Abstract
The Bacillus subtilis divIVA gene encodes a coiled-coil protein that shows weak similarity to eukaryotic tropomyosins. The protein is targeted to the sites of cell division and mature cell poles where, in B.subtilis, it controls the site specificity of cell division. Although clear homologues of DivIVA are present only in Gram-positive bacteria, and its role in division site selection is not conserved in the Gram-negative bacterium, Escherichia coli, a DivIVA–green fluorescent protein (GFP) fusion was targeted accurately to division sites and retained at the cell pole in this organism. Remarkably, the same fusion protein was also targeted to nascent division sites and growth zones in the fission yeast Schizosaccharomyces pombe, mimicking the localization of the endogenous tropomyosin-like cell division protein Cdc8p, and F-actin. The results show that a targeting signal for division sites is conserved across the eukaryote–prokaryote divide.
Keywords: Bacillus subtilis/DivIVA protein/Escherichia coli/fission yeast/tropomyosin
Introduction
Like most other organisms, bacteria proliferate by binary fission. In rod-shaped bacteria, such as Bacillus subtilis and Escherichia coli, division is accomplished by an annular invagination of the cell wall and membrane layers, which is usually placed mid way along the long axis of the cell, giving two daughters of equal size. The mechanisms responsible for division are largely unknown, although many genes required for division have been characterized (reviewed in Bramhill, 1997; Lutkenhaus and Addinall, 1997). Some of the genes were identified by mutations giving a temperature-sensitive filamentous phenotype (fts or divI genes). The precise functions of the products of the division genes are largely unknown. However, FtsZ seems to play a critical role in initiation of division, forming a ring at the site of cell constriction, which apparently contracts as the annulus closes (Bi and Lutkenhaus, 1991; Addinall and Lutkenhaus, 1996; Sun and Margolin, 1998). FtsZ has GTPase activity and can form sheets and filaments similar to those of eukaryotic tubulins, suggesting that it may be a tubulin-like cytoskeletal element (Bramhill and Thompson, 1994; Mukherjee and Lutkenhaus, 1994, 1998; Erickson et al., 1996). The only other bacterial division protein with a known function is the FtsI or PBP3 protein, which is required for the final steps of peptidoglycan synthesis in the septum (Ghuysen, 1991).
The divIVA gene of B.subtilis was identified originally on the basis of a mutation that led to a major reduction in septation frequency and to the formation of some aberrant polar divisions producing small anucleate minicells (Reeve et al., 1973; Mendelson, 1975). More recent work has shown that DivIVA affects division indirectly through the Min system, which is a mechanism that controls the site specificity of cell division (Levin et al., 1992; Varley and Stewart, 1992; Lee and Price, 1993; Cha and Stewart, 1997; Edwards and Errington, 1997; Marston et al., 1998; Marston and Errington, 1999). DivIVA is targeted to the sites of cell division, requiring most of the known cell division genes for this activity. However, unlike other cell division proteins, DivIVA is retained at the mature cell poles after division has been completed. Polar DivIVA helps to sequester a conserved bipartite inhibitor of cell division, comprising the MinC and MinD proteins, at the cell poles. MinCD then prevents division from occurring at these sites, which would produce a minicell. Intriguingly, even though the MinC and MinD proteins are highly conserved and carry out a similar minicell inhibition function in E.coli (De Boer et al., 1989, 1992), the localization of MinCD in E.coli is determined in a quite different manner by MinE protein, which is completely unrelated to DivIVA. MinE forms a central ring-like structure independently of FtsZ but dependent on MinD (Raskin and De Boer, 1997). The role of MinD in directing localization of MinE involves a remarkable pole–pole oscillation, the precise function of which is not quite clear (Hu and Lutkenhaus, 1999; Raskin and De Boer, 1999a,b). Although we cannot exclude the possibility that E.coli uses a DivIVA-like function, in addition to MinE, to control MinCD localization, the dynamic nature of MinD localization would appear to exclude this, and there is no hint of a DivIVA-like protein in the E.coli genome sequence. Despite the lack of an obvious functional homologue of DivIVA, we now show that the pattern of targeting of a DivIVA–green fluorescent protein (GFP) fusion is almost identical in the Gram-negative bacterium, E.coli, to that in its natural Gram-positive host cell.
Analysis of the predicted amino acid sequence of DivIVA has revealed likely homologues in a range of Gram-positive bacteria but not in more distant organisms. Weaker similarities and structural predictions suggest that DivIVA protein has a predominantly coiled-coil structure, particularly resembling tropomyosins, a family of cytoskeletal proteins, found throughout the eukaryotes, which bind to and stabilize F-actin (Smillie, 1979). Intriguingly, the cdc8 gene of fission yeast, Schizosaccharomyces pombe, which encodes one of the eukaryotic proteins most similar to DivIVA, is also required for cell division (Balasubramanian et al., 1992). Its pattern of localization is similar to that of F-actin, occurring at both medial division sites and polar growth zones (Marks and Hyams, 1985; Balasubramanian et al., 1992). To test the possibility that DivIVA targeting to division sites and cell poles is conserved beyond the bacteria, we introduced the DivIVA–GFP fusion into various strains of S.pombe. Remarkably, we have found that expression of the fusion in fission yeast leads to a specific pattern of localization that is similar to those of F-actin and the cdc8 product. These results provide strong evidence for broad conservation of a protein targeting mechanism used to bring certain proteins to nascent and used division sites.
Results
Similarity of DivIVA to α-helical coiled-coil proteins
One of the most important remaining questions about the function of DivIVA in B.subtilis concerns the mechanism whereby it targets to division sites and is retained at these sites in mature cell poles. Sequence database searches of the predicted protein sequence showed that it has likely homologues among a range of Gram-positive bacteria, including closely related organisms, such as Staphylococcus aureus and Streptococcus pneumoniae (Figure 1A), but also the more distant, high G+C Gram-positives, Streptomyces and Mycobacterium (not shown). In at least S.aureus (Chalker et al., 1994) and apparently also Enterococcus faecalis, S.pneumoniae and Streptococcus pyogenes, the protein is encoded by a gene that lies in an identical chromosomal position to that of B.subtilis divIVA, immediately upstream of a conserved gene, ileS, encoding isoleucyl-tRNA synthetase. In general, however, B.subtilis DivIVA is shorter than the other proteins (164 amino acids, as opposed to 200 or more). In addition to weaker similarities to various other prokaryotic proteins, of generally unknown function, weak similarities were detected to a large number of eukaryotic proteins. The unifying feature of these proteins, which included myosins, utrophin and particularly tropomyosins, seemed to be their α-helical coiled-coil structure. The structure of rabbit tropomyosin has been solved (Phillips et al., 1979, 1986). The protein has a classical coiled-coil configuration (reviewed by Lupas, 1996). It folds as a single long α-helix. In solution it is dimeric, the two subunits lying side by side in a parallel configuration, held together mainly by hydrophobic interactions. This general structure is characterized by a repeating heptad motif with the amino acid side chains being predominantly hydrophobic at the first (a) and fourth (d) positions of each heptad, and hydrophilic elsewhere (Lupas et al., 1991). Tests using the programme COILS (Lupas et al., 1991) supported the idea that DivIVA and its relatives also have a high likelihood of having a largely α-helical coiled-coil structure. Figure 1A highlights the repeating motif (a and d positions labelled as directed by the COILS output), which extends over virtually the whole of the region conserved among the bacterial proteins. (Note that in such coiled-coil proteins it is usual for a few of the a and d positions to contain charged residues.) The arrow in Figure 1A points to the position of a ‘skip’ residue (see Lupas, 1996) introduced to optimize the phase of the heptad repeat, as indicated by the COILS output. In support of this interpretation of DivIVA structure, the two divIVA missense mutations that have been isolated so far, each of which generates an alanine to threonine substitution, are both located at proposed hydrophobic core positions within the coiled-coil domain (circles above the sequence in Figure 1A) (Edwards and Errington, 1997; J.Sievers and J.Errington, unpublished results).
Fig. 1. Alignments and structural predictions for DivIVA-like proteins. Proteins similar to DivIVA were identified by BLAST searches and a multiple alignment was generated by CLUSTAL W. Each protein was then tested for the presence of a likely coiled-coil region and the letters a and d were placed above the alignment at positions directed by the COILS output (with minor adjustments to accord with the alignment). The arrow marks the position of a skip residue needed to maintain the phase of the heptad repeat (Lupas, 1996).
A 24 amino acid N-terminal region that is probably not α-helical, based both on the COILS output and the presence of helix-breaking proline residues, is well conserved among the bacterial proteins but does not seem to be conserved in eukaryotes.
Targeting of DivIVA to division sites and cell poles in E.coli
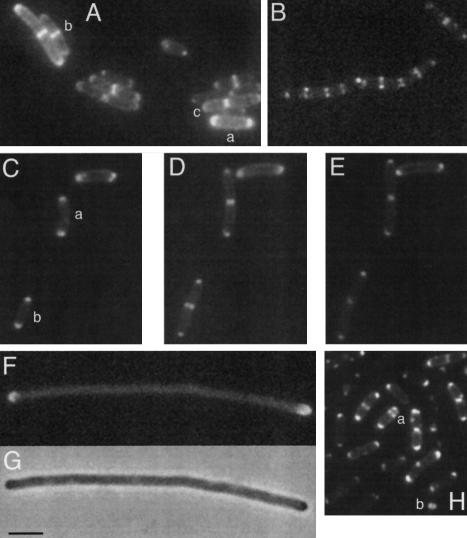
As mentioned above, E.coli does not have an overt homologue of divIVA. We were therefore surprised to find that the divIVA–gfp construct we originally made to examine localization of DivIVA in B.subtilis showed apparent targeting of the product to division sites and mature cell poles in E.coli. To improve the images obtained, we used slow growing E.coli cells cultivated on LB plates or in M9 salts minimal medium (average doubling time ∼80 min in the latter medium). Figure 2A shows a typical field of cells expressing the fusion protein. Virtually all of the cells showed an arc of DivIVA–GFP fluorescence around each pole (e.g. cell labelled a). The longer cells often showed a band of fluorescence at mid-cell (e.g. two adjacent cells labelled b). In some such cells, the band was slightly narrowed and this usually coincided with a clear constriction of the cell (labelled c), indicating that the cell was in the process of dividing. This pattern of localization was similar to that previously reported for DivIVA–GFP in B.subtilis (Figure 2B; Edwards and Errington, 1997).
Fig. 2. DivIVA–GFP is targeted to division sites and cell poles in E.coli. Wild-type and mutant strains of E.coli expressing a DivIVA–GFP fusion were visualized by immunofluorescence microscopy. (A) DivIVA–GFP localization in a static field of E.coli cells (strain D4A). (B) DivIVA–GFP localization in a field of B.subtilis for comparison (strain 1757; see Edwards and Errington, 1997). (C–E) Time lapse images of a field of cells of E.coli cells (strain D4A), with (D) and (E) being taken, respectively, 40 and 80 min after (C). (F and G) DivIVA–GFP localization in filamentous cells of an ftsZts mutant of E.coli (strain Z4A) at the non-permissive temperature. (H) DivIVA–GFP localization in a minB mutant of E.coli (strain M4A). Scale bar represents 2 µm.
To follow the behaviour of DivIVA during cell cycle progression in E.coli, we used time lapse microscopy. Figure 2C–E shows the development of three cells each of which showed fluorescence at both poles to begin with (Figure 2C). After 40 min, when the cells had clearly increased in length, cells a and b both had mid-cell bands of fluorescence, corresponding to the sites of incipient cell division (Figure 2D). By 80 min, these cells had almost completed cell division, as visible from the constrictions at mid-cell (Figure 2E). The fluorescent bands, which were clearly associated with these sites, now had an appearance quite similar to the pattern shown by the mature cell poles. Note also that in one cell that did not grow appreciably during the time course, no change in the GFP pattern occurred.
To show that localization of DivIVA to the mid-cell bands was associated with assembly of the division apparatus, we introduced the divIVA–gfp fusion into a strain carrying the temperature-sensitive mutation, ftsZ84. To preserve the function of the DivIVA–GFP fusion protein, we grew cells of the new strain (Z4A) to mid-exponential phase in M9 salts at 30°C and examined them on agar-coated slides incubated at 37°C. The subsequent reduction of FtsZ function resulted in formation of elongated aseptate filaments. As shown in Figure 2F and G, 4 h after the temperature shift the DivIVA–GFP had clearly maintained its association with the mature poles of the long filaments, but there were no longer any detectable bands within the filaments (50 filaments counted). These results showed that B.subtilis DivIVA is targeted to division sites of E.coli in an FtsZ-dependent manner. As in B.subtilis, it also seems that the protein remains associated with mature poles after division is complete and other members of the division machinery have disappeared. This contrasts strikingly with the FtsZ-independent ring formation by MinE in E.coli (Raskin and De Boer, 1997).
DivIVA–GFP can be recruited to minicell septa
Interestingly, while DivIVA–GFP had maintained its ability to recognize and target to division sites and cell poles in E.coli, it did not appear to affect the correct functioning of the Min system. This was judged from the absence of any filamentous or minicell phenotype in a wild-type strain expressing the fusion. To investigate further the possibility of an interaction with the E.coli Min system, we transformed the divIVA–gfp construct into a minicell mutant (to give strain M4A). Again the targeting of DivIVA was retained (Figure 2H), with the fusion protein localizing to both central and polar (minicell) septa (cell labelled a). Interestingly, minicells were sometimes seen to contain rings of DivIVA (labelled b). We conclude that DivIVA–GFP is recruited to division sites in the absence of a functional Min system. Thus, as in B.subtilis, the subcellular targeting of DivIVA–GFP in E.coli is dependent on FtsZ, and independent of the MinCD system. However, divIVA does not seem to be able to substitute for minE, as we were not able to detect functional complementation in two different minE strains (D.H.Edwards, unpublished results).
Targeting of DivIVA–GFP to the division septum and growth zones in fission yeast
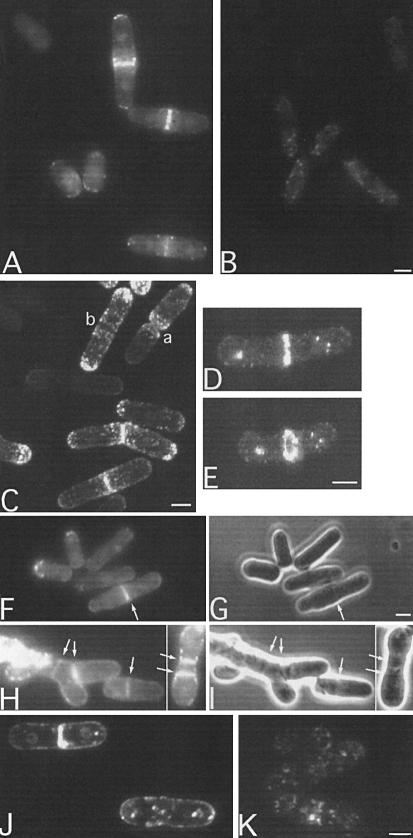
Having found that targeting of DivIVA to cell poles and division sites was rather promiscuous, we were intrigued by the apparent similarities between DivIVA and the Cdc8 protein of S.pombe (Figure 1A). Although many of the similar eukaryotic sequences were from large proteins with long rather repetitive coiled-coil structures, such as myosin, the subfamily of tropomyosins includes proteins of similar size to DivIVA and its Gram-positive homologues. Furthermore, as mentioned above, cdc8 mutants are known to be affected in cell division, and the cdc8 product is targeted to division sites (as well as at the growing poles of the cell) in a manner reminiscent of that of DivIVA (Balasubramanian et al., 1992). As one means of testing for a functional relationship between DivIVA and Cdc8p, we introduced the divIVA–gfp fusion construct and a control fusion made with a truncated copy of divIVA into fission yeast on an expression plasmid, pREP1. Transformant colonies were grown in the absence of thiamine to induce expression from the nmt1 promoter controlling expression of the fusion, and then samples of the cells were observed by fluorescence microscopy. No specific localization was detected in cells bearing the control fusion, with a truncated form of DivIVA fused to GFP (Figure 3B). Remarkably, however, the full-length DivIVA–GFP fusion protein showed a highly localized distribution. Most strikingly, cells that appeared to be in the process of division showed a distinct band of fluorescence across the cell at the site of division (Figure 3A). The cells also frequently showed some speckling that appeared on shifting focus to be associated with the outer, cortical part of the cell. The level of expression of the fusion was variable from cell to cell (because of variability in the copy number of the plasmid; J.Mellor, personal communication), and the brightest cells, presumably having the highest levels of divIVA–gfp expression, sometimes showed staining throughout the cell periphery, including the septum, if present (not shown).
Fig. 3. Targeting of DivIVA–GFP to growth zones and cytokinetic rings in wild-type and mutant strains of S.pombe. All cultures were grown in the absence of leucine and thiamine. (A) Schizosaccharomyces pombe expressing full-length DivIVA fused to GFP (strain ESP1). (B) Schizosaccharomyces pombe expressing a truncated form of DivIVA fused to GFP (strain ESP2). (C) Confocal view of S.pombe strain ESP1 expressing full-length DivIVA fused to GFP. (D) As (C). Close up (1.5× magnified) of a typical cell with a DivIVA–GFP band. (E) Rotation of (D), showing the ring-like configuration of the DivIVA–GFP band. (F–I) Fluorescence (F and H) and phase-contrast (G and I) images of typical cdc8 mutant cells (strain 1815) expressing divIVA–gfp at the permissive (30°C; F and G) and non-permissive temperature (37°C; H and I). Arrows point to the positions of septa. (J and K) Effect of Lat-A on localization of DivIVA–GFP. Live cells of strain ESP1 were visualized either untreated (J) or 30 min after treatment with Lat-A (K). Scale bars represents 4 µm.
To clarify the pattern of localization of DivIVA–GFP, we turned to confocal microscopy. Figure 3C shows a field of cells showing representative cells of the main classes observed. In cells with a deep division furrow, both of the new poles were fluorescent (e.g. cells marked a). Some longer non-dividing cells had a bipolar distribution (b). This would be expected if the protein were associated with growth zones, since, after division, growth is from the new pole only, whereas after new end take off (NETO), bipolar growth ensues (Mitchison and Nurse, 1985; Marks et al., 1987). The general pattern of localization was reminiscent of that of F-actin (Marks and Hyams, 1985) and of the product of the cdc8 gene (Balasubramanian et al., 1992). An enlarged view of a typical dividing cell with a band at mid-cell is shown in Figure 3D. Rotation of such images (Figure 3E) revealed that the DivIVA–GFP was distributed in the form of a ring, strongly suggesting that the protein co-localizes with the contractile ring during division.
To test more directly for function of DivIVA in S.pombe, we introduced the full-length and truncated divIVA–gfp fusions, and a construct expressing divIVA only, into a cdc8 mutant of S.pombe. The resultant strains (1815, 1816 and 1817) were incubated at the non-permissive temperature in the absence of thiamine and tested for complementation on the basis of both growth (optical density) and microscopic appearance. Unfortunately, no evidence for functional complementation was obtained, even though western blot analysis showed that intact DivIVA or DivIVA–GFP was being expressed at significant levels (not shown). However, the cdc8 mutant background also provided us with an opportunity to examine the localization of the DivIVA–GFP fusion protein when cell division was perturbed. As shown in Figure 3F and G, at the permissive temperature, cell shape was more or less normal and DivIVA–GFP bands were again associated with the division septum (arrowed). At the non-permissive temperature, the cdc8 mutant displayed a range of abnormal cell shapes, with multiple septa-like structures, often tilted, and the cells tended to accumulate in this state (Nurse et al., 1976). Strikingly, the DivIVA–GFP fusion protein was still seen at septa, irrespective of their position or orientation (Figure 3H and I). Of >50 cells with a distinct GFP signal that were observed, all contained one or more fluorescent bands of DivIVA–GFP, which always co-localized with the septa. Again, no such ring structures were detected with the truncated form of DivIVA fused to GFP, even though western analysis showed that the proteins were expressed to similar levels (not shown).
The precise pattern of DivIVA–GFP in cdc8 mutant cells differed slightly from that of the wild type in that the speckles appeared to be generally smaller and more dispersed. A similar change in pattern has been reported for cortical actin patches in cdc8 mutants (Gould and Simanis, 1997; Rupes et al., 1999). To check for a direct association between DivIVA–GFP and actin, we treated wild-type cells containing the fusion with latrunculin-A (Lat-A), which rapidly disrupts the actin cytoskeleton (Ayscough et al., 1997). Although the staining pattern for actin became completely diffuse after this treatment (not shown), only minor changes in the DivIVA localization pattern were observed (Figure 3J and K). The clearest difference was that the speckles of fluorescence became less tightly associated with the cortical region of the cell after Lat-A treatment (Figure 3K). The fact that a distinct pattern of DivIVA–GFP localization was retained in the absence of polymerized actin suggests either that DivIVA does not interact directly with actin or that, once targeted, it can retain its localization in the absence of actin.
Discussion
The chromosomal context of the divIVA-like genes in Gram-positive bacteria strongly suggests that these genes are homologous and that they are derived from a common ancestor. The predicted products show relatively poor primary amino acid conservation but they do contain a conserved pattern of predominantly hydrophobic residues that constitutes a pseudo-heptad repeat. Computer predictions strongly suggest that the central region of this polypeptide could form an α-helical coiled-coil in vivo. Such structures have a variety of roles, but are commonly used as a means of forming dimeric or oligomeric structures, as in tropomyosin and the dimeric CRP transcription factor (Lupas et al., 1991).
In B.subtilis, DivIVA has a characteristic pattern of localization in which it first associates with the division apparatus ahead of cell division, and then remains associated with the new cell pole as it changes shape from a flat disc, when the septum is first completed, to a hemisphere when cell separation has occurred. The pattern of localization in E.coli was remarkably similar, even though the mechanism of division is somewhat different, with cytoplasmic membrane, wall and outer membrane layers all constricting in parallel. Consequently, in the images shown in Figure 2A, the mid-cell bands and polar spots were evident in virtually all of the cells. Furthermore, when division site selection was perturbed by a minB mutation, the DivIVA–GFP continued to follow the division machinery to both normal and abnormal positions (Figure 2H). As in B.subtilis, this targeting required the division apparatus, or at least the tubulin-like FtsZ protein, because when FtsZ was inactivated in the ftsZ84 mutant of E.coli, DivIVA targeting to potential division sites no longer occurred (Figure 2F and G). More work is needed to pin down the precise point in assembly of the division apparatus at which DivIVA recruitment occurs in E.coli.
Even more remarkably, strong targeting to division sites was also seen in the eukaryote, S.pombe. Again, the targeting was probably specific because it was retained in cells of a mutant (cdc8), in which division positioning and orientation are perturbed. It is interesting to note that several known septum-associated proteins fail to target in a cdc8 mutant (Gould and Simanis, 1997), so at least some components of the eukaryotic division machinery are not needed for recruitment of DivIVA to the septum. It will be interesting to determine the precise point in the hierarchy of assembly of the S.pombe division machinery at which DivIVA is recruited.
The extreme conservation of DivIVA targeting to the septum across a huge evolutionary divide was unexpected, because, at present, there is little sequence similarity between known components of the prokaryotic and eukaryotic division complexes. The central protein in bacterial cell division, FtsZ, is now recognized as a homologue of eukaryotic tubulin (Nogales et al., 1998). Although microtubules play a much more prominent role in chromosome segregation, via the mitotic spindle, it now seems that they are associated, at least transiently, with the cytokinetic ring in S.pombe (reviewed by Hagan, 1998) and probably other eukaryotes (e.g. Shu et al., 1995). DivIVA–GFP was clearly not recruited to the spindle, but it did target to other sites that strongly resemble the localization of the cortical actin patches that are found at growth zones (Marks and Hyams, 1985; Arai et al., 1998). In principle, actin is a good candidate for the conserved target for DivIVA, since it is known to participate in cytokinesis and to interact with tropomyosins. Moreover, at least one protein with weak similarity to actin is involved in bacterial division, FtsA (Kabsch and Holmes, 1995). FtsA is also strongly conserved between E.coli and B.subtilis (35% identical residues across the whole protein). Estimates of the levels of FtsA in bacterial cells suggest an abundance of between 50 and 200 molecules per cell (Wang and Gayda, 1992), which appears to prohibit it from having a role analogous to that of eukaryotic actin. Experiments with Lat-A, which disrupts filamentous actin, did not affect DivIVA–GFP localization substantially (Figure 3J and K). However, this result is difficult to interpret because Ayscough et al. (1997) have shown that a range of proteins thought to interact directly or indirectly with actin vary considerably in their responses to Lat-A.
Although we favour the idea that DivIVA interacts with one or more components of the division machinery, it is also possible that it is attracted to division sites by some other mechanism. For example, the cell poles could have some biophysical property that sets them apart from the rest of the cell. While this question remains open, experiments with round cell mutants have suggested that simple curvature of the cell membrane is not sufficient to elicit DivIVA targeting (D.H.Edwards, unpublished results).
Whatever the mechanism of targeting, it is evident that the nature of the interaction between DivIVA and its target is an unusual one that does not require a high level of primary sequence conservation. This may be a typical feature of coiled-coil proteins because we have observed a striking lack of sequence conservation in another B.subtilis protein of this class, FtsL (J.Sievers and J.Errington, unpublished results). In principle, target specificity could arise through multiple relatively weak interactions, with most or all individual interactions being redundant. Determining how target specificity is preserved over a huge evolutionary distance is now an important challenge.
Materials and methods
Bacterial and yeast strains
The microbial strains and plasmids used are listed in Table I.
Table I. Bacterial and yeast strains and plasmids.
| Strain/plasmid |
Relevant genotype |
Source/reference |
| E.coli | ||
| DH5α | F– endA1 hsdR17 supE44 thi-1 λ– recA1 gyrA96 relA1 Δ(lacZYA-argF)U169 φ80dlacZΔM15 | Gibco-BRL |
| P678-54 | thy thr leu lacY minA minB gal str thi | Adler et al. (1967) |
| PAT84 | ftsZ84(ts) thr-1 leu6 thi argH1 thy his trp rpsL lacY1malA1 xyl7mtl-2 mel tonA2 supE44 | Hirota et al. (1968) |
| D4A | F– endA1 hsdR17 supE44 thi-1 λ– recA1 gyrA96 relA1 Δ(lacZYA-argF)U169 φ80dlacZΔM15 (pSG1044) divIVA-gfpb | this work |
| M4A | thy thr leu lacY minA minB gal str thi (pSG1044) divIVA-gfpb | this work |
| Z4A | ftsZ84(ts) thr-1 leu6 thi argH1 thy his trp rpsL lacY1malA1 xyl7mtl-2 mel tonA2 supE44 (pSG1044) divIVA-gfpb | this work |
| S.pombe | ||
| cdc8.27 | cdc8.27 h+ | Nurse et al. (1976) |
| Sp178 | ura4-D18 leu1.32 ade6-m210 h– | A.Aranda (Oxford) |
| ESP1 | ura4-d18 leu1.32 ade6-m210 h– (pSG1046) Pnmt1-divIVA-gfpb LEU2 | this work |
| ESP2 | ura4-d18 leu1.32 ade6-m210 h– (pSG1047) Pnmt1-′divIVA-gfpb LEU2 | this work |
| 1814 | cdc8.27 ura4-D18 leu1.32 ade6-m210a | this work |
| 1815 | cdc8.27 ura4-d18 leu1.32 ade6-m210 (pSG1636) Pnmt1-divIVA-gfpc LEU2 | this work |
| 1816 | cdc8.27 ura4-d18 leu1.32 ade6-m210 (pSG1637) Pnmt1- ′divIVA-gfpc LEU2 | this work |
| 1817 | cdc8.27 ura4-d18 leu1.32 ade6-m210 (pSG1621) Pnmt1-divIVA LEU2 | this work |
| Plasmids | ||
| pREP1 | bla Pnmt1 ars1 LEU2 | Maundrell (1993) |
| pREP3X | bla Pnmt1 ars1 LEU2 | Maundrell (1993) |
| pSG1044 | bla cat divIVA-gfpb | Edwards and Errington (1997) |
| pSG1045 | bla cat ′divIVA-gfpb | Edwards and Errington (1997) |
| pSG1046 | bla Pnmt1-divIVA-gfpb ars1 LEU2 | this work |
| pSG1047 | bla Pnmt1-′divIVA-gfpb ars1 LEU2 | this work |
| pSG1151 | bla cat gfpc | Lewis and Marston (1999) |
| pSG1612 | bla cat divIVA-gfpc | this work |
| pSG1621 | bla Pnmt1-divIVA ars1 LEU2 | this work |
| pSG1636 | bla Pnmt1-divIVA-gfpc ars1 LEU2 | this work |
| pSG1637 | bla Pnmt1-′divIVA-gfpc ars1 LEU2 | this work |
| pSG1638 | bla cat ′divIVA-gfpc | this work |
aThe mating type of this strain was not determined.
bS65T-encoding variant of gfp (Heim et al., 1995).
cF64L S65T-encoding variant of gfp (Cormack et al., 1996).
General methods
Methods for growth and manipulation of B.subtilis strains were as described previously (Edwards and Errington, 1997). Escherichia coli strains were grown in LB medium or M9 salts medium (Sambrook et al., 1989). Liquid cultures of S.pombe strains were grown in Edinburgh minimal medium (EMM) or in YEPD medium, supplemented with adenine, uracil and, if necessary, leucine, all at 0.25 µg/ml (Moreno et al., 1991). To repress expression from the nmt1 promoter, thiamine was added at 2 µM. For plate cultures, yeast extract plates (YE; Moreno et al., 1991) or EMM plates (Moreno et al., 1991) were used, supplemented with adenine, uracil, leucine and phloxin B indicator (20 µg/ml), as necessary. Cultures were grown at 30°C, except for experiments with cdc8 mutants, in which a non-permissive temperature of 37°C was used. Lat-A (Calbiochem) was used at a final concentration of 20 µM, as described by Rupes et al. (1999).
Computer analysis
Protein sequence alignments were done with Clustal W version 1.7 (Thompson et al., 1994) and the text was generated with MacBoxshade (version 2.15). For prediction of the secondary structure, Coils version 2.2 was run with an MTK matrix and a window of 28 amino acids (Lupas et al., 1991).
Construction of E.coli strains expressing divIVA–gfp fusions
Escherichia coli strains were transformed with pSG1044 (divIVA–gfpS65T) and selected on ampicillin plates (50 µg/ml). For the minB mutant P678-54 and cold-sensitive FtsZ strain PB143(pDB346), competent cells were prepared using the TransformAid kit (MBI fermentas).
Transformation of S.pombe
Schizosaccharomyces pombe strains were transformed with plasmid DNA by the lithium chloride procedure (Broker, 1987), essentially as described by Moreno et al. (1991). A culture was grown overnight in YEPD medium until stationary phase (A595 of 2.1) at 25°C and then diluted to an A595 of 0.15 in 100 ml of fresh YEPD medium. These cells were grown for 5 h until an optical density of 0.3 A595 was reached, then harvested by centrifugation at 3000 r.p.m. for 5 min. The pellet was washed once in distilled water and resuspended in buffer I (20 mM Tris–HCl pH 7.5, 2 mM EDTA, 200 mM LiCl) to a final volume of 1.2 ml. The cells were grown at 30°C for 1 h with gentle shaking. Plasmid DNA (1 µg) was added to 200 µl of cells. The cultures were incubated without shaking for 30 min at 30°C, then 700 µl of buffer II (40% w/v PEG 4000, 0.1 M LiCl) were added. The cells were heat shocked at 46°C for 25 min then plated on selective medium and incubated at room temperature for 4 days.
Construction of a thermostable divIVA–gfp fusion
A fragment consisting of the whole coding sequence of divIVA, from 138 bp upstream of the divIVA –10 promoter consensus to 8 bp beyond the stop codon, was amplified by PCR using primers 9954 (5′-GCTGTCTACTCGAGGTTTTGGCCGGTGCAGC-3′) and 9955 (5′-CAGAGAAGCTTTTCCTTTTCCTCAAATACAGCG-3′), as described previously (Edwards and Errington, 1997). The XhoI–HindIII-digested fragment was ligated to similarly digested plasmid pSG1151, resulting in plasmid pSG1612. This created a translational DivIVA–GFP fusion, in which a linker (KLDIEFLQ) separated the C-terminus of DivIVA from the N-terminus of the F64L S65T variant of GFP (Cormack et al., 1996). A control fusion was created by EcoRI digestion of pSG1612 and subsequent self-ligation of this vector to give pSG1638, as described by Edwards and Errington (1997). The truncated fusion included only the first 26 codons of divIVA.
Cloning for expression in S.pombe
divIVA was cloned into pREP3X for thiamine-repressible expression in S.pombe. A fragment consisting of the whole divIVA coding sequence, from 25 bp upstream of the start codon until 5 bp after the stop codon, was amplified from the 168CA chromosome with primers 9641 (5′-GATAACCGTACTCGAGTGTAAAAATGGAGGTGGC-3′) and 9640 (5′-GATAATCGGATCCTTTATTCCTTTTCCTC-3′), which introduce XhoI and BamHI sites at the upstream and downstream ends of the fragment, respectively. pREP3X was linearized by XhoI–BamHI digestion and ligated with the similarly digested PCR fragment, giving rise to plasmid pSG1621.
For expression of the DivIVA–GFP fusions in wild-type S.pombe, PCR products were prepared using pSG1044 (divIVA–gfpS65T) or pSG1045 (divIVA′–gfpS65T). Primer 9023 (5′-GATAACCGTACTCGAGTGTAAAAATGGAGGTGGC-3′) anneals 23 bp upstream of divIVA and introduces an XhoI site. Primer 9022 (5′-GAACTAGTAGATCTGAAGTCTGGAC-3′) anneals beyond the C-terminus of gfp, introducing a BglII site after the stop codon. The PCR products generated were digested and inserted into XhoI–BamHI-restricted pREP. The two plasmids derived encoded full-length DivIVA (pSG1046) or truncated DivIVA (pSG1047) fused to GFP, under the control of the thiamine-repressible nmt1 promoter of pREP1.
For expression in the cdc8.27 background, the DivIVA–GFP fusion plasmids were reconstructed using the more thermostable variant of GFP (see above). Fusions were amplified by PCR using pSG1612 or pSG1638, as templates for full-length and truncated GFP fusions, respectively, and primers 9641 and 9956, as before. The PCR fragments were digested with BglII and XhoI and cloned into XhoI–BamHI-digested pREP3X, to give plasmids pSG1636 and pSG1637, respectively.
Construction of a leu1.32 cdc8.27 double mutant of S.pombe
A zygotic cross was performed between a cdc8.27 mutant of h+ mating type (strain cdc8.27) and a leu1.32 mutant of h– mating type (strain Sp178), according to the method described by Moreno et al. (1991). Overlapping patches of each strain to be mated were drawn on a YEPD agar plate, supplemented with all amino acid requirements. The plate was incubated at 25°C for 4 days. A loopful of spores was then collected and resuspended in 1 ml of distilled water. The asci walls were lysed by incubation at 29°C overnight with 200 U/ml of HP-2 β-glucoronidase from Helix pomatia (Sigma). Ten-fold dilutions of these spore suspensions were then plated on YE agar, supplemented with all amino acid requirements and incubated overnight at the permissive temperature (30°C). The germinating colonies were replica plated successively on EMM agar containing adenine and uracil, EMM agar containing adenine, uracil and phloxin B indicator, and EMM agar containing all amino acid requirements. All plates were then incubated overnight at 30°C, except for the plate containing phloxin B, which was incubated at the restrictive temperature (37°C). Clones unable to grow at 37°C or in the absence of leucine were isolated. cdc8.27 mutants grow poorly at 37°C and arrest growth following attempts to divide medially (Nurse et al., 1976; Rupes et al., 1999). The multinucleate mutant cells adopt a ‘bone-shaped’ structure and form malformed, misplaced and often multiple septa under these conditions (Nurse et al., 1976; Gould and Simanis, 1997; Rupes et al., 1999). The cdc8.27 leu1.32 derivative strain (1814) also showed this morphology at both 30 and 37°C, although it was less severe at the lower temperature.
Expression of divIVA or divIVA–gfp in a cdc8.27 mutant of S.pombe
Strain 1814 was transformed with a plasmid (pSG1621) harbouring a complete copy of divIVA under the control of the thiamine-repressible Pnmt1 promoter. This autonomously replicating plasmid also contained the LEU2 marker so S.pombe transformants were selected for leucine prototrophy (Moreno et al., 1991). Strain 1814 was transformed similarly with plasmids pSG1636 and pSG1637, harbouring divIVA–gfp and Δ(EcoRI)divIVA–gfp fusions, to give strains 1815 and 1816, respectively.
Preparation of samples for microscopy
Slides were prepared for visualization of GFP in live S.pombe cells, essentially as described for B.subtilis cells by Glaser et al. (1997) and Sharpe and Errington (1998). Briefly, a thin film of 1.2% agarose was spread over a glass slide and allowed to dry for 3 min at 25°C. A 0.5 µl sample of culture was added to the centre of the slide, followed by a coverslip. The same procedure was followed for visualization of GFP fusions in E.coli except that only 2 µl of cell culture were added to the slide. At high levels of expression, the DivIVA–GFP signal was often disturbed with the occurrence of bright cytoplasmic spots that we assume to be inclusion bodies. Therefore, to reduce the level of expression, bacterial samples were taken from slow growing cells. These were prepared by either growing bacteria at 30°C in M9 salts, or by resuspending an individual colony from a fresh plate in LB.
For time lapse photography, samples were taken from mid-exponential liquid cultures, harvested by centrifugation and resuspended in LB. Samples were placed on the microscope and allowed to grow on the agarose slide for a period of up to 2 h. Fluorescent images were grabbed at 40 min time points, with care being taken to avoid unnecessary exposure to UV.
To examine the effects of Lat-A, cells were observed either unfixed (for DivIVA–GFP), or after formaldehyde fixation and staining with tetramethylrhodamine isothiocyanate (TRITC)–phalloidin (Sigma) (for actin) as described by Balasubramanian et al. (1997).
Fluorescence microscopy, image grabbing and data analysis
Cells were viewed by epifluorescence microscopy using a Zeiss Axiovert 135TV epifluorescence microscope with a 100 W mercury lamp source, a ×100 Plan-Neofluar oil-immersion lens (numerical aperture 1.3), a ×1 or ×1.25 optovar, and a triple band pass filter. All phase-contrast and fluorescence images were acquired using a cooled CCD camera (Digital Pixel Advanced Imaging Systems) with a 1536 × 1024 or a 768 × 512 pixel, 9 µm pitch chip. Exposure times were 1 s for DivIVA–GFP, 0.4–1.0 s for 4′,6-diamidino-2-phenylindole (DAPI). Processing was carried out on the 12 bit images using IPLab Spectrum V3.1 (Signal analytics). Final images were assembled in Adobe Photoshop version 4.0.1.
Confocal images were obtained with a Bio-Rad 1000/1024 hybrid confocal laser scanning microscope (running under LaserSharp 2.1A software) equipped with an argon/krypton laser and coupled to a Nikon Diaphot 200 inverted microscope (×60 PlanApo oil-immersion objective; numerical aperture 1.4). Kalman filtered images (n = 6–10) were collected with minimum iris aperture (0.7 mm). A series of optical sections collected at 200 nm intervals in the z-axis was used to reconstruct the images shown in Figure 3C–E.
Acknowledgments
Acknowledgements
We would like to thank Karen Hansen for her assistance with the yeast work, Jane Mellor, Iain Hagan, Agustin Aranda and Paul Nurse for kindly supplying S.pombe strains and plasmids, and Piet De Boer for E.coli strains and helpful advice. This work was supported by grants from the Biotechnology and Biological Science Research Council and the BIOTECH programme of the EU to J.E. J.E. acknowledges the receipt of a Senior Research Fellowship of the BBSRC. D.E. was kindly supported by the Max-Planck Society.
References
- Addinall S.G. and Lutkenhaus,J. (1996) FtsZ-spirals and -arcs determine the shape of the invaginating septa in some mutants of Escherichia coli.Mol. Microbiol., 22, 231–237. [DOI] [PubMed] [Google Scholar]
- Adler H.I., Fisher,W.D., Cohen,A. and Hardigree,A.A. (1967) Miniature Escherichia coli cells deficient in DNA. Proc. Natl Acad. Sci. USA, 57, 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai R., Nakano,K. and Mabuchi,I. (1998) Subcellular localization and possible function of actin, tropomyosin and actin-related protein 3 (Arp3) in the fission yeast Schizosaccharomyces pombe.Eur. J. Cell Biol., 76, 288–295. [DOI] [PubMed] [Google Scholar]
- Ayscough K.R., Stryker,J., Pokala,N., Sanders,M., Crews,P. and Drubin,D.G. (1997) High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol., 137, 399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian M.K., Helfman,D.M. and Hemmingsen,S.M. (1992) A new tropomyosin essential for cytokinesis in the fission yeast S.pombe.Nature, 360, 84–87. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M.K., McCollum,D. and Gould,K.L. (1997) Cytokinesis in fission yeast Schizosaccharomyces pombe.Methods Enzymol., 283, 494–506. [DOI] [PubMed] [Google Scholar]
- Bi E. and Lutkenhaus,J. (1991) FtsZ ring structure associated with division in Escherichia coli.Nature, 354, 161–164. [DOI] [PubMed] [Google Scholar]
- Bramhill D. (1997) Bacterial cell division. Annu. Rev. Cell. Dev. Biol., 13, 395–424. [DOI] [PubMed] [Google Scholar]
- Bramhill D. and Thompson,C.M. (1994) GTP-dependent polymerization of Escherichia coli FtsZ protein to form tubules. Proc. Natl Acad. Sci. USA, 91, 5813–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broker M. (1987) Transformation of intact Schizosaccharomyces pombe cells with plasmid DNA. Biotechniques, 5, 516–518. [Google Scholar]
- Cha J.-H. and Stewart,G.C. (1997) The divIVA minicell locus of Bacillus subtilis. J. Bacteriol., 179, 1671–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker A.F., Ward,J.M., Fosberry,A.P. and Hodgson,J.E. (1994) Analysis and toxic overexpression in Escherichia coli of a staphylococcal gene encoding isoleucyl-tRNA synthetase. Gene, 141, 103–108. [DOI] [PubMed] [Google Scholar]
- Cormack B.P., Valdivia,R.H. and Falkow,S. (1996) FACS-optimized mutants of the green fluorescent protein (GFP). Gene, 173, 33–38. [DOI] [PubMed] [Google Scholar]
- De Boer P.A.J., Crossley,R.E. and Rothfield,L.I. (1989) A division inhibitor and a topological specificity factor coded for by the minicell locus determine proper placement of the division septum in E.coli.Cell, 56, 641–649. [DOI] [PubMed] [Google Scholar]
- De Boer P.A.J., Crossley,R.E. and Rothfield,L.I. (1992) Roles of MinC and MinD in the site-specific septation block mediated by the MinCDE system of Escherichia coli.J. Bacteriol., 174, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D.H. and Errington,J. (1997) The Bacillus subtilis DivIVA protein targets to the division septum and controls the site specificity of cell division. Mol. Microbiol., 24, 905–915. [DOI] [PubMed] [Google Scholar]
- Erickson H.P., Taylor,D.W., Taylor,K.A. and Bramhill,D. (1996) Bacterial cell division protein FtsZ assembles into protofilament sheets and minirings, structural homologues of tubulin polymers. Proc. Natl Acad. Sci. USA, 93, 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J.-M. (1991) Serine β-lactamases and penicillin-binding proteins. Annu. Rev. Microbiol., 45, 37–67. [DOI] [PubMed] [Google Scholar]
- Glaser P., Sharpe,M.E., Raether,B., Perego,M., Ohlsen,K. and Errington,J. (1997) Dynamic, mitotic-like behaviour of a bacterial protein required for accurate chromosome partitioning. Genes Dev., 11, 1160–1168. [DOI] [PubMed] [Google Scholar]
- Gould K.L. and Simanis,V. (1997) The control of septum formation in fission yeast. Genes Dev., 11, 2939–2951. [DOI] [PubMed] [Google Scholar]
- Hagan I.M. (1998) The fission yeast microtubule cytoskeleton. J. Cell Sci., 111, 1603–1612. [DOI] [PubMed] [Google Scholar]
- Heim R., Cubitt,A.B. and Tsien,R.Y. (1995) Improved green fluorescence. Nature, 373, 663–664. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ryter,A. and Jacob,F. (1968) Thermosensitive mutants of E.coli affected in the process of DNA synthesis and cellular division. Cold Spring Harb. Symp. Quant. Biol., 33, 677–693. [DOI] [PubMed] [Google Scholar]
- Hu Z. and Lutkenhaus,J. (1999) Topological regulation of cell division in Escherichia coli involves rapid pole to pole oscillation of the division inhibitor MinC under the control of MinD and MinE. Mol. Microbiol., 34, 82–90. [DOI] [PubMed] [Google Scholar]
- Kabsch W. and Holmes,K.C. (1995) The actin fold. FASEB J., 9, 167–174. [DOI] [PubMed] [Google Scholar]
- Lee S. and Price,C.W. (1993) The minCD locus of Bacillus subtilis lacks the minE determinant that provides topological specificity to cell division. Mol. Microbiol., 7, 601–610. [DOI] [PubMed] [Google Scholar]
- Levin P.A., Margolis,P.S., Setlow,P., Losick,R. and Sun,D. (1992) Identification of Bacillus subtilis genes for septum placement and shape determination. J. Bacteriol., 174, 6717–6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P.J. and Marston,A.L. (1999) GFP vectors for controlled expression and dual labelling of protein fusions in Bacillus subtilis. Gene, 227, 101–109. [DOI] [PubMed] [Google Scholar]
- Lupas A. (1996) Coiled coils: new structures and new functions. Trends Biochem. Sci., 21, 375–382. [PubMed] [Google Scholar]
- Lupas A., Van Dyke,M. and Stock,J. (1991) Predicting coiled coils from protein sequences. Science, 252, 1162–1164. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. and Addinall,S.G. (1997) Bacterial cell division and the Z ring. Annu. Rev. Biochem., 66, 93–116. [DOI] [PubMed] [Google Scholar]
- Marks J. and Hyams,J.S. (1985) Localization of F-actin through the cell division cycle of Schizosaccharomyces pombe.Eur. J. Cell Biol., 39, 27–32. [Google Scholar]
- Marks J., Hagan,I.M. and Hyams,J.S. (1987) Spatial association of F-actin with growth polarity and septation in the fission yeast Schizosaccharomyces pombe. In Poole,R.K. and Trinci,A.P.J. (eds), Spatial Organization in Eukaryotic Microbes. Society for General Microbiology, IRL Press, Oxford, UK, pp. 119–135. [Google Scholar]
- Marston A.L. and Errington,J. (1999) Selection of the midcell division site in Bacillus subtilis through MinD-dependent polar localization and activation of MinC. Mol. Microbiol., 33, 84–96. [DOI] [PubMed] [Google Scholar]
- Marston A.L., Thomaides,H.B., Edwards,D.H., Sharpe,M.E. and Errington,J. (1998) Polar localization of the MinD protein of Bacillus subtilis and its role in selection of the mid-cell division site. Genes Dev., 12, 3419–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maundrell K. (1993) Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene, 23, 127–130. [DOI] [PubMed] [Google Scholar]
- Mendelson N.H. (1975) Cell division suppression in the Bacillus subtilis divIV-A1 minicell-producing mutant. J. Bacteriol., 121, 1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison J.M. and Nurse,P. (1985) Growth in cell length in the fission yeast Schizosaccharomyces pombe.J. Cell Sci., 75, 357–376. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar,A. and Nurse,P. (1991) Molecular genetic analysis of the fission yeast Schizosaccharomyces pombe.Methods Enzymol., 194, 795–823. [DOI] [PubMed] [Google Scholar]
- Mukherjee A. and Lutkenhaus,J. (1994) Guanine nucleotide-dependent assembly of FtsZ into filaments. J. Bacteriol., 176, 2754–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A. and Lutkenhaus,J. (1998) Dynamic assembly of FtsZ regulated by GTP hydrolysis. EMBO J., 17, 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogales E., Downing,K.H., Amos,L.A. and Löwe,J. (1998) Tubulin and FtsZ form a distinct family of GTPases. Nature Struct. Biol., 5, 451–458. [DOI] [PubMed] [Google Scholar]
- Nurse P., Thuriaux,P. and Nasmyth,K. (1976) Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe.Mol. Gen. Genet., 146, 167–178. [DOI] [PubMed] [Google Scholar]
- Phillips G.N. Jr, Lattman,E.E., Cummins,P., Lee,K.Y. and Cohen,C. (1979) Crystal structure and molecular interactions of tropomyosin. Nature, 278, 413–417. [DOI] [PubMed] [Google Scholar]
- Phillips G.N. Jr, Fillers,J.P. and Cohen,C. (1986) Tropomyosin crystal structure and muscle regulation. J. Mol. Biol., 192, 111–131. [DOI] [PubMed] [Google Scholar]
- Raskin D.M. and De Boer,P.A.J. (1997) The MinE ring: an FtsZ-independent cell structure required for selection of the correct division site in E.coli.Cell, 91, 685–694. [DOI] [PubMed] [Google Scholar]
- Raskin D.M. and De Boer,P.A.J. (1999a) Rapid pole-to-pole oscillation of a protein required for directing division to the middle of Escherichia coli.Proc. Natl Acad. Sci. USA, 96, 4971–4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin D.M. and De Boer,P.A.J. (1999b) MinDE-dependent pole-to-pole oscillation of division inhibitor MinC in Escherichia coli.J. Bacteriol., 181, 6419–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J.N., Mendelson,N.H., Coyne,S.I., Hallock,L.L. and Cole,R.M. (1973) Minicells of Bacillus subtilis. J. Bacteriol., 114, 860–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupes I., Jia,Z. and Young,P.G. (1999) Ssp1 promotes actin depolymerisation and is involved in stress response and new end take-off control in fission yeast. Mol. Biol. Cell, 10, 1495–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Sharpe M.E. and Errington,J. (1998) A fixed distance for separation of newly replicated copies of oriC in Bacillus subtilis: implications for co-ordination of chromosome segregation and cell division. Mol. Microbiol., 28, 981–990. [DOI] [PubMed] [Google Scholar]
- Shu H.B., Li,Z., Palacios,M.J., Li,Q. and Joshi,H.C. (1995) A transient association of γ-tubulin at the midbody is required for the completion of cytokinesis during the mammalian cell division. J. Cell Sci., 108, 2955–2962. [DOI] [PubMed] [Google Scholar]
- Smillie L.B. (1979) Structure and functions of tropomyosins from muscle and non-muscle sources. Trends Biochem. Sci., 4, 151–155. [Google Scholar]
- Sun Q. and Margolin,W. (1998) FtsZ dynamics during the division cycle of live Escherichia coli cells. J. Bacteriol., 180, 2050–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.D., Higgins,D.G. and Gibson,T.J. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res., 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varley A.W. and Stewart,G.C. (1992) The divIVB region of the Bacillus subtilis chromosome encodes homologs of Escherichia coli septum placement (MinCD) and cell shape (MreBCD) determinants. J. Bacteriol., 174, 6729–6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. and Gayda,R.C. (1992) Quantitative determination of FtsA at different growth rates in Escherichia coli using monoclonal antibodies. Mol. Microbiol., 6, 2517–2524. [DOI] [PubMed] [Google Scholar]