Abstract
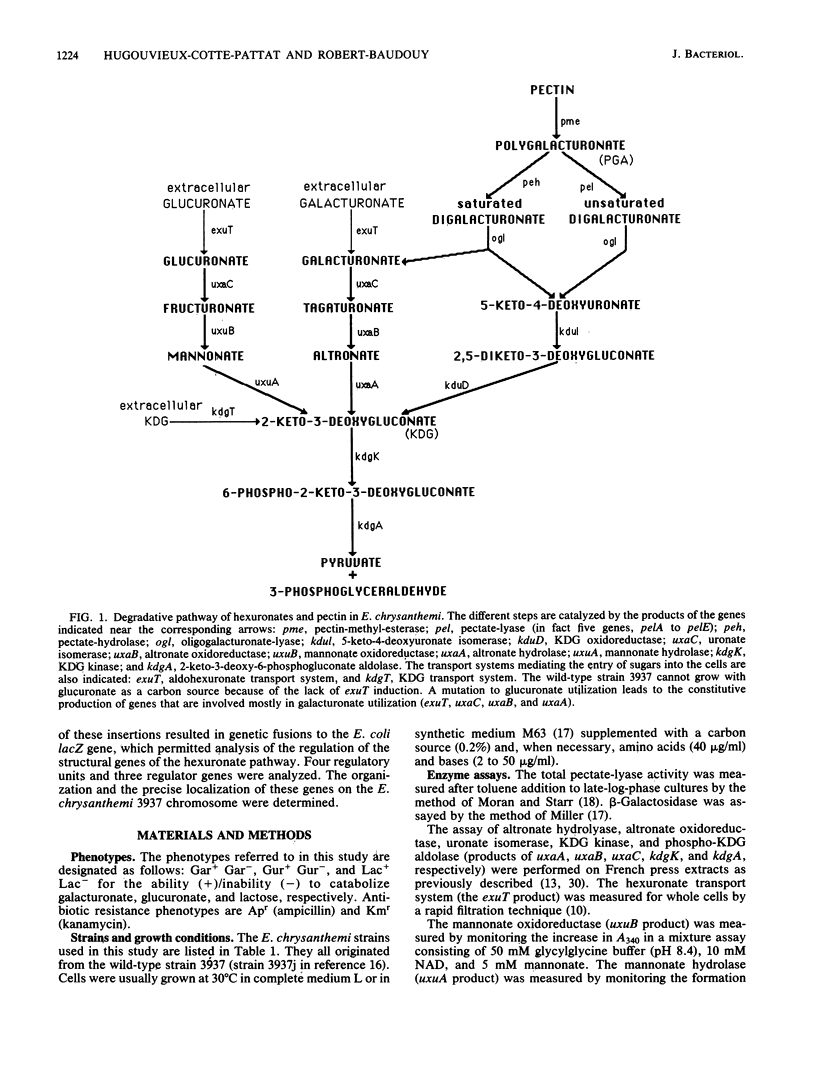
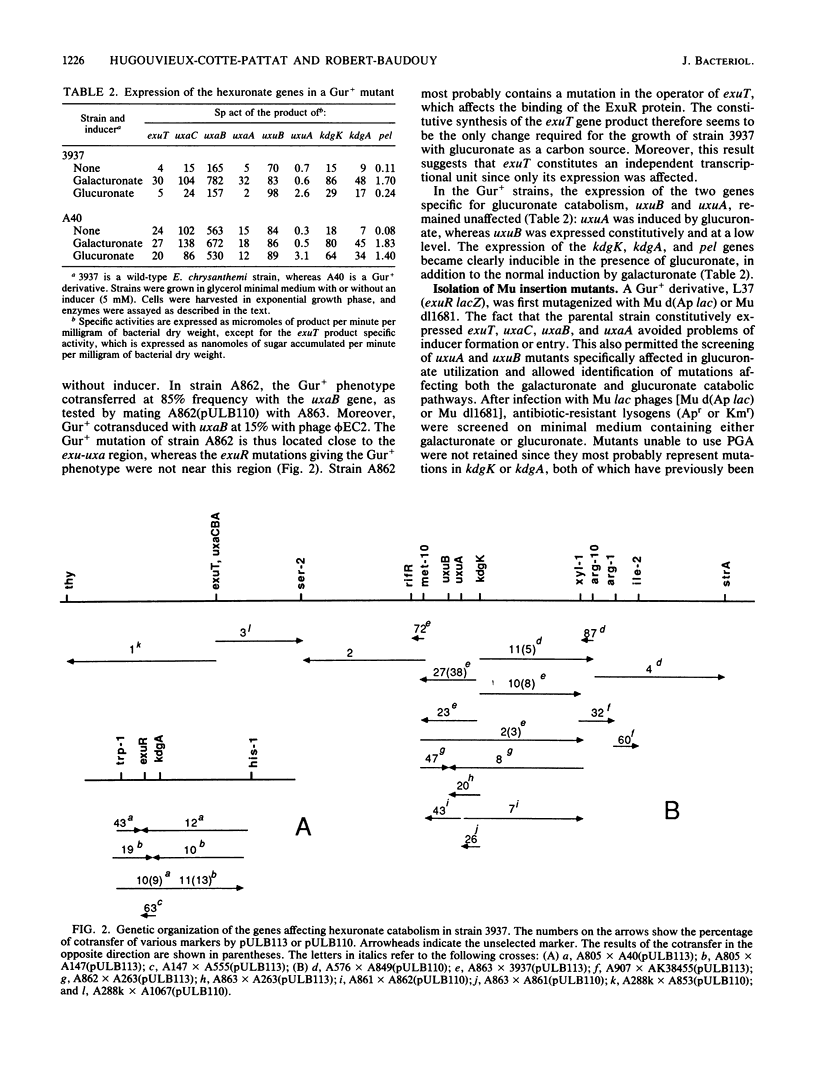
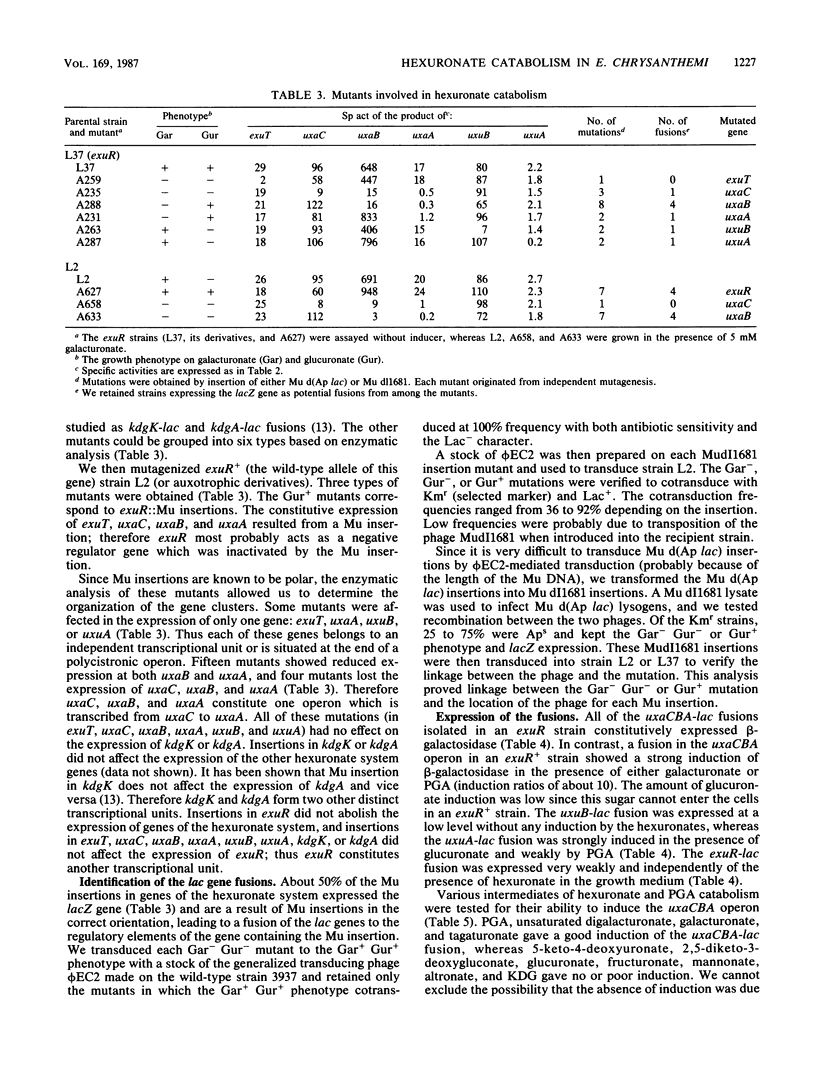
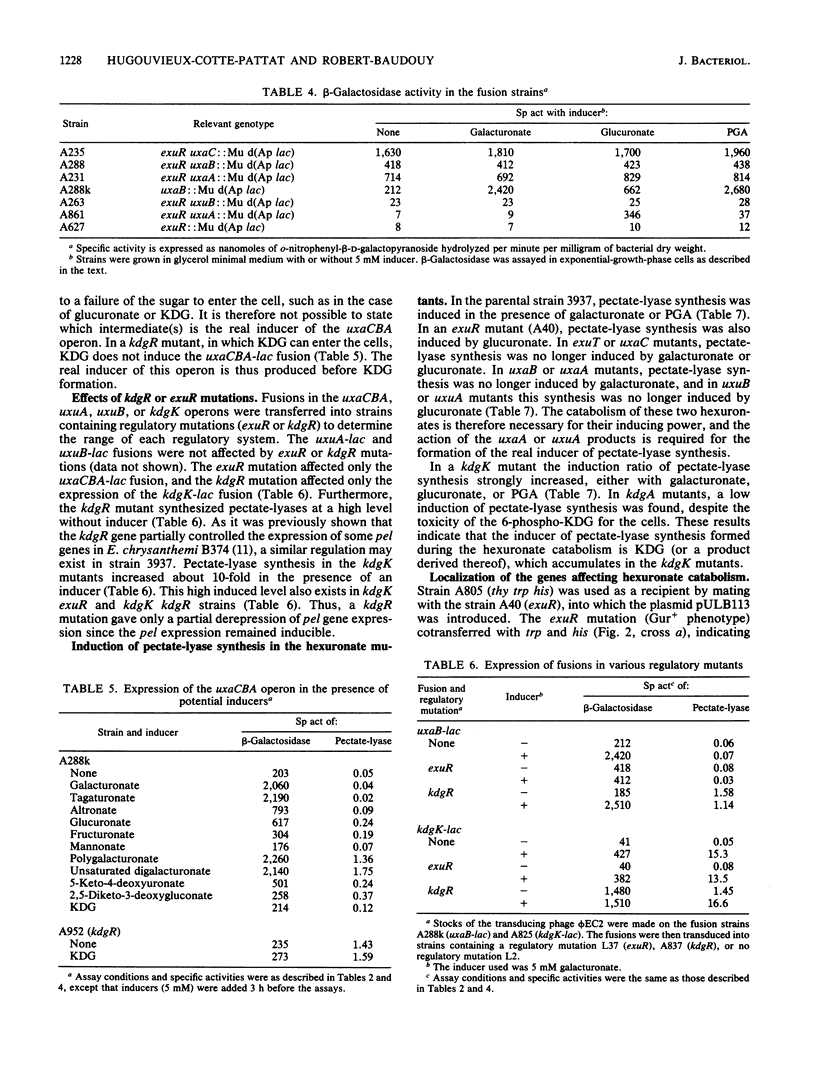
In the phytopathogenic enterobacterium Erwinia chrysanthemi, the catabolism of hexuronates is linked to the degradation of pectic polymers. We isolated Mu lac insertions in each gene of the hexuronate pathway and used genetic fusions with lacZ (the beta-galactosidase gene of Escherichia coli) to study the regulation of this pathway. Three independent regulatory genes (exuR, uxuR, and kdgR) were found. Galacturonate and glucuronate were converted into 2-keto-3-deoxygluconate (KDG) by separate three-step pathways encoded by the uxaC, uxaB, and uxaA genes and the uxaC, uxuB, and uxuA genes, respectively. The two aldohexuronates entered the cell by a specific transport system, encoded by exuT. Wild-type strain 3937 was unable to use glucuronate as a carbon source since glucuronate was unable to induce the exuT expression. Mutants able to use glucuronate possessed an inactivated exuR gene. The product of the regulatory gene exuR negatively controlled the expression of exuT, uxaC, uxaB, and uxaA, which was inducible in the presence of galacturonate. The two genes specifically involved in glucuronate catabolism, uxuA and uxuB, formed two independent transcriptional units regulated separately, uxuB expression was not inducible, whereas uxuA expression was induced in the presence of glucuronate and controlled by the uxuR product. KDG, the common end product of both pathways, is cleaved by the kdgK and kdgA gene products. KDG enters the cell by a specific transport system, encoded by kdgT. The regulatory gene kdgR controlled the expression of kdgT, kdgK, and kdgA and partially that of the pel genes encoding pectate-lyases. The real inducer of pectate-lyase synthesis, originating from catabolism of galacturonate or glucuronate, appeared to be KDG. The genes of E. chrysanthemi affecting hexuronate catabolism are separated into six independent transcriptional units exuT, uxaCBA, uxuA, uxuB, kdgK, and kdgA, but only three gene clusters were localized on the genetic map: exuT-uxaCBA, uxuA-uxuB-kdgK, and kdgA-exuR.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHWELL G., WAHBA A. J., HICKMAN J. Uronic acid metabolism in bacteria. I. Purification and properties of uronic acid isomerase in Escherichia coli. J Biol Chem. 1960 Jun;235:1559–1565. [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Starr M. P. Genetics of Erwinia species. Annu Rev Microbiol. 1980;34:645–676. doi: 10.1146/annurev.mi.34.100180.003241. [DOI] [PubMed] [Google Scholar]
- Collmer A., Bateman D. F. Impaired induction and self-catabolite repression of extracellular pectate lyase in Erwinia chrysanthemi mutants deficient in oligogalacturonide lyase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3920–3924. doi: 10.1073/pnas.78.6.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condemine G., Hugouvieux-Cotte-Pattat N., Robert-Baudouy J. Isolation of Erwinia chrysanthemi kduD mutants altered in pectin degradation. J Bacteriol. 1986 Mar;165(3):937–941. doi: 10.1128/jb.165.3.937-941.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijsegem F., Toussaint A., Schoonejans E. In vivo cloning of the pectate lyase and cellulase genes of Erwinia chrysanthemi. EMBO J. 1985 Mar;4(3):787–792. doi: 10.1002/j.1460-2075.1985.tb03698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugouvieux-Cotte-Pattat N., Quesneau Y., Robert-Baudouy J. Aldohexuronate transport system in Erwinia carotovora. J Bacteriol. 1983 May;154(2):663–668. doi: 10.1128/jb.154.2.663-668.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugouvieux-Cotte-Pattat N., Robert-Baudouy J. Lactose metabolism in Erwinia chrysanthemi. J Bacteriol. 1985 Apr;162(1):248–255. doi: 10.1128/jb.162.1.248-255.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILGORE W. W., STARR M. P. Catabolism of galacturonic and glucuronic acids by Erwinia carotovora. J Biol Chem. 1959 Sep;234:2227–2235. [PubMed] [Google Scholar]
- Kotoujansky A., Diolez A., Boccara M., Bertheau Y., Andro T., Coleno A. Molecular cloning of Erwinia chrysanthemi pectinase and cellulase structural genes. EMBO J. 1985 Mar;4(3):781–785. doi: 10.1002/j.1460-2075.1985.tb03697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotoujansky A., Lemattre M., Boistard P. Utilization of a thermosensitive episome bearing transposon TN10 to isolate Hfr donor strains of Erwinia carotovora subsp. chrysanthemi. J Bacteriol. 1982 Apr;150(1):122–131. doi: 10.1128/jb.150.1.122-131.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran F., Starr M. P. Metabolic regulation of polygalacturonic acid trans-eliminase in Erwinia. Eur J Biochem. 1969 Dec;11(2):291–295. doi: 10.1111/j.1432-1033.1969.tb00772.x. [DOI] [PubMed] [Google Scholar]
- PREISS J., ASHWELL G. Polygalacturonic acid metabolism in bacteria. I. Enzymatic formation of 4-deoxy-L-threo-5-hexoseulose uronic acid. J Biol Chem. 1963 May;238:1571–1583. [PubMed] [Google Scholar]
- Portalier R., Robert-Baudouy J., Stoeber F. Regulation of Escherichia coli K-12 hexuronate system genes: exu regulon. J Bacteriol. 1980 Sep;143(3):1095–1107. doi: 10.1128/jb.143.3.1095-1107.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyssegur J., Stoeber F. Genetic control of the 2-keto-3-deoxy-d-gluconate metabolism in Escherichia coli K-12: kdg regulon. J Bacteriol. 1974 Feb;117(2):641–651. doi: 10.1128/jb.117.2.641-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyssegur J., Stoeber F. Production de 2-céto-3-désoxy-6-phospho-gluconate par un mutant d'Escherichia coli K 12. Bull Soc Chim Biol (Paris) 1970;52(12):1407–1418. [PubMed] [Google Scholar]
- Robert-Baudouy J. M., Portalier R. C., Stoeber F. R. Régulation due métabolisme des hexuronates chez Escherichia coli K12. Modalités de l'induction des enzymes du système hexuronate. Eur J Biochem. 1974 Mar 15;43(1):1–15. doi: 10.1111/j.1432-1033.1974.tb03378.x. [DOI] [PubMed] [Google Scholar]
- Robert-Baudouy J., Portalier R., Stoeber F. Regulation of hexuronate system genes in Escherichia coli K-12: multiple regulation of the uxu operon by exuR and uxuR gene products. J Bacteriol. 1981 Jan;145(1):211–220. doi: 10.1128/jb.145.1.211-220.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr M. P., Chatterjee A. K. The genus Erwinia: enterobacteria pathogenic to plants and animals. Annu Rev Microbiol. 1972;26:389–426. doi: 10.1146/annurev.mi.26.100172.002133. [DOI] [PubMed] [Google Scholar]
- Toussaint A., Schoonejans E. Production and modification of Mu (G-) phage particles in E. coli K12 and Erwinia. Genet Res. 1983 Apr;41(2):145–154. doi: 10.1017/s0016672300021182. [DOI] [PubMed] [Google Scholar]
- Van Gijsegem F., Toussaint A. Chromosome transfer and R-prime formation by an RP4::mini-Mu derivative in Escherichia coli, Salmonella typhimurium, Klebsiella pneumoniae, and Proteus mirabilis. Plasmid. 1982 Jan;7(1):30–44. doi: 10.1016/0147-619x(82)90024-5. [DOI] [PubMed] [Google Scholar]
- Van Gijsegem F., Toussaint A. In vivo cloning of Erwinia carotovora genes involved in the catabolism of hexuronates. J Bacteriol. 1983 Jun;154(3):1227–1235. doi: 10.1128/jb.154.3.1227-1235.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- van Gijsegem F., Hugouvieux-Cotte-Pattat N., Robert-Baudouy J. Isolation and characterization of Erwinia chrysanthemi mutants defective in degradation of hexuronates. J Bacteriol. 1985 Feb;161(2):702–708. doi: 10.1128/jb.161.2.702-708.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


