Abstract
Cells from patients with xeroderma pigmentosum complementation group D (XP-D) and most patients with trichothiodystrophy (TTD) are deficient in excision repair of ultraviolet (UV) radiation-induced DNA damage. Although in both syndromes this defect is based on mutations in the same gene, XPD, only XP-D, not TTD, individuals have an increased risk of skin cancer. Since the reduction in DNA repair capacity is similar in XP-D and TTD patients, it cannot account for the difference in skin cancer risk. The features of XP-D and TTD might therefore be attributable to differences in the immune response following UV-irradiation, a factor which is presumed to be important for photocarcinogenesis. We have measured the capacity of UVB radiation to inhibit expression of the immunological key molecule intercellular adhesion molecule 1 (ICAM-1) in cells from three healthy individuals in comparison to cells from three XP-D and three TTD patients. Cells from XP-D patients, but not from TTD patients, exhibited an increased susceptibility to UVB radiation-induced inhibition of ICAM-1 expression. Transfection of XP-D cells with the wild-type XPD cDNA, but not with XPC cDNA, corrected this abnormal phenotype. Thus, the skin cancer risk in DNA repair-defective individuals correlated with the susceptibility of their cells to UVB radiation-induced inhibition of ICAM-1 expression, rather than with their defect in DNA repair. The XPD protein has dual roles: in DNA repair and transcription. The transcriptional role might be important for the control of expression of immunologically relevant genes and thereby contribute to the skin cancer risk of a DNA-repair-deficient individual.
Keywords: photoimmunology, transfection, skin cancer
Sunlight-induced skin cancer represents the most prevalent malignancy in the Caucasian population, and its incidence is increasing (1). The pathogenesis of photocarcinogenesis is complex and only partially understood. Sunlight is a complete carcinogen, and it is generally accepted that ultraviolet B (UVB; 290–320 nm) radiation-induced DNA mutations constitute the initiation event for the generation of malignant skin cells (2). Studies in animals, however, provide evidence for a second mechanism for the development of clinically apparent skin cancer following UVB radiation exposure. In these studies, UVB radiation at subcarcinogenic doses was found to inhibit the surveillance function of the skin immune system directed against UVB radiation-induced skin tumors (3, 4). The importance of this second mechanism for photocarcinogenesis was demonstrated in tumor transplantation studies in mice, which cannot be carried out in humans. The evidence for a role of UVB radiation-induced immunosuppression in human skin cancer is therefore inevitably circumstantial and includes the observation that immunosuppressed humans who have received renal transplants have an increased frequency of sunlight-induced skin cancers (5–8).
Here we take advantage of two human syndromes associated with defects in excision repair of UV-induced DNA lesions, namely xeroderma pigmentosum complementation group D (XP-D) and trichothiodystrophy (TTD) (9–11). Cells derived from XP-D and the majority of TTD patients have a defect in nucleotide excision repair (12, 13), which results from mutations in the same gene (XPD) (refs. 14–16 and unpublished results of B. C. Broughton and A.R.L.). Despite this similarity and a similar frequency of UV-induced mutations (17), only XP-D patients have an increased risk of developing skin cancer (10, 11). Since the increased risk of skin cancer in XP-D, as compared with TTD patients, cannot be explained simply by differences in DNA repair capacity, we hypothesized that XP-D and TTD cells might differ in some aspect of the immune response after UVB radiation.
To test this hypothesis, the capacity of UVB radiation to suppress transcriptional expression of the intercellular adhesion molecule 1 (ICAM-1) was assessed in comparative studies employing XP-D and TTD cells. ICAM-1 serves as a ligand for leukocyte function-associated antigen 1, and ICAM-1/leukocyte function-associated antigen-1-mediated cell adhesion is essential for a large number of cell-mediated immune responses (18). UVB radiation at physiologically relevant doses inhibits interferon (IFN)-γ-induced ICAM-1 expression in human cells in a UVB-dose-dependent manner (19). This assay provides a model system to investigate in vitro the susceptibility of a given human cell to one aspect of the immune response after UVB radiation.
METHODS
Cell Culture and UVB Irradiation.
In the present study primary human skin fibroblasts from three healthy individuals, three XP-D patients, and three TTD patients (Table 1) were employed. This cell type was studied, because for ethical reasons it is impossible to obtain keratinocytes or epidermal Langerhans cells in sufficient quantities from DNA-repair-deficient individuals. For the same reasons, primary human skin fibroblasts are used routinely for defining the quality and quantity of the DNA-repair defect in patients with DNA-repair-deficiency syndromes. Cells were grown in Eagle’s minimal essential medium/15% fetal calf serum. Assessment of excision repair characteristics in these cells revealed that cells from all three XP-D patients and from two TTD patients exhibited a DNA-repair deficiency, while cells from the third TTD patient (TTD4BR) and from the three healthy individuals had no defect in excision repair (Table 1). Cells (7 × 105) were irradiated with increasing doses of UV radiation (0–100 J/m2) from FS 20 sunlamps (Westinghouse Electric, Pittsburgh, PA), which are known to emit primarily in the UVB range (19). The UVB output was monitored by means of an IL1700 research radiometer and SEE 240 UVB photodetector (International Light, Newburyport, MA) and was approximately 2.4 W/m2 at a tube-to-target distance of 22 cm. Immediately after UV exposure, cells were washed, cultured in medium, and stimulated with 500 units/ml recombinant human (rh) IFN-γ (Genzyme).
Table 1.
Designation and properties of cell strains used in this investigation
| Patient | UDS,* % | ED50,† J/m2 | Amino acid changes in XPD protein‡ | Susceptibility§ | Cancer- prone¶ |
|---|---|---|---|---|---|
| N46 | 100 | 49 | NA | − | − |
| N47 | 100 | 42 | NA | − | − |
| N50 | 100 | 43 | NA | − | − |
| XP67MA (D) | 36 | 20 | Q726am (20) | + | + |
| XP16BR (D) | 16 | 17.5 | R683W, R616P‖ | + | + |
| XP26VI (D) | 20 | 19 | ND | + | + |
| TTD4BR (21) | 100 | 49 | NA | − | − |
| TTD1BI (22) | 50 | 46 | Frameshift 730 (14) | − | − |
| TTD1BEL (12) | 15 | 49 | R722W, R616P (14) | − | − |
UDS, levels of unscheduled DNA synthesis (percent of normal cells) after UVC irradiation. Data were obtained from indicated published data or our own results.
ED50, mean UVB dose required for half-maximal suppression of IFN-γ-induced ICAM-1 mRNA expression, as calculated from three independent experiments for each cell strain. Standard deviations of each ED50 given in this table were <15%.
Results of mutation analysis of the XPD gene in patients. NA, not applicable—no DNA repair defect; ND, not determined.
Susceptible to UVB-radiation-induced suppression of IFN-γ-induced ICAM-1 mRNA expression. Cell strains N46, N47, and N50 were regarded as normal. Cells were defined as susceptible if their ED50 was not within the 95% confidence interval of ED50 calculated from normal cells (35.5–53.9 J/m2). If cells revealed an ED50 within the 95% confidence interval of ED50 calculated from normal cells (N46, N47, N50), cells were defined as normal.
Cancer-prone, personal history of malignant skin tumors during childhood and early adolescence.
Unpublished data of B. C. Broughton and A.R.L.
RNA Extraction and Reverse Transcription–Polymerase Chain Reaction (RT-PCR).
After a 4-h incubation period, cells were harvested, and total RNA was isolated by a modified chloroform/phenol method (23). Because of the limited number of cells from DNA-repair-deficient individuals, inhibition of IFN-γ-induced ICAM-1 mRNA expression by UVB radiation was determined by highly sensitive, semiquantitative, differential RT-PCR (24). Quantification of PCR products, determination of linear amplification ranges with regard to cycle numbers and the amount of cDNA subjected to PCR, and calculation procedures have been described in detail previously (25). In brief, identical amounts of cDNA were subjected to increasing cycle numbers of PCR to obtain the linear amplification range, and then increasing amounts of cDNA (up to 64 times the starting amount) were subjected to PCR of a given cycle number within the linear range to exclude the possibility that increased amounts of a specific cDNA lead to disturbance of the linearity in PCR amplification. For estimation of similar amounts of cDNA used for PCR, samples were screened for expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a “housekeeping” gene. Amplification was found to be linear for up to 34 cycles for the ICAM-1 primer pair and for up to 28 cycles for the GAPDH primer pair. The following primer pairs specific for ICAM-1 and GAPDH were used: ICAM-1: 5′-TGACCAGCCCAAGTTGTTGG-3, 5′-ATCTCTCCTCACCAGCACCG-3′; GAPDH: 5′-CCACCCATGGCAAATTCCATGGCA-3′, 5′-TCTAGACGGCAGGTCAGGTCCACC-3′. Products were quantified by ion-exchange chromatography connected to an on-line UV spectrophotometer (Gynkotek, Germering, Germany), which allowed exact quantification of amplification products at 260 nm. To ensure identity of products, we collected their chromatogram peaks and digested them with an appropriate restriction endonuclease, and fragments were visualized on agarose gel by ethidium bromide staining and fluorescence. Lengths of the restriction fragments were compared with those deduced from published mRNA sequences of ICAM-1 by using pc/gene software, release 6.70 (IntelliGenetics), and were found to match the expected fragment sizes.
Immunofluorescence Flow Cytometry.
Twenty-four hours after IFN-γ addition and UVB irradiation, fibroblast ICAM-1 surface expression was assessed by immunofluorescence flow cytometry using anti-ICAM-1 mAb 84H10 (mIgG1; Dianova, Hamburg, Germany) as described (19, 26).
Construction of Retroviral Vectors Containing XPD or XPC cDNAs.
Retroviral vectors containing XPD (pLXPDSN) or XPC (pLXPCSN) cDNA were constructed as described (27, 28). In brief, the pLXPDSN and pLXPCSN plasmids were constructed by directly inserting the XPD cDNA and XPC cDNA into the EcoRI site of the Moloney murine leukemia virus-based retroviral vector pLXSN. Virus-producing cells were prepared by transfecting the pLXPDSN and pLXPCSN plasmids into the amphotropic packaging cell line ψ-CRIP (29) and by screening G418-resistant clones for virus production. Primary human skin fibroblasts were transduced as follows. Pre-confluent producer cells ψ-CRIP/LXPDSN and ψ-CRIP/LXPCSN were grown for 24 h. Conditioned medium containing the virus was filtered through 0.22-μm-pore nitrocellulose filters and used to transduce recipient cells for 24 h. Two successive transductions were performed. Transduced cells were selected by adding 750 mg/ml active G418 to the medium.
The retroviral vectors were used to transduce primary human skin fibroblasts from an XP-D patient. This patient (XP26VI, Table 1) is a woman, born in 1968. She had early sun sensitivity, photophobia, and skin pigmentation. Although she was highly protected against sun exposure, she developed her first squamous cell carcinoma at 13, and then five skin tumors between 13 and 16. Transduction experiments gave efficient integration, mRNA synthesis, and protein expression of both the XPD and XPC cDNA in LXPDSN- and LXPCSN-transduced XP-D fibroblasts (27). Full correction of the DNA-repair defect—that is, an increased survival after UV-irradiation, a normal level of DNA-repair synthesis, and the reactivation of a UV-irradiated reporter vector—was observed in XP-D cells transduced with the XPD cDNA but not in XP-D cells transduced with the XPC cDNA (28).
RESULTS
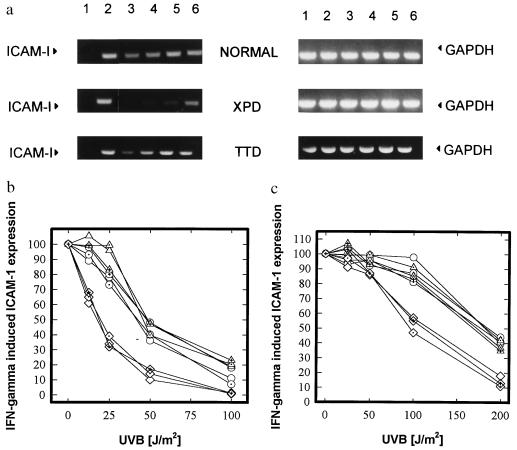
The IFN-γ dose–response and the kinetics of induction of ICAM-1 mRNA and surface expression by rhIFN-γ were essentially identical in all cell lines tested (data not shown), confirming previous findings that cells from DNA-repair-deficient individuals are not deficient in their capacity to respond to IFN-γ stimulation (30). UVB irradiation inhibited IFN-γ-induced ICAM-1 mRNA expression in normal fibroblasts in a dose-dependent manner (mean ED50: 45 J/m2 UVB; Fig. 1 and Table 1). The dose–response curve was shifted toward lower UVB doses for cells from all three XP-D patients (mean ED50 = 19 J/cm2 UVB, Fig. 1 and Table 1), indicating a 2- to 3-fold higher sensitivity of XP-D cells in this assay, as compared with normal cells. In contrast, the responses of the TTD cell lines tested were very similar to those of normal cells (mean ED50 = 48 J/cm2 UVB; Fig. 1 and Table 1), irrespective of whether their DNA repair ability was severely reduced or normal.
Figure 1.
Effect of UVB radiation on the inhibition of IFN-γ-induced ICAM-1 mRNA and surface expression in DNA-repair-deficient versus normal cells. (a) RT-PCR on ICAM-1 (Left) and GAPDH (Right) mRNA expression in normal (N46), XP-D (XP16BR), and TTD (TTD1BEL) cells. Cells were left unstimulated (lane 1) or stimulated with rhIFN-γ (lanes 2–6). In lanes 1 and 2, cells were sham irradiated, in lanes 3–6, cells were exposed to decreasing doses of UVB radiation (lane 3, 100 J/m2; lane 4, 50 J/m2; lane 5, 25 J/m2; and lane 6, 12.5 J/m2). IFN-γ (500 units/ml) was added immediately after UVB radiation exposure and cells were harvested after a 4-h incubation period. Data are shown as fluorescence of ethidium bromide-stained gels and represent one of three essentially identical experiments. (b) Summary of RT-PCR results from three XPD (⋄), three normal (○), and three TTD (▵) cell strains. IFN-γ-induced ICAM-1 mRNA expression is given as percent of ICAM-1 mRNA expression in sham-irradiated, IFN-γ-stimulated cells and is plotted against UVB radiation (J/m2). Each curve represents mean values of three independent experiments. Standard deviation for each mean was less than ±15%. (c) Summary of FACS analysis results from three XPD (⋄), three normal (○), and three TTD (▵) cell strains. IFN-γ was added immediately after UVB radiation exposure and cells were harvested after a 24-h incubation period. IFN-γ-induced ICAM-1 surface expression is given as percent of ICAM-1 surface expression in sham-irradiated, IFN-γ-stimulated cells and plotted against UVB radiation (J/cm2). Each curve represents mean values of three independent experiments. Standard deviation for each mean was less than ±15%.
Analysis of ICAM-1 surface expression in these cell strains revealed a similar dose–response pattern. Higher doses of UVB were necessary to reduce ICAM-1 surface as compared with mRNA expression. However, we again found that XP cells were more sensitive in this assay (mean ED50 = 108 J/cm2; Fig. 1) than normal cells (mean ED50 = 183 J/cm2 UVB, Fig. 1) or TTD cell lines (mean ED50 = 175 J/cm2 UVB; Fig. 1).
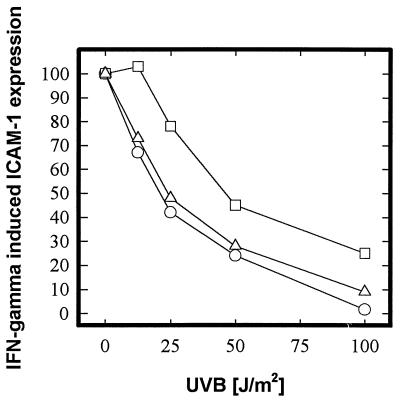
To test whether this phenotype of XP-D cells is determined by the defect in the XPD gene, we used a retroviral vector to transfer a functional copy of the XPD cDNA into cells from an XP-D patient. Transfection corrected not only their DNA-repair defect (27, 28) but also their ICAM-1 response (Fig. 2), increasing their ED50 from 21 J/m2 of UVB to 46 J/m2, a value similar to those for TTD or normal cells (Table 1, Fig. 1). In contrast, transfection of XP-D cells with an XPC cDNA construct had no effect (Fig. 2).
Figure 2.
Effect of UVB radiation on inhibition of IFN-γ-induced ICAM-1 mRNA expression in genetically engineered XP-D cells. Summary of RT-PCR results from XP-D cells transfected with functional XPD cDNA (□), nontransfected XPD cells (○), and XP-D cells transfected with XPC cDNA (▵). Suppression of IFN-γ-induced mRNA expression is given as percent of ICAM-1 mRNA expression in sham-irradiated, IFN-γ-stimulated cells and is plotted against UVB radiation (J/m2). Each curve represents mean values (SD < 15%) of three independent experiments.
DISCUSSION
We have found a substantial (approximately 2-fold) difference in the sensitivities of XP-D and TTD cells to UVB radiation-induced inhibition of ICAM-1 regulation. This difference could be observed regardless of whether ICAM-1 expression was analyzed at the mRNA or surface level.
The increased risk of XP-D patients’ developing skin cancer correlated with the increased susceptibility of cells from these patients to UVB-induced inhibition of expression of this surface marker. In contrast, the normal inhibition of ICAM-1 expression in cells from TTD patients is correlated with the paradoxical observation that these individuals do not develop skin cancer although most of the patients exhibit a DNA-repair defect, which results from mutations within the XPD gene.
We have used fibroblasts for these studies, as they are the only cell type readily available from repair-deficient individuals. We postulate that in human skin similar ICAM-1 inhibition profiles will be found in antigen-presenting cells, and that as a consequence XP, but not TTD, individuals might have a reduced capacity to elicit an immune response toward any precarcinogenic cells.
Previous studies on immune defects in XP cells have given variable results (31). Gaspari et al. (30) found that in 8 of 8 XP patients NK (natural killer) cells were defective in poly(I)⋅poly(C) induction of IFN-γ. Recently an XPA knockout mouse has been generated, and the immune response in these repair-deficient mice is clearly hypersensitive to UVB irradiation (32). If our interpretation of our data is correct, our results strengthen the evidence for a contribution of defective immune functions in carcinogenesis in these patients and indicate that strategies directed at supporting or enhancing immunity against skin tumors may be of benefit for these patients (30–33). Consistent with this assumption is the previously described beneficial effect of intralesional IFN-α application into melanoma of an XP patient (34). In our study, the importance of UVB radiation-induced inhibition of a marker for the immune response has been determined for DNA-repair-deficient individuals. Nevertheless it is tempting to speculate that in normal individuals, UVB radiation-induced depression of the immune response may prove to be an important pathogenetic factor in skin carcinogenesis as well.
Transfection of XP-D cells with the XPD gene, but not with the XPC gene, restored the susceptibility of XP-D cells toward UVB radiation-induced inhibition of IFN-γ-mediated ICAM-1 mRNA expression to normal levels. This experiment clearly indicates that the observed differences in ICAM-1 inhibition depend critically on the function of the XPD protein. It also excludes the possibility that a second gene is responsible for differences in modulation of ICAM-1 expression.
The XPD protein is a component of the transcription factor IIH (TFIIH) complex, which has a dual function: in both DNA repair and basal transcription (35, 36). It has been proposed that subtle alterations in transcription, caused by mutations in TFIIH subunits, can result in deficiencies in certain proteins whose expression is critically dependent on the level of transcription (35, 37). Furthermore it has been suggested that the features of XP largely result from defective DNA repair, whereas many of the features of TTD may be a consequence of transcriptional alterations (14, 35, 37). The finding that the causative mutations in the XPD gene are located at different sites within the gene in XP-D and TTD patients (refs. 14–16 and unpublished results of A.R.L.) is consistent with these ideas. The different effects of UVB on ICAM-1 expression in XP-D and TTD cell strains with similar repair deficiencies may therefore be associated in some way with subtle transcriptional differences between the cell strains. For example, the degree of ICAM-1 inhibition may be dependent not only on the level of unrepaired damage, which would be expected to be similar in XP and TTD individuals, but also on the transcriptional status, which is expected to differ between XPs and TTDs.
The experiments described here focused on DNA-repair-deficiency syndromes based on mutations in the XPD gene. These observations may be relevant also for other DNA-repair-deficiency syndromes, because similar results were obtained when cells from two unrelated XP-G patients were compared. The skin of one patient (XP125LO) was clinically normal and in the other (XP3BR) severely sun-damaged, and these differences were not due to differences in sun protection. Assessment of the ICAM-1 response revealed that XP125LO cells had a normal and XP3BR cells had an approximately 1.8-fold higher sensitivity toward UVB radiation-induced inhibition of IFN-γ-mediated ICAM-1 expression (data not shown). This observation is of particular interest, since the XPG protein is thought to be involved in regulation of transcription (38). Further studies are required to understand the precise mechanisms by which the differences in the photoimmunological phenotype are manifested.
There is increasing evidence that the generation of photoproducts is a prerequisite for UVB radiation-induced immunosuppression (39, 40). We propose that both the repair and the transcriptional functions of certain DNA-repair proteins play a key role in determining the effects of UVB on the immune response and that this affects the risk of a DNA-repair-deficient individual’s developing skin cancer. It is intriguing to assume that a similar link between DNA repair and effects on the immune system may determine the skin cancer risk of normal individuals.
Acknowledgments
The continuous support by B. Bridges (Brighton) and T. Ruzicka (Düsseldorf) is greatly acknowledged. This work was supported by grants from the European Commission, the British–German Academic Research Collaboration, the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie, the Fondation de France, and the Ligue Nationale contre le Cancer (Paris). X.Q. had a fellowship from the Institute Formation Superieure Biomedicale (Villejuif, France).
ABBREVIATIONS
- ICAM-1
intercellular adhesion molecule 1
- IFN
interferon
- XP
xeroderma pigmentosum
- XP-D
XP complementation group D
- TTD
trichothiodystrophy
- RT-PCR
reverse transcription–PCR
- rh
recombinant human
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
References
- 1.Miller D L, Weinstock M A. J Am Acad Dermatol. 1994;27:774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 2.Ziegler A, Lefell D J, Kunala S, Sharma H W, Gailani M, Simon J A, Halperin A J, Baden H P, Shapiro P E, Bale A E, Brash D E. Proc Natl Acad Sci USA. 1993;90:4216–4220. doi: 10.1073/pnas.90.9.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kripke M L. Immunol Rev. 1984;80:87–102. doi: 10.1111/j.1600-065x.1984.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 4.Kripke M L. J Natl Cancer Inst. 1974;53:1333–1336. doi: 10.1093/jnci/53.5.1333. [DOI] [PubMed] [Google Scholar]
- 5.Greene M H, Young T I, Clark J W H. Lancet. 1974;i:1196–1198. [Google Scholar]
- 6.Hoover R, Fraumeni J F. Lancet. 1973;ii:55–57. doi: 10.1016/s0140-6736(73)93256-x. [DOI] [PubMed] [Google Scholar]
- 7.Kinlen L J, Sheil A G, Peto J, Doll R. Br J Med. 1979;2:1461–1466. doi: 10.1136/bmj.2.6203.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bridges B. Carcinogenesis. 1981;2:471–472. doi: 10.1093/carcin/2.5.471. [DOI] [PubMed] [Google Scholar]
- 9.Kraemer K H, Slor H. Clin Dermatol. 1985;2:33–69. doi: 10.1016/0738-081x(85)90096-3. [DOI] [PubMed] [Google Scholar]
- 10.Lehmann A R. Cancer Rev. 1987;7:82–103. [Google Scholar]
- 11.Sarasin A. Cancer J. 1991;4:233–237. [Google Scholar]
- 12.Stefanini M, Lagomarsini P, Giliani S, Nardo T, Botta E, Peserico A, Kleijer W J, Lehmann A R, Sarasin A. Carcinogenesis. 1993;14:1101–1105. doi: 10.1093/carcin/14.6.1101. [DOI] [PubMed] [Google Scholar]
- 13.Mezzina M, Eveno E, Chevallier Lagente O, Benoit A, Carreau M, Vermeulen W, Hoeijmakers J H, Stefanini M, Lehmann A R, Weber C A, Sarasin A. Carcinogenesis. 1994;15:1493–1498. doi: 10.1093/carcin/15.8.1493. [DOI] [PubMed] [Google Scholar]
- 14.Broughton B C, Steingrimsdottir H, Weber C, Lehmann A R. Nat Genet. 1994;7:189–194. doi: 10.1038/ng0694-189. [DOI] [PubMed] [Google Scholar]
- 15.Takayama K, Salazar E P, Lehmann A R, Stefanini M, Thompson L H, Weber C A. Cancer Res. 1995;55:5656–5663. [PubMed] [Google Scholar]
- 16.Takayama K, Salazar E P, Broughton B C, Lehmann A R, Sarasin A, Thompson L H, Weber C A. Am J Hum Genet. 1996;58:263–270. [PMC free article] [PubMed] [Google Scholar]
- 17.Marionnet C, Benoit A, Benhamou S, Sarasin A, Stary A. J Mol Biol. 1995;252:550–562. doi: 10.1006/jmbi.1995.0519. [DOI] [PubMed] [Google Scholar]
- 18.Trefzer U, Krutmann J. In: Photoimmunology. Krutmann J, Elmets C A, editors. Oxford: Blackwell; 1995. pp. 77–90. [Google Scholar]
- 19.Krutmann J, Köck A, Schauer E, Parlow F, Möller A, Kapp A, Förster E, Schöpf E, Luger T A. J Invest Dermatol. 1990;95:127–131. doi: 10.1111/1523-1747.ep12477839. [DOI] [PubMed] [Google Scholar]
- 20.Frederick G D, Amirkhan R H, Schultz R A, Friedberg E C. Hum Mol Genet. 1994;3:1783–1788. doi: 10.1093/hmg/3.10.1783. [DOI] [PubMed] [Google Scholar]
- 21.Tolmie J L, De Bekker D, Dawber R, Galloway C, Gregory D W, Lehmann A R, McClure J, Pollit R J, Stephenson J B P. Clinical Dysmorphology. 1994;3:1–14. [PubMed] [Google Scholar]
- 22.Broughton B C, Lehmann A R, Harcourt S A, Arlett C F, Sarasin A, Kleijer W L, Beemer F A, Nairn R, Mitchell D L. Mutat Res. 1990;235:33–40. doi: 10.1016/0921-8777(90)90020-6. [DOI] [PubMed] [Google Scholar]
- 23.Chomzynski P, Sacchi N. Anal Biochem. 1987;162:156–161. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 24.Grewe M, Gyufko K, Schöpf E, Krutmann J. Lancet. 1994;343:25–26. doi: 10.1016/s0140-6736(94)90879-6. [DOI] [PubMed] [Google Scholar]
- 25.Henninger H P, Hoffmann R, Grewe M, Schulz-Specking A, Decker K. Biol Chem Hoppe-Seyler. 1993;374:625–632. doi: 10.1515/bchm3.1993.374.7-12.625. [DOI] [PubMed] [Google Scholar]
- 26.Grether-Beck S, Olaizola-Horn S, Schmitt H, Grewe M, Jahnke A, Johnson J P, Briviba K, Sies H, Krutmann J. Proc Natl Acad Sci USA. 1996;93:14586–14591. doi: 10.1073/pnas.93.25.14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carreau M, Quilliet X, Eveno E, Salvetti A, Danos O, Heard J-M, Mezzina M, Sarasin A. Hum Gene Ther. 1995;6:1307–1316. doi: 10.1089/hum.1995.6.10-1307. [DOI] [PubMed] [Google Scholar]
- 28.Quilliet X, Chevallier-Lagente O, Eveno E, Stojkovic T, Destee A, Sarasin A, Mezzina M. Mutat Res. 1996;364:161–169. doi: 10.1016/s0921-8777(96)00024-9. [DOI] [PubMed] [Google Scholar]
- 29.Danos O, Mulligan R. Proc Natl Acad Sci USA. 1988;85:6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaspari A A, Fleisher T A, Kraemer K H. J Clin Invest. 1993;92:1135–1142. doi: 10.1172/JCI116682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bridges B A. Carcinogenesis. 1981;2:471–472. doi: 10.1093/carcin/2.5.471. [DOI] [PubMed] [Google Scholar]
- 32.Miyauchi-Hashimoto H, Tanaka K, Horio T. J Invest Dermatol. 1996;107:343–348. doi: 10.1111/1523-1747.ep12363295. [DOI] [PubMed] [Google Scholar]
- 33.Norris P G, Limb G A, Hamblin A S, Lehmann A R, Arlett C F, Cole J, Waugh A P W, Hawk J L M. J Invest Dermatol. 1990;94:94–100. doi: 10.1111/1523-1747.ep12873952. [DOI] [PubMed] [Google Scholar]
- 34.Turner M L C, Moshell A N, Cornett D W, Stern J B, Roth M J, DiGiovanna J, Horn T D, Kraemer K H. Arch Dermatol. 1994;130:1491–1502. [PubMed] [Google Scholar]
- 35.Bootsma D, Hoeijmakers J H J. Nature (London) 1993;363:114–115. doi: 10.1038/363114a0. [DOI] [PubMed] [Google Scholar]
- 36.Schaeffer L, Moncollin V, Roy R, Staub A, Mezzina M, Sarasin A, Weerda G, Hoeijmakers J H J, Egly J-M. EMBO J. 1994;13:2388–2392. doi: 10.1002/j.1460-2075.1994.tb06522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vermeulen W, van Vuuren A J, Chipoulet M, Schaeffer L, Appeldoorn E, Weeda G, Jaspers N G J, Priestley A, Arlett C F, Lehmann A R, Stefanini M, Mezzina M, Sarasin A, Bootsma D, Egly J-M, Hoeijmakers J H L. Cold Spring Harbor Symp Quant Biol. 1994;59:317–329. doi: 10.1101/sqb.1994.059.01.036. [DOI] [PubMed] [Google Scholar]
- 38.Friedberg E C. BioEssays. 1996;18:731–738. doi: 10.1002/bies.950180908. [DOI] [PubMed] [Google Scholar]
- 39.Applegate L A, Ley R A, Alcalay J, Kripke M L. J Exp Med. 1989;170:1117–1131. doi: 10.1084/jem.170.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kripke M L, Cox P A, Alas L G, Yarosh D B. Proc Natl Acad Sci USA. 1992;89:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]