Abstract
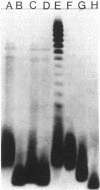
Mutations which prevent absorption of the bacteriophage K20 to Escherichia coli K-12 were selected by using an altered OmpF protein which confers the ability to grow on maltodextrin in the absence of the LamB maltoporin. The mutations map in the rfa gene cluster and alter the structure of the lipopolysaccharide core.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher M. G., Schnaitman C. A. Regulation of the OmpA outer membrane protein of Escherichia coli. J Bacteriol. 1981 Sep;147(3):972–985. doi: 10.1128/jb.147.3.972-985.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S. A., Decloux A. Isolation and characterization of outer membrane permeability mutants in Escherichia coli K-12. J Bacteriol. 1985 Jan;161(1):361–367. doi: 10.1128/jb.161.1.361-367.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R. Porin from bacterial and mitochondrial outer membranes. CRC Crit Rev Biochem. 1985;19(2):145–190. doi: 10.3109/10409238509082542. [DOI] [PubMed] [Google Scholar]
- Clément J. M., Lepouce E., Marchal C., Hofnung M. Genetic study of a membrane protein: DNA sequence alterations due to 17 lamB point mutations affecting adsorption of phage lambda. EMBO J. 1983;2(1):77–80. doi: 10.1002/j.1460-2075.1983.tb01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
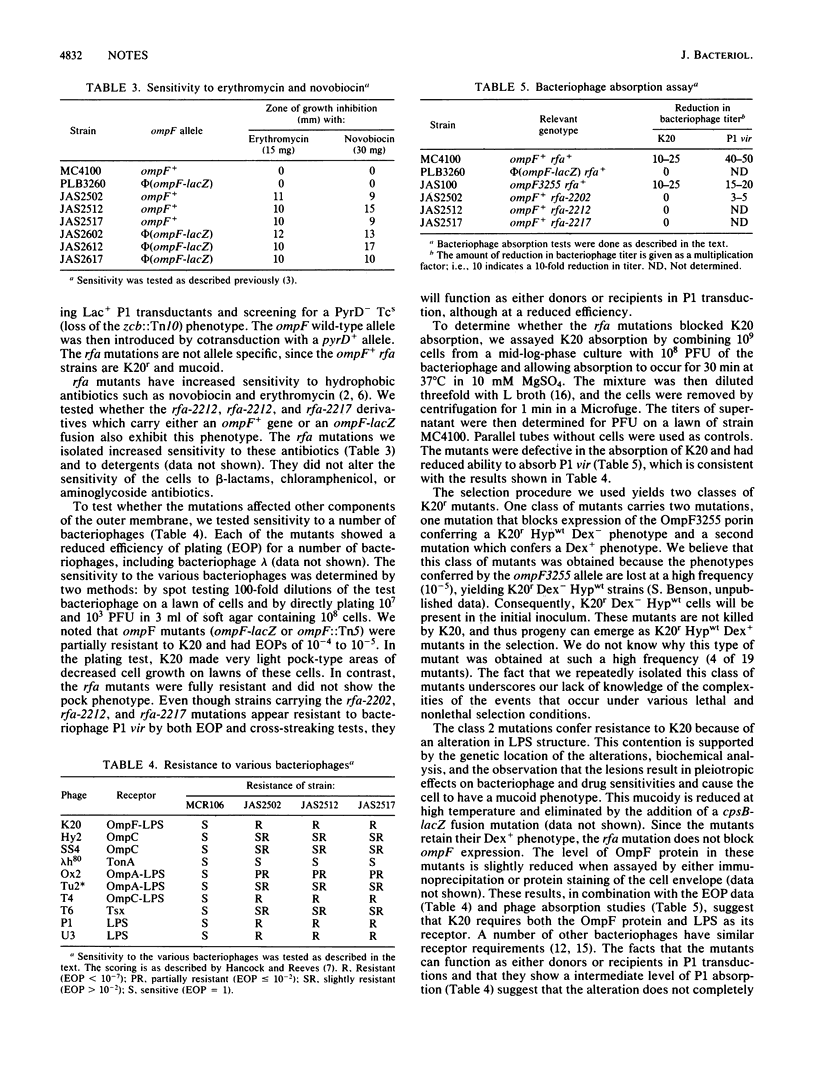
- Coleman W. G., Jr, Deshpande K. S. New cysE-pyrE-linked rfa mutation in Escherichia coli K-12 that results in a heptoseless lipopolysaccharide. J Bacteriol. 1985 Mar;161(3):1209–1214. doi: 10.1128/jb.161.3.1209-1214.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Reeves P. Bacteriophage resistance in Escherichia coli K-12: general pattern of resistance. J Bacteriol. 1975 Mar;121(3):983–993. doi: 10.1128/jb.121.3.983-993.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona R., Henning U. Host range mutants of bacteriophage Ox2 can use two different outer membrane proteins of Escherichia coli K-12 as receptors. J Bacteriol. 1984 Aug;159(2):579–582. doi: 10.1128/jb.159.2.579-582.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona R., Klose M., Henning U. Escherichia coli K-12 outer membrane protein (OmpA) as a bacteriophage receptor: analysis of mutant genes expressing altered proteins. J Bacteriol. 1984 Aug;159(2):570–578. doi: 10.1128/jb.159.2.570-578.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975 Apr 25;250(8):2911–2919. [PubMed] [Google Scholar]
- Mutoh N., Furukawa H., Mizushima S. Role of lipopolysaccharide and outer membrane protein of Escherichia coli K-12 in the receptor activity for bacteriophage T4. J Bacteriol. 1978 Nov;136(2):693–699. doi: 10.1128/jb.136.2.693-699.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa M., Clément J. M. Location of a phage binding region on an outer membrane protein. FEBS Lett. 1980 Nov 17;121(1):127–129. doi: 10.1016/0014-5793(80)81280-4. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Tsugita A., Schwartz M., Rosenbusch J. P. Topology of phage lambda receptor protein. Mapping targets of proteolytic cleavage in relation to binding sites for phage or monoclonal antibodies. J Biol Chem. 1984 Jun 25;259(12):7570–7576. [PubMed] [Google Scholar]
- Schweizer M., Hindennach I., Garten W., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane. Interaction of protein II with lipopolysaccharide. Eur J Biochem. 1978 Jan 2;82(1):211–217. doi: 10.1111/j.1432-1033.1978.tb12013.x. [DOI] [PubMed] [Google Scholar]
- Tommassen J., van der Ley P., van Zeijl M., Agterberg M. Localization of functional domains in E. coli K-12 outer membrane porins. EMBO J. 1985 Jun;4(6):1583–1587. doi: 10.1002/j.1460-2075.1985.tb03820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Schwartz M. Mutations that alter the transport function of the LamB protein in Escherichia coli. J Bacteriol. 1982 Jul;151(1):15–21. doi: 10.1128/jb.151.1.15-21.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L. Novel regulatory mutants of the phosphate regulon in Escherichia coli K-12. J Mol Biol. 1986 Sep 5;191(1):39–58. doi: 10.1016/0022-2836(86)90421-3. [DOI] [PubMed] [Google Scholar]