Abstract
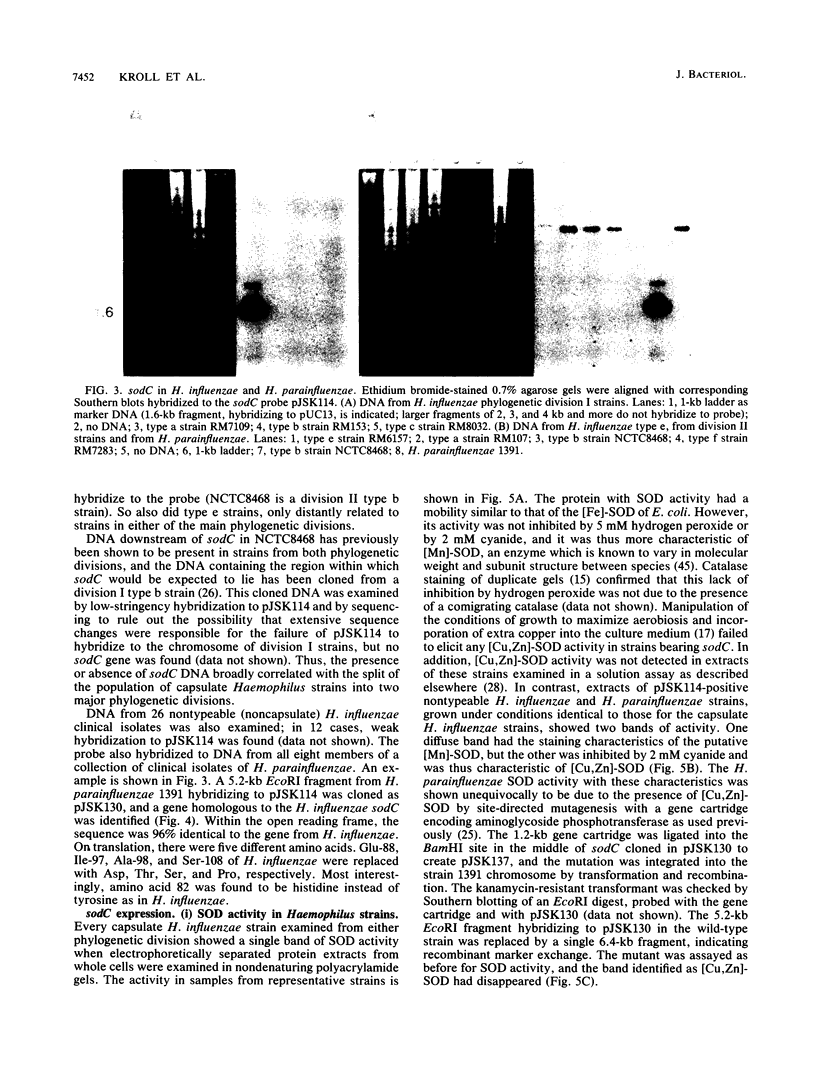
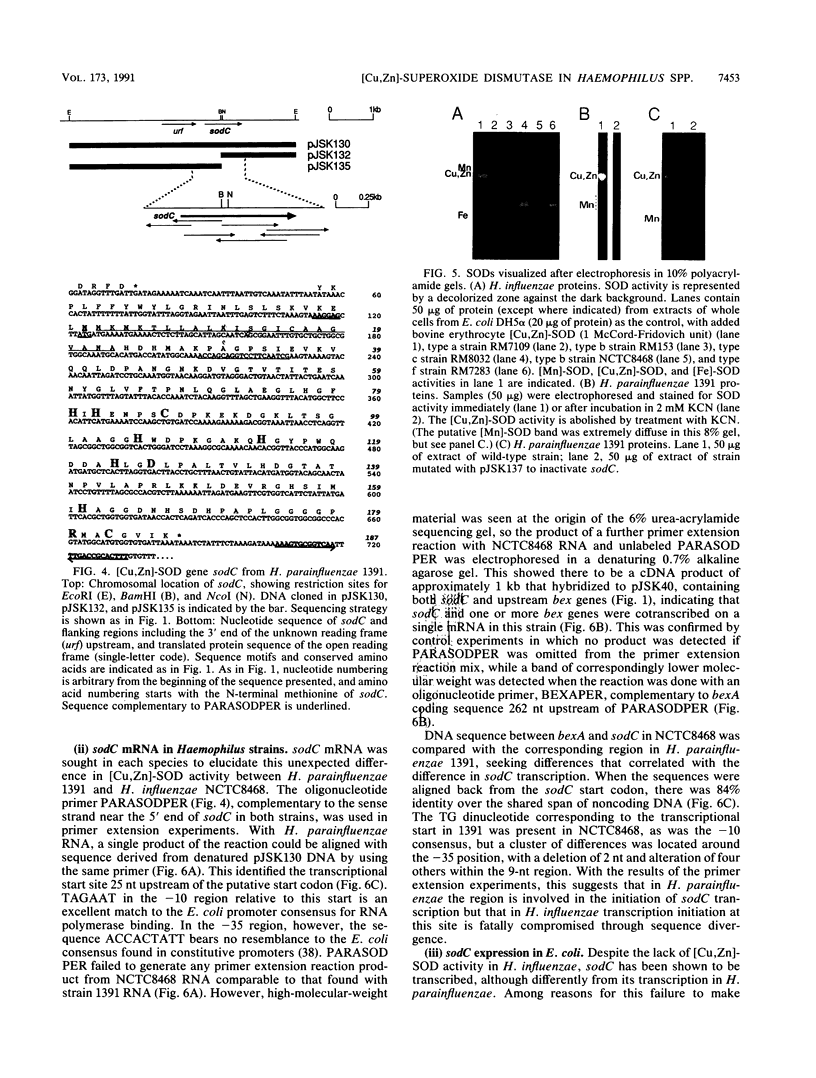
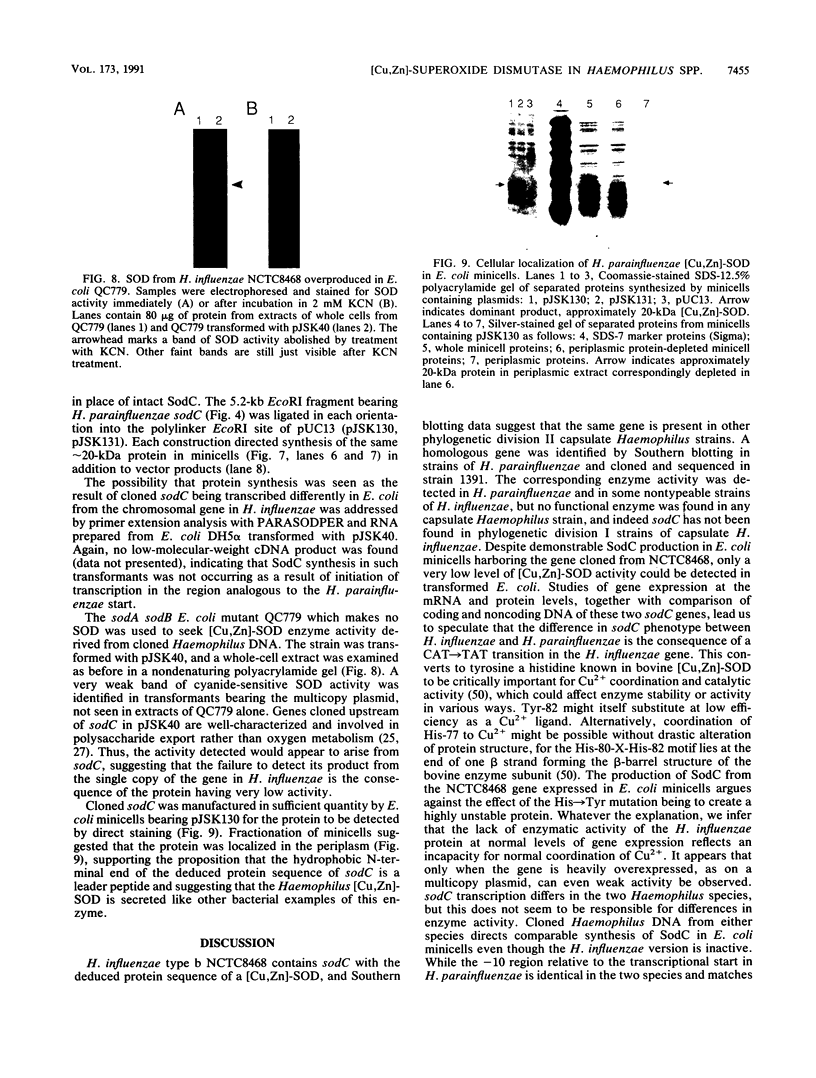
Copper-zinc superoxide dismutase ([Cu,Zn]-SOD) is widely found in eukaryotes but has only rarely been identified in bacteria. Here we describe sodC, encoding [Cu,Zn]-SOD in Haemophilus influenzae and H. parainfluenzae, frequent colonists and pathogens of the human respiratory tract. In capsulate H. influenzae, sodC was found in only one division of the bacterial population, and although the protein it encoded was clearly [Cu,Zn]-SOD from its deduced sequence, it lacked enzymatic activity. In H. parainfluenzae, in contrast, active enzyme was synthesized which appeared to be secreted beyond the cytoplasm when the gene was expressed in Escherichia coli minicells. The origin of gene transcription differed between the Haemophilus species, but protein synthesis from cloned genes in vitro was comparable. A C-T transition was found in the H. influenzae sequence compared with the H. parainfluenzae sequence, leading to a histidine, known to be crucial in eukaryotic [Cu,Zn]-SOD for copper ion coordination and so for enzymatic activity, to be changed to tyrosine. This is speculated to be the cause of inactivity of the H. influenzae enzyme. Secreted SODs have only been described in a few bacterial species, and this is the first identification of [Cu,Zn]-SOD in a common human upper respiratory tract colonist. The role of secreted bacterial SODs is unknown, and we speculate that in Haemophilus species the enzyme may confer survival advantage by accelerating dismutation of superoxide of environmental origin to hydrogen peroxide, disruptive to the normal mucociliary clearance process in the host.
Full text
PDF
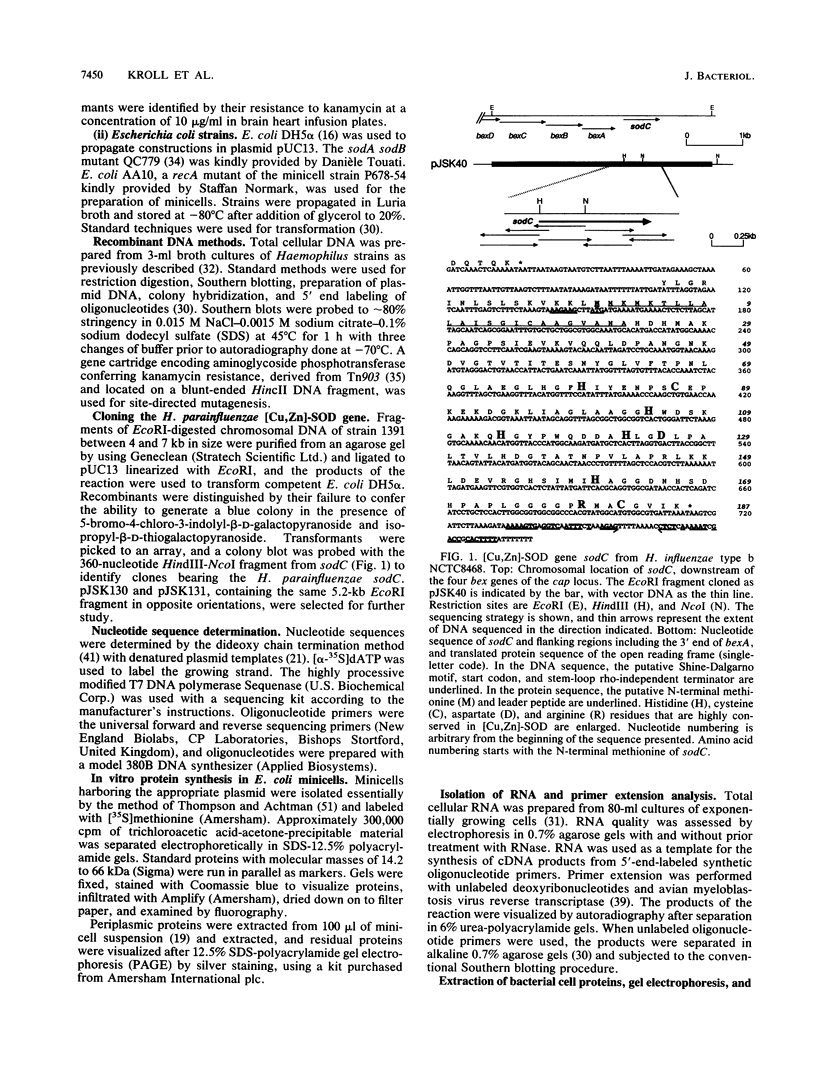




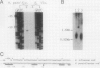
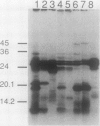
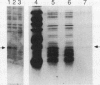
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannister J. V., Bannister W. H., Rotilio G. Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit Rev Biochem. 1987;22(2):111–180. doi: 10.3109/10409238709083738. [DOI] [PubMed] [Google Scholar]
- Beaman B. L., Scates S. M., Moring S. E., Deem R., Misra H. P. Purification and properties of a unique superoxide dismutase from Nocardia asteroides. J Biol Chem. 1983 Jan 10;258(1):91–96. [PubMed] [Google Scholar]
- Beaman L., Beaman B. L. Monoclonal antibodies demonstrate that superoxide dismutase contributes to protection of Nocardia asteroides within the intact host. Infect Immun. 1990 Sep;58(9):3122–3128. doi: 10.1128/iai.58.9.3122-3128.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beck B. L., Tabatabai L. B., Mayfield J. E. A protein isolated from Brucella abortus is a Cu-Zn superoxide dismutase. Biochemistry. 1990 Jan 16;29(2):372–376. doi: 10.1021/bi00454a010. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Broome-Smith J. K., Spratt B. G. A vector for the construction of translational fusions to TEM beta-lactamase and the analysis of protein export signals and membrane protein topology. Gene. 1986;49(3):341–349. doi: 10.1016/0378-1119(86)90370-7. [DOI] [PubMed] [Google Scholar]
- Burman W. J., Martin W. J., 2nd Oxidant-mediated ciliary dysfunction. Possible role in airway disease. Chest. 1986 Mar;89(3):410–413. doi: 10.1378/chest.89.3.410. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., McCord J. M., Fridovich I. Preparation and assay of superoxide dismutases. Methods Enzymol. 1978;53:382–393. doi: 10.1016/s0076-6879(78)53044-9. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dunlap P. V., Steinman H. M. Strain variation in bacteriocuprein superoxide dismutase from symbiotic Photobacterium leiognathi. J Bacteriol. 1986 Feb;165(2):393–398. doi: 10.1128/jb.165.2.393-398.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- Grace S. C. Phylogenetic distribution of superoxide dismutase supports an endosymbiotic origin for chloroplasts and mitochondria. Life Sci. 1990;47(21):1875–1886. doi: 10.1016/0024-3205(90)90399-c. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Visualization of catalase on acrylamide gels. Anal Biochem. 1974 Mar;58(1):57–62. doi: 10.1016/0003-2697(74)90440-0. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hartman J. R., Geller T., Yavin Z., Bartfeld D., Kanner D., Aviv H., Gorecki M. High-level expression of enzymatically active human Cu/Zn superoxide dismutase in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7142–7146. doi: 10.1073/pnas.83.19.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M. Microbial superoxide dismutases. Adv Genet. 1989;26:65–97. doi: 10.1016/s0065-2660(08)60223-0. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. M., Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalmarsson K., Marklund S. L., Engström A., Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll J. S., Hopkins I., Moxon E. R. Capsule loss in H. influenzae type b occurs by recombination-mediated disruption of a gene essential for polysaccharide export. Cell. 1988 May 6;53(3):347–356. doi: 10.1016/0092-8674(88)90155-9. [DOI] [PubMed] [Google Scholar]
- Kroll J. S., Loynds B. M., Moxon E. R. The Haemophilus influenzae capsulation gene cluster: a compound transposon. Mol Microbiol. 1991 Jun;5(6):1549–1560. doi: 10.1111/j.1365-2958.1991.tb00802.x. [DOI] [PubMed] [Google Scholar]
- Kroll J. S., Loynds B., Brophy L. N., Moxon E. R. The bex locus in encapsulated Haemophilus influenzae: a chromosomal region involved in capsule polysaccharide export. Mol Microbiol. 1990 Nov;4(11):1853–1862. doi: 10.1111/j.1365-2958.1990.tb02034.x. [DOI] [PubMed] [Google Scholar]
- Kroll J. S., Moxon E. R. Capsulation in distantly related strains of Haemophilus influenzae type b: genetic drift and gene transfer at the capsulation locus. J Bacteriol. 1990 Mar;172(3):1374–1379. doi: 10.1128/jb.172.3.1374-1379.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford P., Williams A. E., Kroll J. S. Superoxide dismutases of pathogenic and non-pathogenic Streptococcus suis type 2 isolates. FEMS Microbiol Lett. 1991 Jan 15;61(2-3):347–350. doi: 10.1016/0378-1097(91)90578-x. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Deich R. A., Connelly C. Cloning of chromosomal DNA from Haemophilus influenzae. Its use for studying the expression of type b capsule and virulence. J Clin Invest. 1984 Feb;73(2):298–306. doi: 10.1172/JCI111214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Kroll J. S., Moxon E. R., Selander R. K. Evolutionary genetics of the encapsulated strains of Haemophilus influenzae. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7758–7762. doi: 10.1073/pnas.85.20.7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natvig D. O., Imlay K., Touati D., Hallewell R. A. Human copper-zinc superoxide dismutase complements superoxide dismutase-deficient Escherichia coli mutants. J Biol Chem. 1987 Oct 25;262(30):14697–14701. [PubMed] [Google Scholar]
- Oka A., Sugisaki H., Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981 Apr 5;147(2):217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- Parker M. W., Blake C. C. Iron- and manganese-containing superoxide dismutases can be distinguished by analysis of their primary structures. FEBS Lett. 1988 Mar 14;229(2):377–382. doi: 10.1016/0014-5793(88)81160-8. [DOI] [PubMed] [Google Scholar]
- Puget K., Michelson A. M. Isolation of a new copper-containing superoxide dismutase bacteriocuprein. Biochem Biophys Res Commun. 1974 Jun 4;58(3):830–838. doi: 10.1016/s0006-291x(74)80492-4. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- Rather P. N., Moran C. P., Jr Compartment-specific transcription in Bacillus subtilis: identification of the promoter for gdh. J Bacteriol. 1988 Nov;170(11):5086–5092. doi: 10.1128/jb.170.11.5086-5092.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind G. B., Gould G. A., Ahmad F., Croughan M. J., Calder M. A. Haemophilus parainfluenzae and H influenzae respiratory infections: comparison of clinical features. Br Med J (Clin Res Ed) 1985 Sep 14;291(6497):707–708. doi: 10.1136/bmj.291.6497.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simurda M. C., van Keulen H., Rekosh D. M., LoVerde P. T. Schistosoma mansoni: identification and analysis of an mRNA and a gene encoding superoxide dismutase (Cu/Zn). Exp Parasitol. 1988 Oct;67(1):73–84. doi: 10.1016/0014-4894(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Sriranganathan N., Boyle S. M., Schurig G., Misra H. Superoxide dismutases of virulent and avirulent strains of Brucella abortus. Vet Microbiol. 1991 Feb 15;26(4):359–366. doi: 10.1016/0378-1135(91)90029-f. [DOI] [PubMed] [Google Scholar]
- Steinman H. M. Bacteriocuprein superoxide dismutase of Photobacterium leiognathi. Isolation and sequence of the gene and evidence for a precursor form. J Biol Chem. 1987 Feb 5;262(4):1882–1887. [PubMed] [Google Scholar]
- Steinman H. M. Bacteriocuprein superoxide dismutases in pseudomonads. J Bacteriol. 1985 Jun;162(3):1255–1260. doi: 10.1128/jb.162.3.1255-1260.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman H. M. Copper-zinc superoxide dismutase from Caulobacter crescentus CB15. A novel bacteriocuprein form of the enzyme. J Biol Chem. 1982 Sep 10;257(17):10283–10293. [PubMed] [Google Scholar]
- Steinman H. M., Ely B. Copper-zinc superoxide dismutase of Caulobacter crescentus: cloning, sequencing, and mapping of the gene and periplasmic location of the enzyme. J Bacteriol. 1990 Jun;172(6):2901–2910. doi: 10.1128/jb.172.6.2901-2910.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoscheck C. M. Increased uniformity in the response of the coomassie blue G protein assay to different proteins. Anal Biochem. 1990 Jan;184(1):111–116. doi: 10.1016/0003-2697(90)90021-z. [DOI] [PubMed] [Google Scholar]
- Tainer J. A., Getzoff E. D., Beem K. M., Richardson J. S., Richardson D. C. Determination and analysis of the 2 A-structure of copper, zinc superoxide dismutase. J Mol Biol. 1982 Sep 15;160(2):181–217. doi: 10.1016/0022-2836(82)90174-7. [DOI] [PubMed] [Google Scholar]
- Thompson R., Achtman M. The control region of the F sex factor DNA transfer cistrons: restriction mapping and DNA cloning. Mol Gen Genet. 1978 Oct 24;165(3):295–304. doi: 10.1007/BF00332530. [DOI] [PubMed] [Google Scholar]
- Van Camp W., Bowler C., Villarroel R., Tsang E. W., Van Montagu M., Inzé D. Characterization of iron superoxide dismutase cDNAs from plants obtained by genetic complementation in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9903–9907. doi: 10.1073/pnas.87.24.9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignais P. M., Terech A., Meyer C. M., Henry M. F. Isolation and characterization of a protein with cyanide-sensitive superoxide dismutase activity from the prokaryote, Paracoccus denitrificans. Biochim Biophys Acta. 1982 Mar 4;701(3):305–317. doi: 10.1016/0167-4838(82)90233-3. [DOI] [PubMed] [Google Scholar]