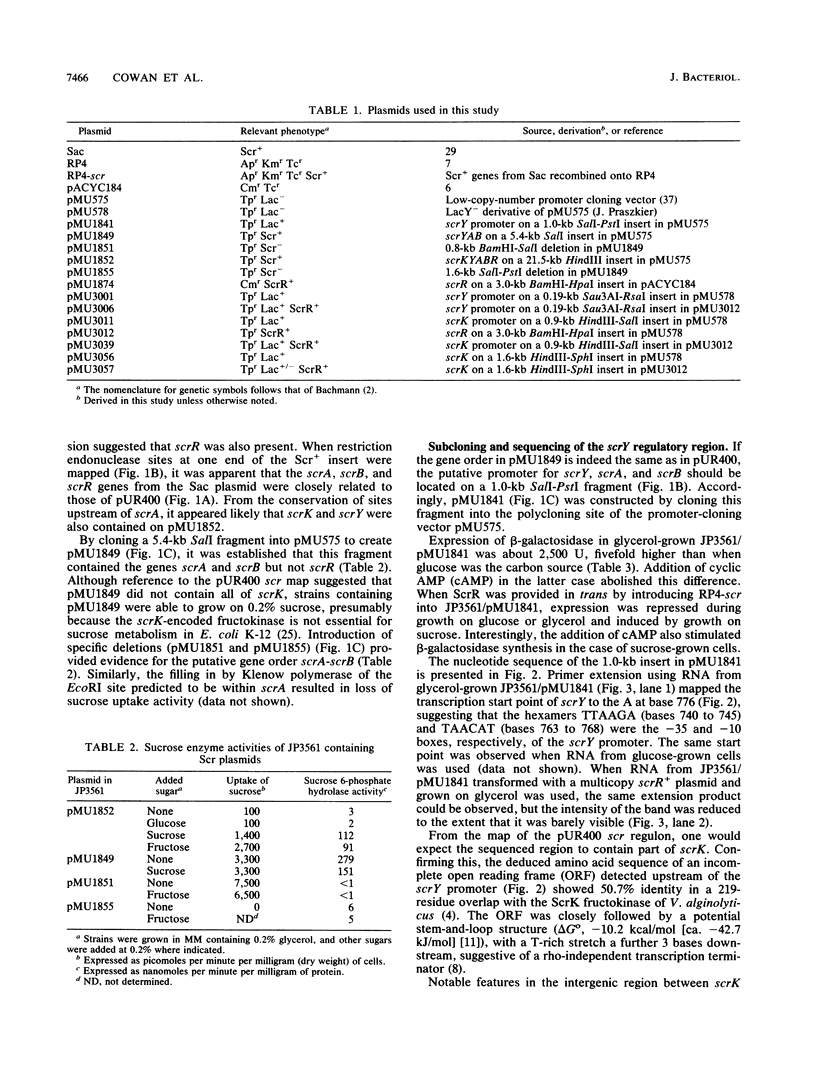
Abstract
Sucrose genes from a Salmonella thompson plasmid were cloned in Escherichia coli K-12. A physical map and a genetic map of the genes were constructed, revealing strong homology with the scr regulon from the Salmonella typhimurium plasmid pUR400. Two promoters were examined after being subcloned into transcriptional fusion vectors. Primer extension analysis and site-directed mutagenesis were used to identify the precise location of the promoter of scrY, scrA, and scrB. Transcription from this promoter was regulated over a 1,000-fold range by the combined effects of ScrR-mediated repression and catabolite repression. A putative cyclic AMP receptor protein binding site centered 72.5 bp upstream of the start point of transcription of scrY appeared to be essential for full activity of the scrY promoter. Transcription from the putative scrK promoter was far less sensitive to repression by ScrR. In ScrR+ cells, readthrough transcription from the putative scrK promoter into scrY accounted for less than 10% of scrY expression.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett K. H., Trust T. J. Plasmid-specified sucrose fermentation in Salmonella arizonae. J Gen Microbiol. 1980 Nov;121(1):255–257. doi: 10.1099/00221287-121-1-255. [DOI] [PubMed] [Google Scholar]
- Blatch G. L., Scholle R. R., Woods D. R. Nucleotide sequence and analysis of the Vibrio alginolyticus sucrose uptake-encoding region. Gene. 1990 Oct 30;95(1):17–23. doi: 10.1016/0378-1119(90)90408-j. [DOI] [PubMed] [Google Scholar]
- Blatch G. L., Woods D. R. Nucleotide sequence and analysis of the Vibrio alginolyticus scr repressor-encoding gene (scrR). Gene. 1991 May 15;101(1):45–50. doi: 10.1016/0378-1119(91)90222-w. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner R., Lengeler J. W. DNA sequence of the gene scrA encoding the sucrose transport protein EnzymeII(Scr) of the phosphotransferase system from enteric bacteria: homology of the EnzymeII(Scr) and EnzymeII(Bgl) proteins. Mol Microbiol. 1988 Jan;2(1):9–17. [PubMed] [Google Scholar]
- Freier S. M., Kierzek R., Jaeger J. A., Sugimoto N., Caruthers M. H., Neilson T., Turner D. H. Improved free-energy parameters for predictions of RNA duplex stability. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9373–9377. doi: 10.1073/pnas.83.24.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston K., Bell A., Kolb A., Buc H., Busby S. Stringent spacing requirements for transcription activation by CRP. Cell. 1990 Aug 24;62(4):733–743. doi: 10.1016/0092-8674(90)90118-x. [DOI] [PubMed] [Google Scholar]
- Gaston K., Kolb A., Busby S. Binding of the Escherichia coli cyclic AMP receptor protein to DNA fragments containing consensus nucleotide sequences. Biochem J. 1989 Jul 15;261(2):649–653. doi: 10.1042/bj2610649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardesty C., Ferran C., DiRienzo J. M. Plasmid-mediated sucrose metabolism in Escherichia coli: characterization of scrY, the structural gene for a phosphoenolpyruvate-dependent sucrose phosphotransferase system outer membrane porin. J Bacteriol. 1991 Jan;173(2):449–456. doi: 10.1128/jb.173.2.449-456.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson G. S., Davidson B. E. Nucleotide sequence and transcription of the phenylalanine and tyrosine operons of Escherichia coli K12. J Mol Biol. 1984 Dec 25;180(4):1023–1051. doi: 10.1016/0022-2836(84)90269-9. [DOI] [PubMed] [Google Scholar]
- Kieny M. P., Lathe R., Lecocq J. P. New versatile cloning and sequencing vectors based on bacteriophage M13. Gene. 1983 Dec;26(1):91–99. doi: 10.1016/0378-1119(83)90039-2. [DOI] [PubMed] [Google Scholar]
- Lengeler J. W., Mayer R. J., Schmid K. Phosphoenolpyruvate-dependent phosphotransferase system enzyme III and plasmid-encoded sucrose transport in Escherichia coli K-12. J Bacteriol. 1982 Jul;151(1):468–471. doi: 10.1128/jb.151.1.468-471.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Palchaudhuri S., Rahn S., Santos D. S., Maas W. K. Characterization of plasmids in a sucrose-fermenting strain of Escherichia coli. J Bacteriol. 1977 Jun;130(3):1402–1403. doi: 10.1128/jb.130.3.1402-1403.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Poy F., Jacobson G. R., Kuramitsu H. K. Characterization and sequence analysis of the scrA gene encoding enzyme IIScr of the Streptococcus mutans phosphoenolpyruvate-dependent sucrose phosphotransferase system. J Bacteriol. 1989 Jan;171(1):263–271. doi: 10.1128/jb.171.1.263-271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K., Ebner R., Altenbuchner J., Schmitt R., Lengeler J. W. Plasmid-mediated sucrose metabolism in Escherichia coli K12: mapping of the scr genes of pUR400. Mol Microbiol. 1988 Jan;2(1):1–8. doi: 10.1111/j.1365-2958.1988.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Schmid K., Ebner R., Jahreis K., Lengeler J. W., Titgemeyer F. A sugar-specific porin, ScrY, is involved in sucrose uptake in enteric bacteria. Mol Microbiol. 1991 Apr;5(4):941–950. doi: 10.1111/j.1365-2958.1991.tb00769.x. [DOI] [PubMed] [Google Scholar]
- Schmid K., Schupfner M., Schmitt R. Plasmid-mediated uptake and metabolism of sucrose by Escherichia coli K-12. J Bacteriol. 1982 Jul;151(1):68–76. doi: 10.1128/jb.151.1.68-76.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholle R. R., Robb S. M., Robb F. T., Woods D. R. Nucleotide sequence and analysis of the Vibrio alginolyticus sucrase gene (scrB). Gene. 1989 Aug 1;80(1):49–56. doi: 10.1016/0378-1119(89)90249-7. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Parsell Z. Transmissible substrate-utilizing ability in enterobacteria. J Gen Microbiol. 1975 Mar;87(1):129–140. doi: 10.1099/00221287-87-1-129. [DOI] [PubMed] [Google Scholar]
- Sprenger G. A., Lengeler J. W. Analysis of sucrose catabolism in Klebsiella pneumoniae and in Scr+ derivatives of Escherichia coli K12. J Gen Microbiol. 1988 Jun;134(6):1635–1644. doi: 10.1099/00221287-134-6-1635. [DOI] [PubMed] [Google Scholar]
- Staden R. Further procedures for sequence analysis by computer. Nucleic Acids Res. 1978 Mar;5(3):1013–1016. doi: 10.1093/nar/5.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Sequence data handling by computer. Nucleic Acids Res. 1977 Nov;4(11):4037–4051. doi: 10.1093/nar/4.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Le Coq D., Aymerich S. Induction of saccharolytic enzymes by sucrose in Bacillus subtilis: evidence for two partially interchangeable regulatory pathways. J Bacteriol. 1989 Mar;171(3):1519–1523. doi: 10.1128/jb.171.3.1519-1523.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P., Holst B., Søgaard-Andersen L., Martinussen J., Nesvera J., Douthwaite S. R. Design of cAMP-CRP-activated promoters in Escherichia coli. Mol Microbiol. 1991 Feb;5(2):433–437. doi: 10.1111/j.1365-2958.1991.tb02126.x. [DOI] [PubMed] [Google Scholar]
- Wehmeier U., Sprenger G. A., Lengeler J. W. The use of lambda plac-Mu hybrid phages in Klebsiella pneumoniae and the isolation of stable Hfr strains. Mol Gen Genet. 1989 Feb;215(3):529–536. doi: 10.1007/BF00427052. [DOI] [PubMed] [Google Scholar]
- Wohlhieter J. A., Lazere J. R., Snellings N. J., Johnson E. M., Synenki R. M., Baron L. S. Characterization of transmissible genetic elements from sucrose-fermenting Salmonella strains. J Bacteriol. 1975 May;122(2):401–406. doi: 10.1128/jb.122.2.401-406.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Pittard J. Molecular analysis of the regulatory region of the Escherichia coli K-12 tyrB gene. J Bacteriol. 1987 Oct;169(10):4710–4715. doi: 10.1128/jb.169.10.4710-4715.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Aubenton Carafa Y., Brody E., Thermes C. Prediction of rho-independent Escherichia coli transcription terminators. A statistical analysis of their RNA stem-loop structures. J Mol Biol. 1990 Dec 20;216(4):835–858. doi: 10.1016/s0022-2836(99)80005-9. [DOI] [PubMed] [Google Scholar]
- de Crombrugghe B., Busby S., Buc H. Cyclic AMP receptor protein: role in transcription activation. Science. 1984 May 25;224(4651):831–838. doi: 10.1126/science.6372090. [DOI] [PubMed] [Google Scholar]



