Abstract
Foxp3+ regulatory T (T reg) cells play a key role in controlling immune pathological re actions. Many develop their regulatory activity in the thymus, but there is also evidence for development of Foxp3+ T reg cells from naive precursors in the periphery. Recent studies have shown that transforming growth factor (TGF)-β can promote T reg cell development in culture, but little is known about the cellular and molecular mechanisms that mediate this pathway under more physiological conditions. Here, we show that after antigen activation in the intestine, naive T cells acquire expression of Foxp3. Moreover, we identify a population of CD103+ mesenteric lymph node dendritic cells (DCs) that induce the devel opment of Foxp3+ T reg cells. Importantly, promotion of T reg cell responses by CD103+ DCs is dependent on TGF-β and the dietary metabolite, retinoic acid (RA). These results newly identify RA as a cofactor in T reg cell generation, providing a mechanism via which functionally specialized gut-associated lymphoid tissue DCs can extend the repertoire of T reg cells focused on the intestine.
Regulatory T (T reg) cells are known to play an important role in the control of destructive inflammatory responses (1). Most frequently studied is the naturally occurring population of CD4+CD25+Foxp3+ T reg cells that develops in the thymus. These cells are important in the control of a wide range of immune-mediated pathologies, including autoimmunity, colitis, and chronic infection. Nevertheless, T cells with regulatory function can also be generated in the periphery from the naive T cell pool after, for example, the oral administration of antigen or the targeting of peptide ligands to DCs in vivo (2–4). Foxp3+ T cells can also be generated in the presence of TGF-β (5–10).
Although peripheral T cells can begin to express Foxp3 and acquire regulatory function, the relevance of this pathway under normal physiological conditions remains unclear. This is compounded by the fact that induced Foxp3+ T cells look phenotypically similar to the naturally occurring population. Consequently, it is difficult to determine what proportion of peripheral CD25+Foxp3+ T reg cells had their regulatory function imprinted in the periphery.
The ability to induce T reg cell populations from the naive pool may be of particular benefit in the intestine. The extensive immune system here must cope with the challenge of mounting protective immunity to occasional pathogens while remaining tolerant to dietary antigen and the commensal flora. It may therefore be crucial to generate T reg cells specific for these types of antigen, in addition to those T reg cells selected for their high affinity to self-antigen in the thymus.
DCs are thought to play an important role in the generation of T reg cell responses. DCs present in the gut-associated lymphoid tissue (GALT) possess several functional specializations that suggest they may be capable of inducing regulatory-type responses (11, 12). However, a clear role for these cells in the extrathymic development of Foxp3+ T reg cells remains to be demonstrated.
Here, we demonstrate that a population of CD103+ DCs found in the mesenteric LNs (MLNs) of normal mice can promote the conversion of naive T cells into Foxp3+ T reg cells. This occurred without any further manipulation of the DC population and was dependent on TGF-β and the vitamin A metabolite, retinoic acid (RA). These results highlight one pathway by which T reg cells may be generated in the periphery under normal conditions.
RESULTS AND DISCUSSION
Oral administration of OVA induces Foxp3+ T cells from naive precursors
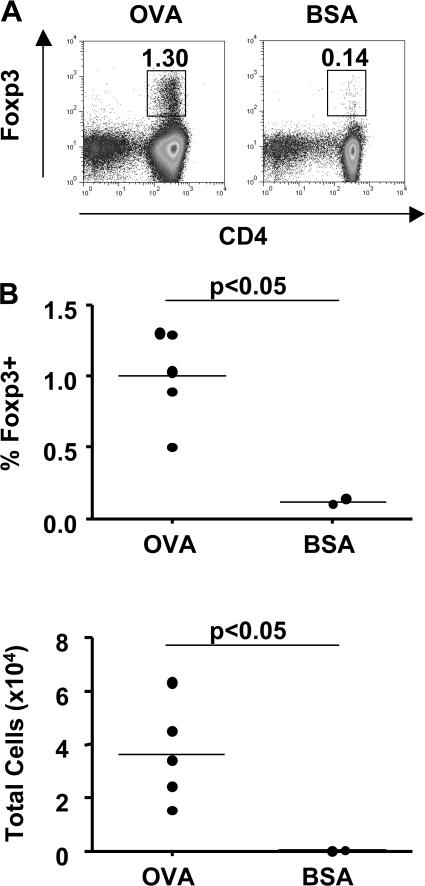
Oral administration of antigen has been shown to increase T reg cell populations in a variety of experimental settings (3, 4, 13). However, whether this represents de novo induction or expansion of a preformed pool of Foxp3+ T reg cells is not clear. Here, we have investigated the induction of Foxp3 expression in antigen-specific T cells after oral administration of OVA to DO11.10 SCID mice. Placing a TCR transgene on the SCID background prevents endogenous rearrangement of TCR chains, resulting in all peripheral T cells having a single antigen specificity. As the antigen recognized by the DO11.10 TCR is exogenous, T cells from unfed DO11.10 SCID mice are a uniformly naive population and do not express Foxp3. DO11.10 SCID mice are therefore a useful tool to investigate induction of Foxp3 expression rather than expansion of a preexisting population. When drinking water was supplemented with OVA, but not BSA, we observed a dramatic increase in MLN cellularity, including a proportionate increase in the number of CD4+ T cells. This was accompanied by the formation of a small population of Foxp3+ T cells in the MLN (Fig. 1). Populations of Foxp3+ T cells were also detected in the spleen and colonic lamina propria (LP) of OVA-fed mice, although the increase in T cell numbers was moderate in comparison to the MLN (unpublished data). Foxp3+ T cells were still detectable 5 d after the removal of antigen from the drinking water (Fig. S1, A and B, available at http://www.jem.org/cgi/content/full/jem.20070590/DC1).
Figure 1.
Induction of Foxp3+ T cells after oral administration of antigen. DO11.10 SCID mice were given OVA or BSA in drinking water for 5 d. (A) Single cell suspensions of MLNs stained for CD4 and Foxp3 and analyzed by FACS. Numbers represent the proportion of Foxp3+ cells among the CD4+ population. (B) Percentage of Foxp3+ cells among the CD4+ population, and total number of Foxp3+ cells in OVA- and BSA-fed mice. Data are representative of two similar independent experiments.
Expression of Foxp3 also occurred in naive peripheral DO11.10 SCID CD4+ T cells adoptively transferred into congenic Ly9.2 BALB/c mice (Fig. S1, C–E). This supports the conclusion that Foxp3+ T reg cells can arise from the peripheral T cell pool rather than from immature thymocytes, which may have occurred if antigen-loaded peripheral DCs migrated to the thymus of DO11.10 SCID mice. Furthermore, this finding confirms that the presence of a normal T reg cell repertoire does not inhibit the peripheral generation of Foxp3+ T reg cells.
Collectively, our results suggest that the GALT is one site at which the peripheral induction of Foxp3+ T reg cells can occur. Although potential mediators for the peripheral induction of Foxp3 expression have been studied, the factors governing their formation at this particular anatomical site remain unknown. Antigen administered by the oral route is likely to be picked up by DCs present in the Peyer's patches or LP. Upon encounter with T cells, most likely in the MLNs, such antigen-loaded DCs may preferentially direct the induction or expansion of T cells with regulatory properties. In line with this, several previous studies have suggested that intestinal DCs may be tolerogenic (11, 14). Moreover, a recent study has demonstrated a role for the migration of intestinal DCs through the lymph to the MLN in the induction of oral tolerance (15). It therefore seemed likely that the induction of Foxp3 expression we observed upon the feeding of OVA could be mediated by DCs present in the MLNs. We therefore sought to investigate the role of MLN DCs in the induction of Foxp3+ T reg cells.
CD103+ DCs isolated from the MLNs promote the conversion of naive CD4+ T cells into Foxp3+ T cells
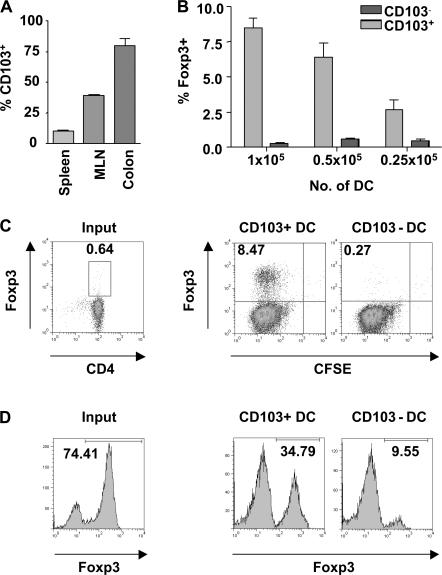
We have previously demonstrated that expression of CD103 on DCs is required for the CD4+CD25+ T reg cell–mediated control of experimental colitis (16). This suggested that CD103+ DCs may be functionally specialized to drive T reg cell responses. CD103 is expressed by a proportion of DCs isolated from the MLNs and colonic LP of normal mice and is also present on rat lymph DCs migrating from the intestine in the steady state (Fig. 2 A) (17–19). Fittingly, in CCR7−/− mice, where the migration of DCs from tissue to the LN is impaired, the number of CD103+ DCs in the MLNs is greatly reduced (19). This suggests that a proportion of the CD103+ DCs present in the MLNs migrate there constitutively from the intestine, making them likely candidates for the generation of T reg cell responses. We compared the ability of CD103+ and CD103− DCs isolated from the MLNs of normal BALB/c mice to induce expression of Foxp3 in splenic CD4+ T cells isolated from DO11.10 SCID mice. Proliferation and accumulation of T cells was comparable in the presence of both CD103+ and CD103− DCs (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20070590/DC1). However, it was striking that only the CD103+ DC population was able to induce expression of Foxp3, which was detectable by FACS at days 3 and 7 of culture (Fig. 2, B and C, and not depicted). Induction of Foxp3 was dependent on the number of DCs present in culture, with a reduction in the number of CD103+ DCs leading to a decrease in the proportion of T cells expressing Foxp3 (Fig. 2 B). CD103+ MLN DCs isolated from OVA-fed mice also drove expression of Foxp3 when cultured with DO11.10 SCID CD4+ T cells, indicating that CD103+ DCs are capable of taking up and presenting orally administered antigen (Fig. S3). However, very little CD4+ T cell accumulation was observed in the presence of CD103− DCs or when mice were fed an irrelevant antigen.
Figure 2.
Induction of Foxp3+ T cells in the presence of MLN CD103+ DCs. (A) Single cell suspensions of spleen, MLNs, and colonic LP from BALB/c mice were prepared, and the proportion of CD103+ cells among the CD11chigh population was determined. (B) 0.25–1 × 105 CD103+ or CD103− MLN DCs were cultured with CFDA SE-labeled DO11.10 CD4+ T cells and 0.2 μg/ml OVA peptide. At day 7 of culture, T cells were stained for Foxp3 and CD4 and analyzed by FACS. The graph shows the percentage of Foxp3+ cells among CD4+ T cells in the presence of varying numbers of either DC subset. Data is representative of three independent experiments. (C) FACS plots showing the percentage of T cells expressing Foxp3 at the start of culture and after 7 d of culture with 105 CD103+ or CD103− MLN DCs. Plots are gated on CD4+ cells, and numbers represent the proportion of CD4+ cells in each quadrant. (D) CD4+CD25+ T cells from DO11.10 mice were isolated and cultured with 105 CD103+ or CD103− MLN DCs and 0.2 μg/ml OVA peptide. Before culture, and at day 6 of culture, T cells were stained for CD4, Foxp3, and clonotypic TCR (KJ-1.26). Plots are gated on KJ-1.26+CD4+ cells. Numbers represent the percentage of Foxp3+ cells among the KJ-1.26+CD4+ population. Data are representative of two independent experiments.
The ability of CD103+ DCs to maintain a preexisting population of Foxp3+CD4+CD25+ T cells in culture was also investigated. CD4+CD25+ T cells were obtained from the spleens of DO11.10 BALB/c mice, and expression of Foxp3 in the TCR transgenic (KJ-1.26+) fraction was investigated. At the beginning of culture, 75% of KJ-1.26+ T cells expressed Foxp3. Over the period of culture this proportion decreased, probably as a result of outgrowth of contaminating Foxp3− T cells rather than a genuine down-regulation of Foxp3. However, the proportion of T cells expressing Foxp3 by day 7 of culture was greater in the presence of CD103+ DCs (Fig. 2 D). This suggests that in addition to inducing Foxp3 expression in naive T cells, CD103+ DCs favor the maintenance of existing Foxp3+ cells over expansion of any contaminating Foxp3− population.
We had previously observed that CD103+ MLN DCs directed the migration of T cells to the intestine without inducing production of the proinflammatory cytokine IFN-γ (16). We now demonstrate that CD103+ MLN DCs are capable both of converting naive T cells into Foxp3+ T reg cells and of supporting conventional CD4+CD25+Foxp3+ T reg cells. Collectively, these data indicate that CD103+ MLN DCs are capable of driving T reg cell responses focused on the intestine. Because expression of CD103 on DCs was required for the CD4+CD25+ T reg cell–mediated control of colitis, we speculate that both the transferred CD4+CD25+ T reg cells and the Foxp3+ T reg cells generated peripherally from naive T cells may play an important role in the prevention of intestinal pathology.
TGF-β enhances the conversion of naive T cells into Foxp3+ cells in the presence of CD103+ DCs
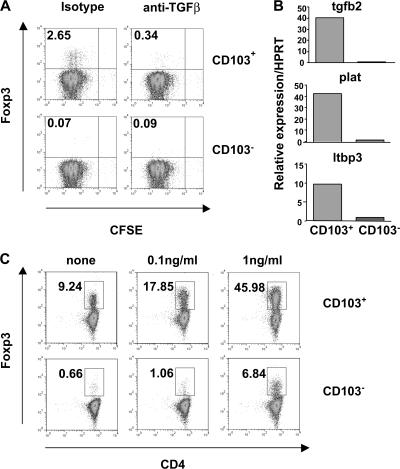
TGF-β1 is important in maintaining functional Foxp3+CD4+CD25+ T reg cells in the periphery and can also induce Foxp3 expression in naive T cells (5–10, 20). Therefore, the role of TGF-β in the induction of Foxp3 by CD103+ DCs was investigated. Naive T cells were again cultured with CD103+ or CD103− DCs. Inclusion of a blocking mAb against TGF-β completely blocked the induction of Foxp3, indicating that the conversion of naive T cells into Foxp3+ T reg cells by CD103+ DCs is mediated by TGF-β (Fig. 3 A). A possible explanation for the functional difference between CD103+ and CD103− DC subsets is that CD103+ DCs produce higher levels of active TGF-β than the CD103− population. Analysis of gene expression by freshly isolated CD103+ and CD103− MLN DCs revealed several differences in the expression of mRNA related to TGF-β and its secretion and activation (Fig. 3 B). Specifically, CD103+ DCs expressed higher levels of tgfb2, plat (tissue plasminogen activator), and latent TGF-β binding protein 3 (ltbp3). LTBP3 is important for the efficient secretion and appropriate localization of latent TGF-β, whereas tissue plasminogen activator plays a role in the activation of latent TGF-β (21).
Figure 3.
Induction of Foxp3 is dependent on TGF-β. (A) CD4+ T cells from DO11.10 SCID mice were cultured with 5 × 104 CD103+ or CD103− MLN DCs, 0.2 μg/ml OVA peptide, and 50 μg/ml anti–TGF-β or isotype control. At day 6 of culture, T cells were stained for CD4 and Foxp3 and analyzed by FACS. Representative plots from two independent experiments are gated on CD4+ cells, and numbers represent the percentage of CD4+ cells in each quadrant. (B) CD103+ and CD103− DCs were sorted from the MLNs of BALB/c mice. Tgfb2, ltbp3, and plat gene expression was assayed by quantitative PCR and normalized relative to expression of HPRT. Data shown are representative of two independent experiments. (C) CD4+ T cells from DO11.10 SCID mice were cultured with 105 CD103+ or CD103− MLN DCs, 0.2 ug/ml OVA peptide, and the indicated concentrations of rhTGF-β. At day 6 of culture, T cells were stained for CD4 and Foxp3 and analyzed by FACS. Representative plots from three similar experiments are gated on CD4+ cells, and numbers represent the percentage of Foxp3+ cells among CD4+ T cells.
Although sufficient endogenous TGF-β was present in cultures containing CD103+ DCs to mediate the conversion of ∼10% of naive T cells into Foxp3+ T cells, we investigated the effect of adding varying concentrations of exogenous TGF-β on the induction of Foxp3. The addition of TGF-β to cultures containing CD103+ DCs enhanced the conversion of naive T cells into Foxp3+ T cells, so that inclusion of 1 ng/ml TGF-β resulted in the expression of Foxp3 by ∼50% of T cells. However, although inclusion of TGF-β in cultures containing CD103− DCs allowed for the generation of a minor Foxp3+ T cell population, the percentage of T cells expressing Foxp3 was still considerably lower than in the presence of CD103+ DCs (Fig. 3 C).
Although CD103+ DCs express genes involved in the secretion and activation of latent TGF-β, this cannot account for their enhanced ability to induce Foxp3 in the presence of exogenous TGF-β. The exogenous TGF-β used in these experiments was already in its active form, bypassing the need for the production of factors involved in the conversion of latent into active TGF-β. Even the provision of very high concentrations of active TGF-β did not enable CD103− DCs to induce similar levels of Foxp3 to CD103+ DCs, suggesting either that CD103− DCs are producing an inhibitory factor or lack an important cofactor.
RA acts as a cofactor for Foxp3 induction
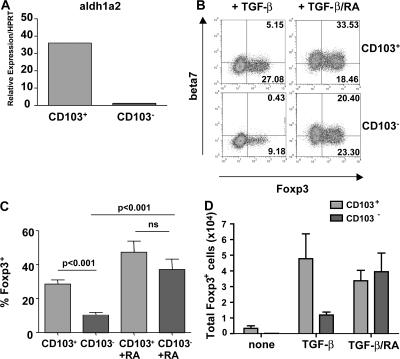
We and others have previously demonstrated that CD103+ DCs promote the expression of gut homing receptors on T cells (16, 19). Expression of gut homing receptors on T cells is enhanced by the vitamin A metabolite RA (22). Accordingly, we now show that CD103+ DCs express aldh1a2, a retinal dehydrogenase involved in the conversion of retinal into RA (Fig. 4 A). As a result, we investigated whether the provision of vitamin A was an important factor in the conversion of naive T cells into Foxp3+ T cells. As expected, the culture of T cells with exogenous TGF-β and CD103+ DCs led to the generation of a greater proportion of Foxp3+ T cells than did culture with TGF-β and CD103− DCs (Fig. 4, B–D). However, the addition of both TGF-β and RA to cultures led to the generation of a similar proportion and number of Foxp3+ T cells in the presence of both CD103− and CD103+ DCs (Fig. 4, B-D). Foxp3+ T cells generated in this way were still detectable after 17 d in culture, regardless of whether or not exogenous TGF-β and RA continued to be provided (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20070590/DC1). It would therefore appear that RA is a necessary cofactor for the efficient TGF-β–mediated conversion of naive T cells into Foxp3+ T reg cells. In addition, inclusion of synthetic RA receptor inhibitors (LE540 and LE135) blocked the spontaneous induction of Foxp3 seen in the presence of CD103+ MLN DCs (Fig. S5). The ability of CD103+ DCs to metabolize vitamin A is therefore likely to account for their ability to drive the spontaneous conversion of naive T cells into Foxp3+ T cells in the absence of any exogenous factors.
Figure 4.
RA acts as a cofactor for Foxp3 induction. (A) CD103+ and CD103− DCs were sorted from the MLNs of BALB/c mice. Aldh1a2 gene expression was assayed by quantitative PCR and normalized relative to expression of HPRT. Data shown are representative of two independent experiments. (B) CD4+ T cells from DO11.10 SCID mice were cultured with 5 × 104 CD103+ or CD103− MLN DCs, 0.2 μg/ml OVA peptide, and 2 ng/ml rhTGF-β. Some wells were additionally supplemented with 100 nM RA. T cells were stained for β7 integrin, CD4, and Foxp3 and analyzed by FACS. Plots are gated on CD4+ cells, and numbers represent the percentage of CD4+ cells in each quadrant. Data are representative of two independent experiments. (C) Graph depicts pooled data from the experiments described in B. Bars show the percentage of CD4+ cells expressing Foxp3. (D) Graph depicts absolute numbers of Foxp3+ T cells in culture under the indicated conditions.
Converted Foxp3+ T cells suppress naive T cell proliferation in vitro
Expression of Foxp3 can be transiently up-regulated upon T cell activation in the absence of an accompanying acquisition of regulatory properties (23). It was therefore important to test the ability of Foxp3+ T cells generated in the presence of CD103+ MLN DCs to suppress the proliferation of naive T cells. Because live cells cannot be sorted on the basis of Foxp3 expression in the absence of a reporter gene, we could not test the suppressive function of the Foxp3+ T cells generated using the experimental setup described above. Instead, GFP−CD4+ T cells were isolated from Foxp3-GFP mice and activated in the presence of CD103+ DCs and anti-CD3. This led to the generation of a similar proportion of Foxp3+ T cells as in the antigen- specific system. Again, this induction of Foxp3 could be inhibited by the inclusion of anti–TGF-β or RA receptor inhibitors and could be enhanced by the inclusion of rhTGF-β and RA (Fig. S5). Foxp3+ T cells generated in the presence of CD103+ DCs and rhTGF-β were then sorted on the basis of GFP expression and their ability to suppress the proliferation of naive T cells compared with that of freshly isolated Foxp3+ T reg cells. Foxp3+ T cells generated in this way were able to suppress the proliferation of naive T cells, indicating that they represent a true regulatory population (Fig. S5).
CD103− DCs produce proinflammatory cytokines
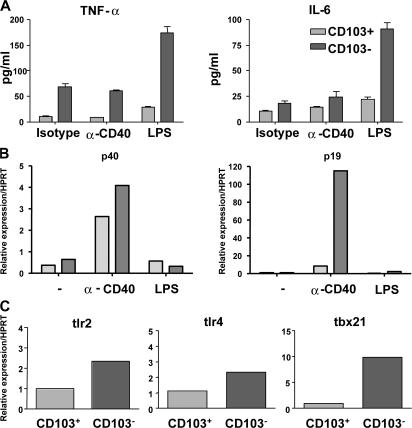
We have demonstrated a clear role for MLN CD103+ DCs in the induction of Foxp3 and regulatory function in naive T cells. However, it is crucial that protective immunity to intestinal pathogens can also be mounted. To this end, we investigated the functional properties of the reciprocal CD103− DC population. Purified CD103+ and CD103− MLN DCs were cultured overnight either alone or in the presence of LPS or an agonist anti-CD40 mAb. Cytokines in the supernatants were quantitated by cytometric bead array, whereas levels of cytokine mRNA in the DCs were determined by quantitative PCR. CD103− DCs produced higher levels of TNF-α regardless of whether DCs were left unstimulated or activated with LPS or anti-CD40. However, treatment with LPS did increase the levels of TNF-α present in the supernatants of CD103− DC (Fig. 5 A). LPS treatment also led to the enhanced production of IL-6 by CD103− DCs (Fig. 5 A). Furthermore, CD103− DCs produce substantially higher levels of IL-23p19 mRNA than CD103+ DCs after stimulation through CD40. Only a comparatively moderate increase in the expression of IL-12p40 was observed (Fig. 5 B). Consistent with their increased production of TNF-α and IL-6 in response to LPS stimulation, CD103− DCs also expressed slightly higher levels of Toll-like receptor (TLR)4 mRNA than CD103+ DCs. Further evidence for the more proinflammatory phenotype of CD103− DCs included the enhanced expression of tlr2 and tbx21, which encodes Tbet (Fig. 5 C).
Figure 5.
CD103− DCs produce proinflammatory cytokines. CD103+ and CD103− DCs were sorted from the MLN of BALB/c mice and cultured overnight in the presence of anti-CD40, an isotype control, or LPS. (A) Supernatants were harvested, and cytokine concentrations were analyzed by cytometric bead array. Graphs are representative of two independent experiments and depict cytokine concentrations in pg/ml. (B) IL-12p40 and IL-23p19 gene expression was assayed by quantitative PCR and normalized relative to expression of HPRT. Data are representative of two independent experiments. (C) CD103+ and CD103− DCs were sorted from the MLN of BALB/c mice. Tlr2, tlr4, and tbx21 gene expression was assayed by quantitative PCR and normalized relative to expression of HPRT. Data are representative of two independent experiments.
The data presented here raise the question of why the functional properties of CD103+ and CD103− DCs differ so dramatically. It is likely that the CD103+ DC subset has arrived in the MLNs from the intestine and, consequently, its journey through the immunosuppressive intestinal environment may have left an impact on its function. We hypothesize that the interaction between CD103 on DCs and E-cadherin on epithelial cells may tether the DCs close to the intestinal epithelium. This would allow them to be conditioned, perhaps by epithelial cell–derived factors, to go on to drive regulatory-type responses in the draining LNs. An example of this is the conditioning of DCs by thymic stromal lymphopoietin produced by intestinal epithelial cells (24). A key feature of gut conditioning in our studies is the capacity of CD103+ DCs to produce or activate TGF-β and to provide RA, both essential cofactors for the emergence of Foxp3+ T cells. In this way, gut-derived DCs respond to environmental cues to promote noninflammatory immune-suppressive responses that may contribute to intestinal homeostasis. On the other hand, CD103− DCs may have arrived in the LNs through the blood and therefore escaped gut conditioning. This would leave them poised to respond to inflammatory stimuli through the production of proinflammatory cytokines.
Our results demonstrate the presence of two functionally distinct DC subsets present in the MLNs of normal mice. CD103− DCs are poised to deal with pathogens through the production of proinflammatory cytokines, whereas CD103+ DCs induce Foxp3+ T reg cells, suggesting a mechanism by which tolerance to harmless non–self-antigen may be maintained. The ability to induce or expand populations of T reg cells in culture increases the likelihood that they could be used therapeutically, for example, in the treatment of inflammatory bowel disease. Identification of the key factors involved in the induction of T reg cells by CD103+ DCs suggests ways in which this process can be made more efficient. In this regard, the finding that RA enhances the TGF-β–mediated induction of Foxp3 may have important therapeutic implications.
MATERIALS AND METHODS
Mice.
BALB/c, DO11.10 BALB/c, and DO11.10 SCID TCR transgenic mice were maintained in microisolator cages in a specific pathogen-free animal facility at the University of Oxford. Experiments were performed according to the UK Animals (Scientific Procedures) Act of 1986. Foxp3 eGFP reporter mice (Foxp3GFP) were originally obtained from M. Oukka (Harvard Medical School, Boston, MA) (25) and were maintained in the NIH's animal facilities under specific pathogen-free conditions.
Antibodies.
The following antibodies were used for cell purification: anti–mouse CD8 (clone YTS169), MHC class II (TIB120), Mac-1 (M1/70), and B220 (RA3-6B2; all purified from hybridoma supernatant by affinity chromatography); and biotinylated anti–mouse CD25 (7D4), anti–mouse CD11c (clone HL3), anti–mouse CD103 (M290), and anti–mouse CD3e (145-2C11; all from BD Biosciences). For use in in vitro cultures, anti–mouse TGF-β1/2 (1D11.16.8) and anti–mouse CD40 (FGK45) were purified from hybridoma supernatant by affinity chromatography. Anti–mouse CD3 used for in vitro stimulation of T cells was clone 145-2C11 (BD Biosciences). Anti–mouse CD4 (L3T4), anti–mouse β7 integrin (M293), and anti–mouse α4β7 (DATK32; all from BD Biosciences); anti–mouse Foxp3 (FJK-16S; eBioscience); and biotinylated or FITC-labeled anti-clonotypic TCR (KJ-1.26) were used for analysis of T cell populations.
Adoptive transfer and oral feeding of antigen.
CD4+ T cells were isolated from splenic single cell suspensions from DO11.10-SCID mice by labeling with magnetic anti-CD4 beads (Miltenyi Biotec) and separating the cell populations using LS MACS columns according to the manufacturer's instructions. 2.5 × 106 cells were injected i.v. into Ly9.2 BALB/c recipients. After 24 h, drinking water was supplemented with 20 mg/ml Grade VI OVA (Sigma-Aldrich) as described previously (13). Antigen was administered to intact DO11.10 SCID mice in the same way. At the time points indicated in the relevant figure legends, organs were harvested and the presence of Foxp3+ T cells was determined by FACS.
Preparation and culture of CD11chigh subsets.
MLNs from BALB/c mice were cut into small fragments and incubated in RPMI with 10% FCS, 15 mM Hepes, and 1 mg/ml collagenase type VIII (Sigma-Aldrich) for 40 min at 37°C in a shaking incubator. After adding EDTA for an additional 5 min, the solution was filtered through a nylon mesh and washed in HBSS with 0.1% BSA. Cell suspensions were incubated with an anti-FcR antibody (clone 24G2; eBioscience), followed by anti-CD11c MACS beads (Miltenyi Biotec). CD11c+ cells were positively selected on an LS MACS column according to the manufacturer's instructions (Miltenyi Biotec). Cells were labeled with anti-CD3e, anti-CD103, anti-CD11c, and 7-AAD and sorted on a MoFlo sorter (DakoCytomation) to >98% purity. For analysis of cytokine production, DC subsets were cultured overnight in DMEM supplemented with 10% FCS, 2 mM L-glutamine, and 100 U of penicillin and streptomycin. 1 ug/ml LPS (Sigma-Aldrich) or 10 ug/ml anti–mouse CD40 was added to some wells. Supernatants were analyzed for cytokines using a Cytometric Bead Array mouse inflammation kit according to the manufacturer's instructions (BD Biosciences). Cells were prepared for quantitative PCR analysis as described below.
Preparation of T cell populations.
CD4+ T cells were isolated from splenic single cell suspensions from DO11.10 SCID mice by labeling with magnetic anti-CD4 beads (Miltenyi Biotec) and separating the cell populations using LS MACS columns according to the manufacturer's instructions. The positively selected CD4+ T cells were labeled with 10 μM CFDA SE using a Vybrant CFDA SE Cell Tracer kit (Invitrogen). CD4+CD25+ T cells were isolated from the spleens of DO11.10 BALB/c mice. Single cell suspensions were depleted of CD8+, MHC class II+, Mac-1+, and B220+ cells by negative selection using sheep anti–rat-coated Dynabeads (Dynal) as described previously (26). The resulting CD4-enriched cells were stained with biotinylated anti–mouse CD25, followed by Straptavidin-coated MACS beads and sorted by AutoMACS (Miltenyi Biotec).
T cell differentiation assay.
2 × 105 T cells were cultured together with 2.5 × 104–105 of the sorted CD11chigh subsets and 0.2 μg/ml OVA peptide in complete RPMI (10% FCS, 2 mM L-glutamine, 0.05 mM 2-mercaptoethanol, and 100 U of penicillin and streptomycin) for 4 d. Cultures were supplemented with fresh medium containing 100 U/ml recombinant human IL-2 (PeproTech) and incubated for an additional 2–3 d. Alternatively, 105 CD4+eGFP− T cells were cultured with 104 purified CD103+ or CD103− MLN DCs and 1 μg/ml of soluble anti-CD3 for 5 d. 5 ng/ml of recombinant human IL-2 was added to cultures every other day beginning on day 2 as described previously (25). On day 5, cells were stained with PE-Cy7–conjugated anti-CD4 (RM4-5), and Foxp3+ cells were detected by eGFP expression. Recombinant human TGF-β1 (R&D Systems), anti–mouse TGF-β1/2, all-trans RA (Sigma-Aldrich), or the RA receptor inhibitors, LE540 (Wako Chemicals USA) and LE135 (Tocris Bioscience), were added to culture wells in some cases. Concentrations are indicated in the relevant figure legends.
Suppression assay.
The ability of Foxp3+ T cells generated in the presence of CD103+ DCs to suppress T cell proliferation was determined as described previously (27). In brief, sorted CD4+GFP+ T reg cells were cultured with 5 × 104 freshly isolated CD4+GFP− T cells, 105 antigen-presenting cells, and 0.5 μg/ml anti-CD3 (145-2C11; BD Biosciences). Antigen-presenting cells were prepared by depleting splenocytes of CD90+ cells by isolation kit and autoMACs (Miltenyi Biotec), followed by irradiation. Cultures were incubated for 72 h, with the inclusion of 1 μCi/well [3H]TdR (MP Biomedicals) for the final 8 h.
Quantitation of gene expression using real-time PCR.
Total RNA was purified from sorted cells using RNAeasy kits (QIAGEN). cDNA synthesis was performed using Superscript III reverse transcriptase and Oligo dT primers (both from Invitrogen). Quantitative PCR reactions were performed either using quantitect Primer Assays with SYBR green PCR mastermix (QIAGEN) or the following reagents: IL-23 p19 primer AGCGGGACATATGAATCTACTAAGAGA, GTCCTAGTAGGGAGGTGTGAAGTTG, and FAM/TAMRA-labeled probe CCAGTTCTGCTTGCAAAGGATCCGC; IL-12 p40 primers GACCATCACTGTCAAAGAGTTTCTAGAT, AGGAAA GTCTTGTTTTTGAAATTTTTTAA, and FAM/TAMRA-labeled probe CCACTCACATCTGCTGCTCCACAAGAAG; HPRT primers GACCGGTCCCGTCATGC and TCATAACCTGGTTCATCATCGC; and VIC/TAMRA-labeled probe ACCCGCAGTCCCAGCGTCGTC.
cDNA samples were assayed in triplicate using a Chromo4 detection system (MJ Research), and gene expression levels for each individual sample were normalized to HPRT. Mean relative gene expression was determined and the differences were calculated using the 2−ΔC(t) method (28).
Statistics.
An unpaired student's t test was performed in Prism (Graphpad) in all cases. Where appropriate, mean ± SD is represented on graphs.
Online supplemental material.
Fig. S1 shows the generation of Foxp3+ T cells in the GALT. Foxp3+ T cells are maintained in the MLN of DO11.10 SCID mice after the removal of antigen and can be generated after the transfer of DO11.10 SCID T cells to normal mice. Fig. S2 shows the accumulation and proliferation of CD4+ T cells in the presence of CD103+ and CD103− DCs. Fig. S3 shows that CD103+ DCs can acquire and present orally administered antigen. Fig. S4 shows that Foxp3+ T cells continue to accumulate in culture without the continued provision of TGF-β and RA. Fig. S5 shows that induction of Foxp3 by CD103+ DCs is inhibited by RA receptor inhibitors, and that Foxp3+ T cells generated in vitro can suppress T cell proliferation. The online supplemental material can be found at http://www.jem.org/cgi/content/full/jem.20070590/DC1.
Supplemental Material
Acknowledgments
We would like to thank Nigel Rust for cell sorting and the staff of our animal facility for care of the mice.
This study was funded by the Wellcome Trust and the European Union (Euro-Thymaide FP6 Integrated Project; LSHB-CT-2003-503410), with J.L. Coombes and K.R.R. Siddiqui in receipt of Medical Research Council studentships.
The authors have no conflicting financial interests.
References
- 1.Coombes, J.L., N.J. Robinson, K.J. Maloy, H.H. Uhlig, and F. Powrie. 2005. Regulatory T cells and intestinal homeostasis. Immunol. Rev. 204:184–194. [DOI] [PubMed] [Google Scholar]
- 2.Kretschmer, K., I. Apostolou, D. Hawiger, K. Khazaie, M.C. Nussenzweig, and H. von Boehmer. 2005. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 6:1219–1227. [DOI] [PubMed] [Google Scholar]
- 3.Sun, J.B., S. Raghavan, A. Sjoling, S. Lundin, and J. Holmgren. 2006. Oral tolerance induction with antigen conjugated to cholera toxin B subunit generates both Foxp3+CD25+ and Foxp3−CD25− CD4+ regulatory T cells. J. Immunol. 177:7634–7644. [DOI] [PubMed] [Google Scholar]
- 4.Thorstenson, K.M., and A. Khoruts. 2001. Generation of anergic CD25+CD4+ T cells with immunoregulatory potential in vivo following induction of peripheral tolerance with intravenous or oral antigen. J. Immunol. 167:188–195. [DOI] [PubMed] [Google Scholar]
- 5.Chen, W., W. Jin, N. Hardegen, K.J. Lei, L. Li, N. Marinos, G. McGrady, and S.M. Wahl. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cobbold, S.P., R. Castejon, E. Adams, D. Zelenika, L. Graca, S. Humm, and H. Waldmann. 2004. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J. Immunol. 172:6003–6010. [DOI] [PubMed] [Google Scholar]
- 7.Fantini, M.C., C. Becker, G. Monteleone, F. Pallone, P.R. Galle, and M.F. Neurath. 2004. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 172:5149–5153. [DOI] [PubMed] [Google Scholar]
- 8.Fu, S., N. Zhang, A.C. Yopp, D. Chen, M. Mao, H. Zhang, Y. Ding, and J.S. Bromberg. 2004. TGF-beta induces Foxp3+ T-regulatory cells from CD4+ CD25- precursors. Am. J. Transplant. 4:1614–1627. [DOI] [PubMed] [Google Scholar]
- 9.Rao, P.E., A.L. Petrone, and P.D. Ponath. 2005. Differentiation and expansion of T cells with regulatory function from human peripheral lymphocytes by stimulation in the presence of TGF-{beta }. J. Immunol. 174:1446–1455. [DOI] [PubMed] [Google Scholar]
- 10.Wan, Y.Y., and R.A. Flavell. 2005. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc. Natl. Acad. Sci. USA. 102:5126–5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chirdo, F.G., O.R. Millington, H. Beacock-Sharp, and A.M. Mowat. 2005. Immunomodulatory dendritic cells in intestinal lamina propria. Eur. J. Immunol. 35:1831–1840. [DOI] [PubMed] [Google Scholar]
- 12.Johansson, C., and B.L. Kelsall. 2005. Phenotype and function of intestinal dendritic cells. Semin. Immunol. 17:284–294. [DOI] [PubMed] [Google Scholar]
- 13.Zhang, X., L. Izikson, L. Liu, and H.L. Weiner. 2001. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J. Immunol. 167:4245–4253. [DOI] [PubMed] [Google Scholar]
- 14.Bilsborough, J., T.C. George, A. Norment, and J.L. Viney. 2003. Mucosal CD8alpha+ DC, with a plasmacytoid phenotype, induce differentiation and support function of T cells with regulatory properties. Immunology. 108:481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Worbs, T., U. Bode, S. Yan, M.W. Hoffmann, G. Hintzen, G. Bernhardt, R. Forster, and O. Pabst. 2006. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J. Exp. Med. 203:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Annacker, O., J.L. Coombes, V. Malmstrom, H.H. Uhlig, T. Bourne, B. Johansson-Lindbom, W.W. Agace, C.M. Parker, and F. Powrie. 2005. Essential role for CD103 in the T cell–mediated regulation of experimental colitis. J. Exp. Med. 202:1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilshaw, P.J. 1993. Expression of the mucosal T cell integrin alpha M290 beta 7 by a major subpopulation of dendritic cells in mice. Eur. J. Immunol. 23:3365–3368. [DOI] [PubMed] [Google Scholar]
- 18.Brenan, M., and M. Puklavec. 1992. The MRC OX-62 antigen: a useful marker in the purification of rat veiled cells with the biochemical properties of an integrin. J. Exp. Med. 175:1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson-Lindbom, B., M. Svensson, O. Pabst, C. Palmqvist, G. Marquez, R. Forster, and W.W. Agace. 2005. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202:1063–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marie, J.C., J.J. Letterio, M. Gavin, and A.Y. Rudensky. 2005. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 201:1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annes, J.P., J.S. Munger, and D.B. Rifkin. 2003. Making sense of latent TGFbeta activation. J. Cell Sci. 116:217–224. [DOI] [PubMed] [Google Scholar]
- 22.Iwata, M., A. Hirakiyama, Y. Eshima, H. Kagechika, C. Kato, and S.Y. Song. 2004. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 21:527–538. [DOI] [PubMed] [Google Scholar]
- 23.Gavin, M.A., T.R. Torgerson, E. Houston, P. DeRoos, W.Y. Ho, A. Stray-Pedersen, E.L. Ocheltree, P.D. Greenberg, H.D. Ochs, and A.Y. Rudensky. 2006. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc. Natl. Acad. Sci. USA. 103:6659–6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rimoldi, M., M. Chieppa, V. Salucci, F. Avogadri, A. Sonzogni, G.M. Sampietro, A. Nespoli, G. Viale, P. Allavena, and M. Rescigno. 2005. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 6:507–514. [DOI] [PubMed] [Google Scholar]
- 25.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T.B. Strom, M. Oukka, H.L. Weiner, and V.K. Kuchroo. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238. [DOI] [PubMed] [Google Scholar]
- 26.Powrie, F., M.W. Leach, S. Mauze, L.B. Caddle, and R.L. Coffman. 1993. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 5:1461–1471. [DOI] [PubMed] [Google Scholar]
- 27.Thornton, A.M., and E.M. Shevach. 1998. CD4+ CD25+ immuno regulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfaffl, M.W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.