |
| Women (%) | 55 | 55 | 56 |
| Age (yr) | 58 ± 15 | 58 ± 15 | 57 ± 15 |
| Total tobacco consumption (pack-yearsa) | 23 ± 24 | 22 ± 24 | 23 ± 23 |
| Current or former smoker (%) | 78 | 76 | 78 |
| Body mass index (kg/m2) | 25 ± 4 | 25 ± 4 | 25 ± 4 |
| Alcohol (g/wk) | 112 ± 147 | 113 ± 151 | 106 ± 133 |
| Plasma cholesterol (mmol/liter) | 6.2 ± 1.3 | 6.1 ± 1.3 | 6.1 ± 1.3 |
| Systolic blood pressure (mmHg) | 139 ± 23 | 138 ± 22 | 137 ± 22 |
| Physically inactive (%) | 12 | 12 | 14 |
Variables expressed as mean ± standard deviation or proportion were collected at the 1991–1994 examination of the Copenhagen City Heart Study. Statistical comparisons between the three genotype groups were made using two-sided Mann-Whitney U test, Pearson's χ2 test, or Student's t test on untransformed or log-transformed parameters as appropriate. No parameter had differences that were statistically significant among the three genotype groups.
Pack-years indicates the average number of packs of cigarettes smoked daily by a person multiplied by the number of years that person has been a smoker.
Longevity
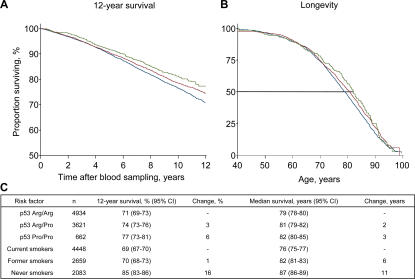
The overall 12-yr survival after blood sampling was increased in Arg/Pro heterozygotes with 3% (P = 0.003) and in Pro/Pro homozygotes with 6% (P = 0.002) versus Arg/Arg homozygotes (Fig. 1, A and C). Death incidences from any cause were 270, 238, and 210 per 10,000 person-years (the number of collective years that the individuals in a particular group were observed) in Arg/Arg homozygotes, Arg/Pro heterozygotes, and Pro/Pro homozygotes, respectively (Table II). This corresponds to gender- and age-adjusted hazard ratios for death from any cause in Arg/Pro heterozygotes and Pro/Pro versus Arg/Arg homozygotes of 0.88 (95% confidence interval [CI]: 0.81–0.96; P = 0.005) and 0.82 (0.68–0.97; P = 0.02), respectively. In accordance with this, the frequency of Pro/Pro homozygotes and the Pro allele appeared to increase with increasing age toward 90 yr (Figs. S2 and S3, available at http://www.jem.org/cgi/content/full/jem.20062476/DC1). The frequency of Pro alleles rises when the participants with advancing age begin to die in considerable numbers. This is also what can be seen in the Kaplan-Meyer curves in Fig. 1 and in the Cox regressions in Table II.
Figure 1.
12-yr survival and longevity of the general population by p53 Arg72Pro genotype. Death endpoints were collected from the Danish Civil Registration System, which is 100% complete. (A) For 12-yr survival, follow-up started at blood sampling and ended at death, emigration, or March 11, 2004, whichever came first. (B) For longevity using left-truncated age, follow-up started at blood sampling and ended at death, emigration, or March 11, 2004, whichever came first. (C) Comparison of the effect of p53 Arg72Pro genotype with that of smoking status on 12-yr survival and median survival in the same population. Arg/Arg homozygotes are in blue, Arg/Pro heterozygotes are in red, and Pro/Pro homozygotes are in green.
Table II.
Mortality, mortality after disease, and morbidity according to p53 Arg72Pro genotype in the general population
|
|
|
|
Arg/Arg
|
Arg/Pro
|
Pro/Pro
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Endpoint | Participants | Incidents | Incidence n/10,000 person-yearsa |
HR | Incidence n/10,000 person-yearsa |
HR (95% CI) |
p-value | Incidence n/10,000 person-yearsa |
HR (95% CI) |
p-value |
| Mortality | 9,219 | 2,264 | 270 | 1.0 | 238 | 0.88 (0.81–0.96) |
0.005a | 210 | 0.82 (0.68–0.97) |
0.02 |
| Mortality after disease | ||||||||||
| Cancer | 2,048 | 1,074 | 680 | 1.0 | 562 | 0.87 (0.77–0.98) |
0.03 | 507 | 0.74 (0.56–0.98) |
0.03 |
| Cardiovascular disease | 2,849 | 1,416 | 560 | 1.0 | 498 | 0.95 (0.85–1.06) |
0.36 | 480 | 0.85 (0.69–1.05) |
0.14 |
| Other | 7,429 | 2,075 | 197 | 1.0 | 173 | 0.88 (0.81–0.97) |
0.007a | 148 | 0.81 (0.68–0.98) |
0.03 |
| Morbidity | ||||||||||
| Cancer | 9,218 | 2,048 | 170 | 1.0 | 178 | 1.10 (1.01–1.21) |
0.03 | 142 | 0.91 (0.76–1.10) |
0.32 |
| Cardiovascular disease | 9,218 | 2,849 | 137 | 1.0 | 132 | 0.96 (0.89–1.04) |
0.31 | 128 | 0.99 (0.86–1.15) |
0.93 |
| Other | 9,214 | 7,429 | 589 | 1.0 | 586 | 0.99 (0.95–1.04) |
0.80 | 607 | 1.03 (0.94–1.12) |
0.55 |
Hazard ratios were adjusted for gender and age in all analyses. Numbers of participants vary slightly because some participants were excluded because of development of disease before follow-up. International Classification of Diseases 8 and 10 codes are as follows: for cardiovascular disease, 390-458, I00-I99; and for cancer, 140-209, C00-C97. HR, hazard ratio; CI, confidence interval.
Person-years indicates the number of collective years that the individuals in a particular group were observed.
bRemained statistically significant (P < 0.05) after Bonferroni correction for multiple comparison for two tests on mortality, six tests on mortality after disease, and six tests on morbidity.
This reduction in risk of death translates into an increased median survival of 2 and 3 yr for Arg/Pro heterozygotes and Pro/Pro versus Arg/Arg homozygotes (Fig. 1, B and C). For comparison, the median survival was 6 and 11 yr longer in former smokers and people who had never been smokers versus smokers (Fig. 1 C). Thus, the effect of the Arg72Pro polymorphism is equivalent to a quarter of the mean survival difference between smokers and nonsmokers, the most important environmental factor affecting longevity in developed countries.
In accordance with our data, in the Leiden 85-plus study, Pro/Pro versus Arg/Arg homozygotes were associated with a 1.41-fold (95% CI: 1.03–1.92) increased survival among 1,226 participants 85 yr or older followed for 5–10 yr (10). A 1.41-fold–increased survival is equivalent to a 0.71-fold risk of mortality, where we observed a 0.82-fold risk of mortality in Pro/Pro versus Arg/Arg homozygotes (Table II). In contrast to our study, the former study (10) did not observe decreased mortality in Arg/Pro heterozygotes versus Arg/Arg homozygotes; however, that study had less statistical power than the present study.
Genetic variations in modifiers of p53 like ATM and MDM2 could have an impact on the effect of p53 Arg72Pro on mortality. However, the Ser49Cys and Ser707Pro polymorphisms of ATM and the −309 T/G polymorphism of the MDM2 promoter did not interact with the effect of the Arg72Pro polymorphism on mortality (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20062476/DC1).
Survival after a diagnosis of cancer
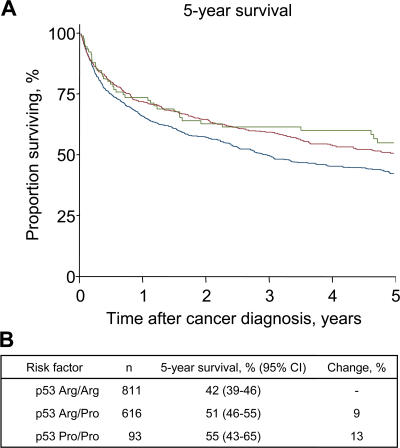
We next examined whether the increased longevity was explained by a better prognosis in Arg/Pro heterozygotes and Pro/Pro versus Arg/Arg homozygotes after the diagnosis of cancer, cardiovascular disease, or other life-threatening diseases. The cumulative 5-yr mortality after a cancer diagnosis was reduced in Arg/Pro heterozygotes and Pro/Pro versus Arg/Arg homozygotes by 9% (P = 0.003) and 13% (P = 0.03; Fig. 2). The reduced 5-yr mortality corresponds to a decreased gender- and age-adjusted hazard ratio for death after a diagnosis of cancer of 0.87 (95% CI: 0.77–0.98) in Arg/Pro heterozygotes and 0.74 (0.56–0.98) in Pro/Pro versus Arg/Arg homozygotes (Table II). The equivalent hazard ratios for death were 0.95 (0.85–1.06) and 0.85 (0.69–1.05) after a diagnosis of cardiovascular disease and 0.88 (0.81–0.97) and 0.81 (0.68–0.98) after a diagnosis of another life-threatening disease, respectively. Thus, the increased longevity associated with the Arg72Pro polymorphism may be due to increased survival after a diagnosis of cancer or other life-threatening diseases.
Figure 2.
5-yr survival of the general population after a cancer diagnosis by p53 Arg72Pro genotype. Cancer diagnosis and death endpoints were collected from the Danish Cancer Registry and the Danish Civil Registration System, which are 98 and 100% complete. Only cancers diagnosed 1 yr before blood sampling and afterward were included. (A) For 5-yr survival after a cancer diagnosis, follow-up started at cancer diagnosis and ended at death, emigration, or March 11, 2004, whichever came first. (B) Effect of p53 Arg72Pro genotype on 5-yr survival after a cancer diagnosis. Arg/Arg homozygotes are in blue, Arg/Pro heterozygotes are in red, and Pro/Pro homozygotes are in green.
In the Leiden 85-plus study, Pro/Pro versus Arg/Arg homozygotes had a hazard ratio of 0.59 (95% CI: 0.41–0.85) for death from all causes but cancer, but a nonsignificant increase in mortality from cancer (10). However, this data is not directly comparable with ours, as we studied mortality after a diagnosis of cancer and other life-threatening diseases. In other words, we studied prognosis among those with disease, whereas the former study addressed cause-specific mortality. Nevertheless, we cannot totally exclude the possibility that our finding of increased survival after a diagnosis of cancer or other life-threatening diseases could represent a chance finding, as p-values only range from 0.03 to 0.007, and because this has not previously been shown by another independent group.
Genetic variations in modifiers of p53 (ATM and MDM2) did not interact with the effect of the Arg72Pro polymorphism on mortality after a diagnosis of cancer (Fig. S4). Inflammatory conditions and leukocyte activation release nitric oxide, a known activator of p53. Therefore, diseases with inflammatory elements could be particularly affected by the influence of the p53 Arg72Pro polymorphism. Interestingly, mortality after a diagnosis of respiratory diseases with a known inflammatory component was reduced in both Arg/Pro heterozygotes and Pro/Pro vs. Arg/Arg homozygotes (Table S1, available at http://www.jem.org/cgi/content/full/jem.20062476/DC1).
Different cancers have a different percentage of somatic p53 mutations. Therefore, the impact of the Arg72Pro polymorphism upon mortality after a cancer diagnosis could depend on cancer subtype with a different percentage of somatic p53 mutations. However, when we regrouped all types of cancers in three groups according to frequency of somatic p53 mutations as reported for each cancer type by the International Agency for Research on Cancer (low, middle, and high; references 11–14), the impact of the germline Arg72Pro polymorphism upon mortality was independent of frequency of somatic p53 mutations (Table S2, available at http://jem.org/cgi/content/full/jem.20062476/DC1; P = 0.98 on robust interaction test in Cox regression). Accordingly, for patients with tumors with high levels of somatic p53 mutations, where all the impact in the tumor of the germline Arg72Pro polymorphism might be expected to be eliminated, we speculate that the reduced mortality in Pro/Pro versus Arg/Arg homozygotes could be a characteristic of the entire person harboring the tumor rather than of the tumor itself.
Risk of cancer
Thereafter, we examined whether the increased longevity by TP53 Arg72Pro genotype was explained by decreased risk of developing cancer, cardiovascular disease, or other life-threatening disease (Table II). The gender- and age-adjusted hazard ratios in Arg/Pro heterozygotes and Pro/Pro versus Arg/Arg homozygotes were 1.10 (1.01–1.21) and 0.91 (0.76–1.10) for risk of cancer, 0.96 (0.89–1.04) and 0.99 (0.86–1.15) for risk of cardiovascular disease, and 0.99 (0.95–1.04) and 1.03 (0.94–1.12) for risk of other life-threatening diseases, respectively. Furthermore, there was no evidence for decreased risk of any cancer subtype in Arg/Pro heterozygotes and Pro/Pro versus Arg/Arg homozygotes (Table III). Thus, in accordance with a recent study (15), the Arg72Pro substitution did not associate with a decreased risk of cancer.
Table III.
Risk of cancer subgroups according to p53 Arg72Pro genotype in the general population
|
|
|
|
Arg/Arg
|
Arg/Pro
|
Pro/Pro
|
||
|---|---|---|---|---|---|---|---|
| Endpoint | Participants | Events | HR | HR (95% CI) |
p-value | HR (95% CI) |
p-value |
| Gastrointestinal cancer | 9,219 | 391 | 1.0 | 1.10 (0.89–1.34) |
0.38 | 0.89 (0.58–1.37) |
0.59 |
| Hematologic cancer | 9,218 | 112 | 1.0 | 2.01 (1.36–2.98) |
<0.001a | 1.49 (0.70–3.17) |
0.30 |
| Respiratory cancer | 9,219 | 271 | 1.0 | 0.91 (0.71–1.17) |
0.47 | 0.82 (0.49–1.38) |
0.46 |
| Urologic cancer | 9,217 | 185 | 1.0 | 1.10 (0.82–1.48) |
0.53 | 0.73 (0.37–1.45) |
0.37 |
| Female cancer | 5,155 | 477 | 1.0 | 1.06 (0.88–1.28) |
0.51 | 0.71 (0.46–1.07) |
0.10 |
| Male cancer | 4,114 | 136 | 1.0 | 1.11 (0.79–1.58) |
0.55 | 1.15 (0.59–2.23) |
0.68 |
| Other cancer | 9,219 | 659 | 1.0 | 1.09 (0.93–1.28) |
0.30 | 1.00 (0.73–1.37) |
0.98 |
Hazard ratios were adjusted for gender (when relevant) and age in all analyses. Follow-up started January 1, 1947, at the establishment of the Danish Cancer Registry or at birth, whichever came last, and ended at the date of the first relevant cancer diagnosis, death, emigration, or December 31, 2002, whichever came first. Numbers of participants vary slightly because some participants were excluded as a result of the development of disease before follow-up. HR, hazard ratio; CI, confidence interval.
Remained statistically significant (P < 0.05) after Bonferroni correction for multiple comparison for 14 tests.
In a recent meta-analysis of 61 individual case-control studies with 10,700 cases of mainly cervical, lung, breast, head, and neck cancer and 12,300 controls (10), Pro/Pro versus Arg/Arg homozygotes had an aggregated odds ratio for increased cancer risk of 1.11 (95% CI: 1.02–1.22). However, in another recent study including nine individual case-control studies with 8,700 cases with breast cancer versus 10,600 controls (15), Pro/Pro versus Arg/Arg homozygotes had an aggregated odds ratio for increased breast cancer risk of 1.03 (0.92–1.15). Finally, in our study in the general population, including 2,000 cases with any cancer subtype and 7,200 individuals without cancer during the 55-yr follow-up, we observed a hazard ratio of any cancer in Pro/Pro versus Arg/Arg homozygotes of 0.91 (0.76–1.10). Thus, although we only had 40% statistical power to exclude a hazard ratio of 1.11 for increased risk of cancer in Pro/Pro versus Arg/Arg homozygotes, the overall evidence does not favor increased cancer risk in Pro/Pro versus Arg/Arg homozygotes.
Surprisingly, the risk of hematologic cancer increased twofold in Arg/Pro heterozygotes versus Arg/Arg homozygotes (P < 0.001), with a similar trend in Pro/Pro homozygotes (Table III). This twofold-increased risk was significant even after correction for multiple testing and therefore might represent a real rather than a chance finding; however, this needs to be confirmed in another independent study.
The p53 pathway has elements of sexual dimorphism: female Li-Fraumeni patients with p53 mutations develop tumors earlier and with a higher frequency when adjusted for age (16), and the promoter of MDM2, a p53 interaction partner, contains a functional estrogen receptor signal in the DNA (17). Therefore, the effect of the p53 Arg72Pro polymorphism on risk of cancer in women could depend on menopausal status. However, we did not detect any statistically significant interaction between the Arg72Pro polymorphism and gender or menopausal status on risk of cancer (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20062476/DC1).
Potential limitations
For all the analyses described, multifactorial adjustment for other potential confounders, as shown in Table I, did not change the estimates (unpublished data). Furthermore, because of Mendelian randomization, our results are unlikely to be confounded by yet other environmental or lifestyle factors (18). Finally, because the p53 Arg72Pro polymorphism is well known to be functional at the cellular level, influencing apoptotic potential as well as cellular arrest in G1 of the cell cycle (5–7), the effects observed in the present study are most likely directly due to the Arg72Pro polymorphism rather than to another polymorphism nearby in linkage disequilibrium with this polymorphism.
Although performed in a large, well-characterized cohort of the general population with long-term follow-up (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20062476/DC1), our study has limitations. Because participants were only genotyped if they participated in the 1991–1994 examination of the Copenhagen City Heart Study, a selection bias might have occurred if death or morbidity prevented certain individuals from participating in this examination. However, we found Hardy-Weinberg equilibrium in the genotype distribution, making selection bias against any of the genotypes unlikely. Should selection bias have occurred in this study, it would tend to result in a conservative estimate concerning mortality, and therefore cannot explain our result. Misclassification of disease status, but not mortality, could also have occurred. However, such misclassification most likely is minimal because we have 100% follow-up of the participants and because systematic registration of diseases causing hospitalization as well as death are registered in the entire country.
Mechanism
p53 can act through several pathways when reacting to cellular stress. Apoptosis, cell cycle arrest at the G1 checkpoint, and cellular senescence are all mechanisms triggered by activated p53 (19). These mechanisms are all beneficial when the organism is young, but in older organisms, such effects probably reduce longevity and augment cancer risk (20), so-called antagonistic pleiotropy (19, 21). In accordance with this concept, p53 accelerates aging when responding to cellular stress (22). The Arg allele of the Arg72Pro polymorphism increases p53-induced apoptosis, whereas the Pro allele effectuates cell cycle arrest in the G1 phase (5–7). Our findings suggest that Arg/Pro heterozygotes and Pro/Pro versus Arg/Arg homozygotes have reduced mortality, which could result from a generally decreased aging process caused by decreased proapoptotic activity and increased cell cycle–arresting abilities of p53.
Conclusions
Although our results suggest that p53 Arg72Pro is not associated with risk of cancer or any other disease, this polymorphism has a profound gene dose–dependent beneficial effect on 5-yr survival after a diagnosis of cancer, on survival after other life-threatening diseases, and on longevity. Thus, the increased longevity may be due to a general increased robustness after a diagnosis of any life-threatening disease. We speculate that the decreased proapoptotic and increased cell cycle–arresting abilities of the Pro versus Arg allele (5–7) might be beneficial for a person experiencing any critical illness. This suggests that the TP53 Arg72Pro polymorphism is an important gain-of-function genetic variant affecting the entire person.
MATERIALS AND METHODS
Using a prospective study of the Danish general population, we examined 9,219 participants ages 20–95 yr from the Copenhagen City Heart Study participating in the 1991–1994 examination (Fig. S1; references 23–25). Height and weight were measured, and participants were questioned about smoking habits, alcohol consumption, and physical activity. Blood was drawn for cholesterol measurement and DNA extraction. All participants gave written informed consent. Herlev University Hospital and the Danish ethical committee of Copenhagen and Frederiksberg approved the study (No. 100.2039/91), which was conducted according to the Helsinki Declaration.
Endpoints.
We collected information on morbidity and mortality from three different population registries: information on mortality (23) was obtained from the Danish Civil Registration System; information on morbidity was obtained from the Danish National Hospital Discharge Register (26) and subdivided according to the Global Burden of Disease classification (27); and information on diagnoses of invasive cancer was obtained from the Danish Cancer Registry (28).
Cancer diagnoses were classified according to criteria from the International Classification of Diseases, Seventh Edition (29), and divided into seven different subgroups: (a) gastrointestinal cancer, including oral, esophagus, stomach, small intestine, liver and biliary tract, pancreatic, colon, rectum, and anal cancer; (b) hematologic cancer, including non-Hodgkin's lymphoma, Hodgkin's disease, multiple myeloma, and leukemia; (c) respiratory cancer, including larynx and lung cancer; (d) urologic cancer, including kidney and bladder cancer; (e) female cancer, including breast, cervix uteri, corpus uteri, ovarian, and vaginal cancer; (f) male cancer, including testis and prostate cancer; and (g) other cancers, including melanoma, nonmelanoma skin cancer, sarcoma, brain, and other central nervous system cancers, thyroid and other endocrine cancers, metastasis with unknown primary tumors, and other tumors.
Genotyping.
We genotyped 9,219 participants for p53 Arg72Pro, an amino acid–changing polymorphism caused by a G215C substitution. By PCR, a 331-basepair fragment, corresponding to exon 4, was amplified from genomic DNA using an intronic forward and exonic reverse primer (forward, 5′-CATCTACAGTCCCCCTTGC-3′; reverse, 5-GCTTCCATGAGACTTCAATGCC-3′). After thermo cycling, genotypes were determined using the Nanogen system (30). ATM and MDM2 polymorphisms were genotyped using endpoint PCR (Taqman; Applied Biosystems). Probes and primers were designed by using Primer Express software (Applied Biosystems) and are available from the authors upon request.
Statistical analysis.
The statistical software STATA (version 8.2) was used. Two-tailed P < 0.05 was considered significant. We used Mann-Whitney U test and Pearson's χ2 test. Kaplan-Meier curves plotted overall survival and survival after a cancer diagnosis as a function of follow-up time as well as left-truncated age. Hazard ratios for morbidity and mortality were calculated using Cox proportional hazards regression analysis. We tested for proportionality of hazards over time to ensure that this assumption of the Cox proportional hazards regression model was fulfilled based on Schonefeld residuals. Individuals with endpoints developed before study entry were excluded from analysis.
Multifactorial adjustments for cardiovascular and other disease included gender, age, tobacco consumption, smoking habits, systolic blood pressure, alcohol consumption, total cholesterol, body mass index, and physical activity at the time of blood sampling. Multifactorial adjustment for risk of cancer included the aforementioned variables excluding total cholesterol and systolic blood pressure.
Online supplemental material.
Fig. S1 shows the design of the study. Fig. S2 shows p53 Arg72Pro genotype frequency as a function of age in the general population. Fig. S3 shows the frequency of the p53 Arg72Pro Pro allele and frequency of deaths per 5-yr age group as functions of age. Fig. S4 shows the overall hazard ratio of death and the hazard ratio of death after a cancer diagnosis by Arg72Pro genotype, stratified by two ATM genotypes and one MDM2 genotype. Fig. S5 shows risk of cancer and cancer subgroups by Arg72Pro genotype, stratified by gender and menopausal status. Table S1 shows mortality after other diseases and morbidity of other diseases according to p53 Arg72Pro genotype in the general population. Table S2 shows mortality after a diagnosis of cancer according to low, middle, and high p53 somatic mutation frequencies and p53 Arg72Pro germline genotype in the general population. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20062476/DC1.
Supplemental Material
Acknowledgments
This work was supported by the Danish Heart Foundation, Chief Physician Johan Boserup and Lise Boserup's Fund, the Danish Medical Research Council, the Research Fund at Rigshospitalet, Copenhagen University Hospital, and the Copenhagen County Research Fund. These are public or nonprofit organizations supporting science in general. They had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, and approval of the manuscript.
The authors have no conflicting financial interests.
D. Dynnes Ørsted and S.E. Bojesen contributed equally to this paper.
References
- 1.Rudin, C.M., and C.B. Thompson. Apoptosis and cancer. In The Metabolic and Molecular Bases of Inherited Disease. C.R. Scriver, A.L. Beaudet, W.S. Sly, and D. Valle, editors. McGraw-Hill, New York. 631–643.
- 2.Malkin, D. 2001. Li-Fraumeni syndrome. In The Metabolic & Molecular Bases of Inherited Disease. C.R. Scriver, A.L. Beaudet, W.S. Sly, and D. Valle, editors. McGraw-Hill, New York. 849–863.
- 3.Hogdall, E.V., C.K. Hogdall, L. Christensen, E. Glud, J. Blaakaer, J.E. Bock, J. Vuust, B. Norgaard-Pedersen, and S.K. Kjaer. 2002. Distribution of p53 codon 72 polymorphisms in ovarian tumour patients and their prognostic significance in ovarian cancer patients. Anticancer Res. 22:1859–1864. [PubMed] [Google Scholar]
- 4.Marin, M.C., C.A. Jost, L.A. Brooks, M.S. Irwin, J. O'Nions, J.A. Tidy, N. James, J.M. McGregor, C.A. Harwood, I.G. Yulug, et al. 2000. A common polymorphism acts as an intragenic modifier of mutant p53 behaviour. Nat. Genet. 25:47–54. [DOI] [PubMed] [Google Scholar]
- 5.Dumont, P., J.I. Leu, P.A. Della III, D.L. George, and M. Murphy. 2003. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat. Genet. 33:357–365. [DOI] [PubMed] [Google Scholar]
- 6.Pim, D., and L. Banks. 2004. p53 polymorphic variants at codon 72 exert different effects on cell cycle progression. Int. J. Cancer. 108:196–199. [DOI] [PubMed] [Google Scholar]
- 7.Bergamaschi, D., Y. Samuels, A. Sullivan, M. Zvelebil, H. Breyssens, A. Bisso, G. Del Sal, N. Syed, P. Smith, M. Gasco, et al. 2006. iASPP preferentially binds p53 proline-rich region and modulates apoptotic function of codon 72-polymorphic p53. Nat. Genet. 38:1133–1141. [DOI] [PubMed] [Google Scholar]
- 8.Tyner, S.D., S. Venkatachalam, J. Choi, S. Jones, N. Ghebranious, H. Igelmann, X. Lu, G. Soron, B. Cooper, C. Brayton, et al. 2002. p53 mutant mice that display early ageing-associated phenotypes. Nature. 415:45–53. [DOI] [PubMed] [Google Scholar]
- 9.Ferbeyre, G., and S.W. Lowe. 2002. Ageing: the price of tumour suppression? Nature. 415:26–27. [DOI] [PubMed] [Google Scholar]
- 10.van Heemst, D., S.P. Mooijaart, M. Beekman, J. Schreuder, A.J. de Craen, B.W. Brandt, P.E. Slagboom, and R.G. Westendorp. 2005. Variation in the human TP53 gene affects old age survival and cancer mortality. Exp. Gerontol. 40:11–15. [DOI] [PubMed] [Google Scholar]
- 11.Olivier, M., R. Eeles, M. Hollstein, M.A. Khan, C.C. Harris, and P. Hainaut. 2002. The IARC TP53 database: new online mutation analysis and recommendations to users. Hum. Mutat. 19:607–614. [DOI] [PubMed] [Google Scholar]
- 12.Soto, J.L., C.M. Cabrera, S. Serrano, and M.A. Lopez-Nevot. 2005. Mutation analysis of genes that control the G1/S cell cycle in melanoma: TP53, CDKN1A, CDKN2A, and CDKN2B. BMC Cancer. 5:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elnenaei, M.O., A.M. Gruszka-Westwood, R. A'Hernt, E. Matutes, B. Sirohi, R. Powles, and D. Catovsky. 2003. Gene abnormalities in multiple myeloma; the relevance of TP53, MDM2, and CDKN2A. Haematologica. 88:529–537. [PubMed] [Google Scholar]
- 14.Maggio, E.M., E. Stekelenburg, A. van den Berg, and S. Poppema. 2001. TP53 gene mutations in Hodgkin lymphoma are infrequent and not associated with absence of Epstein-Barr virus. Int. J. Cancer. 94:60–66. [DOI] [PubMed] [Google Scholar]
- 15.The Breast Cancer Association Consortium. 2006. Commonly studied single-nucleotide polymorphisms and breast cancer: results from the breast cancer association consortium. J. Natl. Cancer Inst. 98:1382–1396. [DOI] [PubMed] [Google Scholar]
- 16.Wu, C.C., S. Shete, C.I. Amos, and L.C. Strong. 2006. Joint effects of germ-line p53 mutation and sex on cancer risk in Li-Fraumeni syndrome. Cancer Res. 66:8287–8292. [DOI] [PubMed] [Google Scholar]
- 17.Bond, G.L., K.M. Hirshfield, T. Kirchhoff, G. Alexe, E.E. Bond, H. Robins, F. Bartel, H. Taubert, P. Wuerl, W. Hait, et al. 2006. MDM2 SNP309 accelerates tumor formation in a gender-specific and hormone-dependent manner. Cancer Res. 66:5104–5110. [DOI] [PubMed] [Google Scholar]
- 18.Davey Smith, G., S. Ebrahim, S. Lewis, A.L. Hansell, L.J. Palmer, and P.R. Burton. 2005. Genetic epidemiology and public health: hope, hype, and future prospects. Lancet. 366:1484–1498. [DOI] [PubMed] [Google Scholar]
- 19.Lombard, D.B., K.F. Chua, R. Mostoslavsky, S. Franco, M. Gostissa, and F.W. Alt. 2005. DNA repair, genome stability, and aging. Cell. 120:497–512. [DOI] [PubMed] [Google Scholar]
- 20.Krtolica, A., and J. Campisi. 2002. Cancer and aging: a model for the cancer promoting effects of the aging stroma. Int. J. Biochem. Cell Biol. 34:1401–1414. [DOI] [PubMed] [Google Scholar]
- 21.Campisi, J. 2003. Cancer and ageing: rival demons? Nat. Rev. Cancer. 3:339–349. [DOI] [PubMed] [Google Scholar]
- 22.Campisi, J. 2004. Fragile fugue: p53 in aging, cancer and IGF signaling. Nat. Med. 10:231–232. [DOI] [PubMed] [Google Scholar]
- 23.Dahl, M., A. Tybjaerg-Hansen, P. Schnohr, and B.G. Nordestgaard. 2004. A population-based study of morbidity and mortality in mannose-binding lectin deficiency. J. Exp. Med. 199:1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bojesen, S.E., A. Tybjærg-Hansen, and B.G. Nordestgaard. 2003. Integrin beta3 Leu33Pro homozygosity and risk of cancer. J. Natl. Cancer Inst. 95:1150–1157. [DOI] [PubMed] [Google Scholar]
- 25.Weischer, M., S.E. Bojesen, A. Tybjærg-Hansen, C.K. Axelsson, and B.G. Nordestgaard. 2007. Increased risk of cancer associated with CHECK2*1100delC. J. Clin. Oncol. 25:57–63. [DOI] [PubMed] [Google Scholar]
- 26.Juel, K., and K. Helweg-Larsen. 1999. The Danish registers of causes of death. Dan. Med. Bull. 46:354–357. [PubMed] [Google Scholar]
- 27.Mathers, C.D. 2002. Global Burden of Disease 2000. Second edition. World Health Organization, Geneva, Switzerland. 108 pp.
- 28.Storm, H.H., E.V. Michelsen, I.H. Clemmensen, and J. Pihl. 1997. The Danish Cancer Registry—history, content, quality and use. Dan. Med. Bull. 44:535–539. [PubMed] [Google Scholar]
- 29.World Health Organization. 1952. Third Report of the Expert Committee on Health Statistics. WHO Technical Report Series, No. 53. World Health Organization, Geneva, Switzerland. [PubMed]
- 30.Sethi, A.A., A. Tybjærg-Hansen, R.V. Andersen, and B.G. Nordestgaard. 2004. Nanogen microelectronic chip for large-scale genotyping. Clin. Chem. 50:443–446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.