Abstract
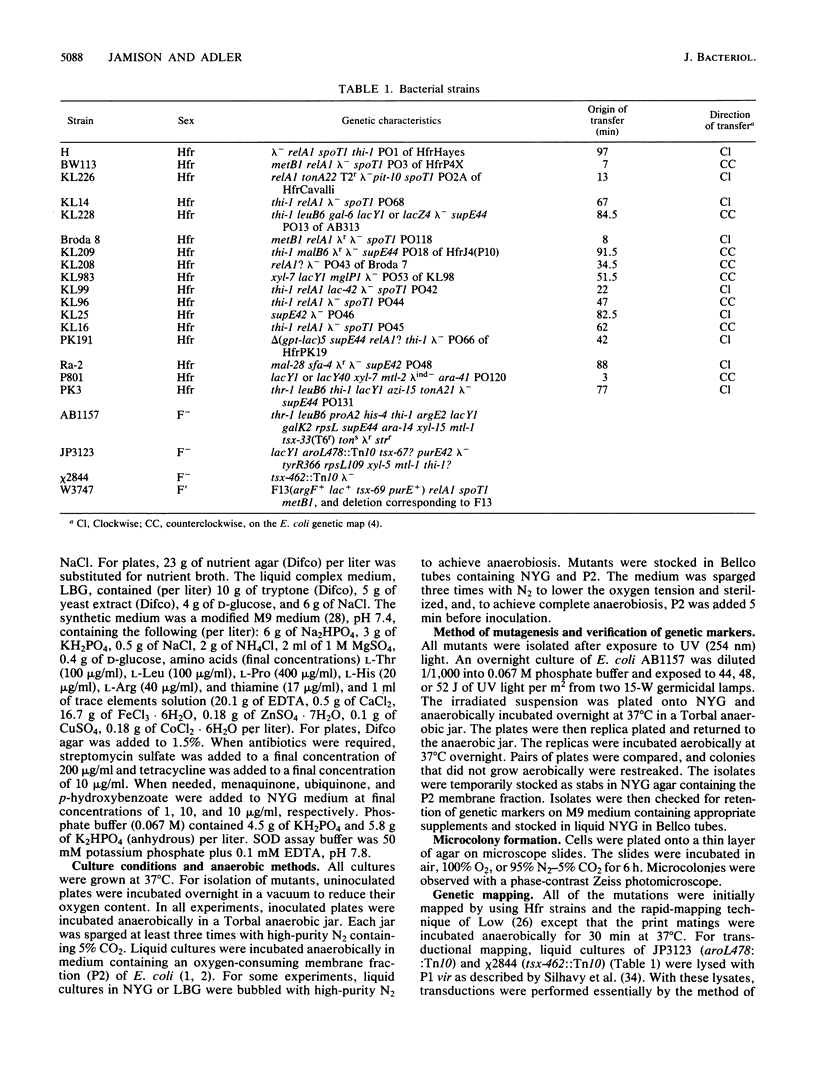
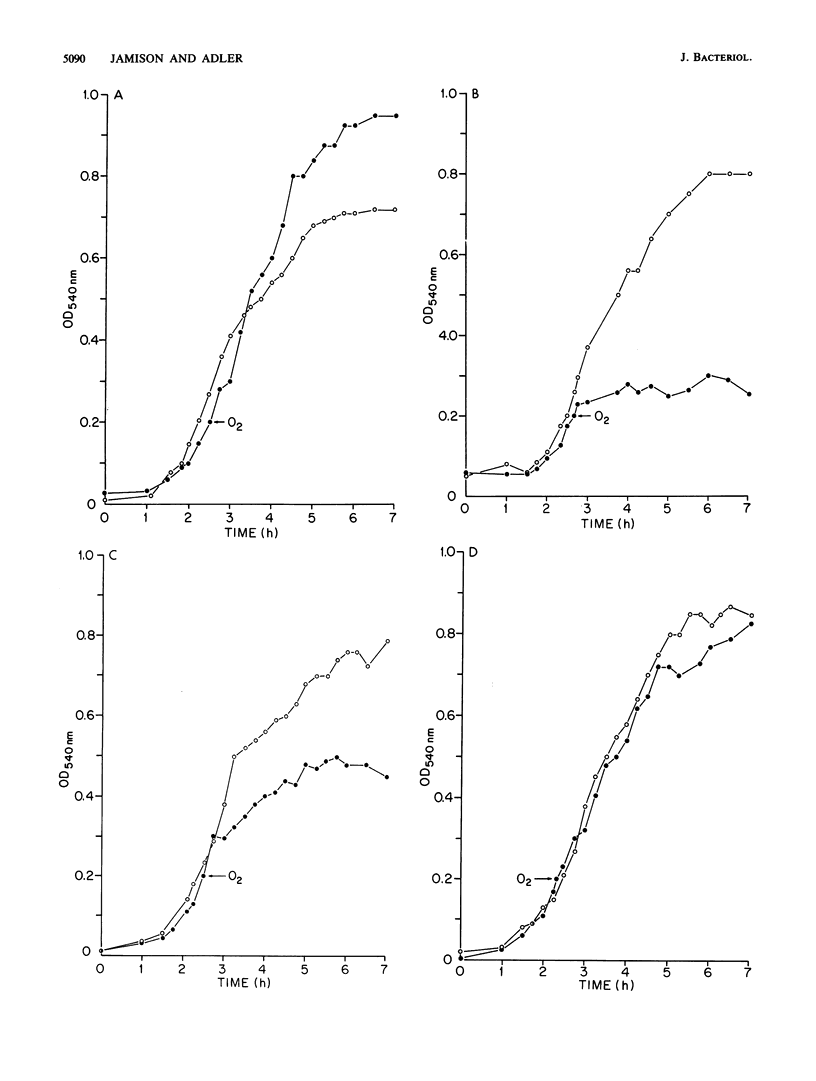
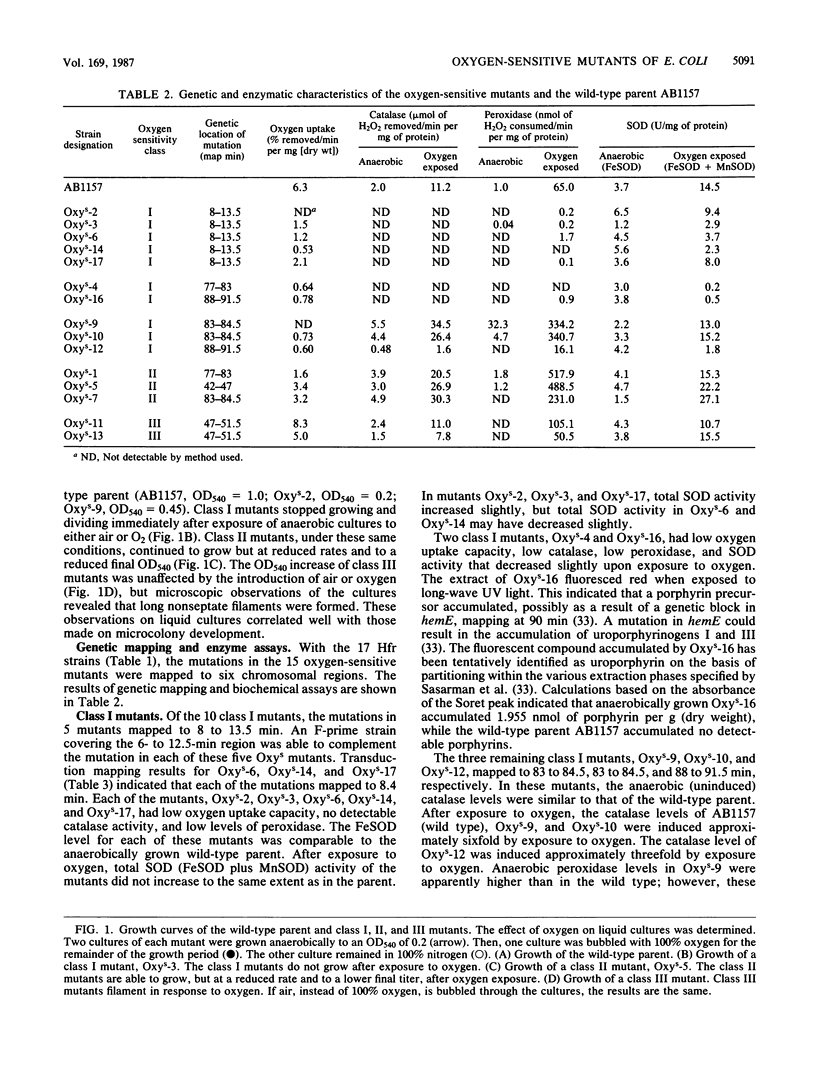
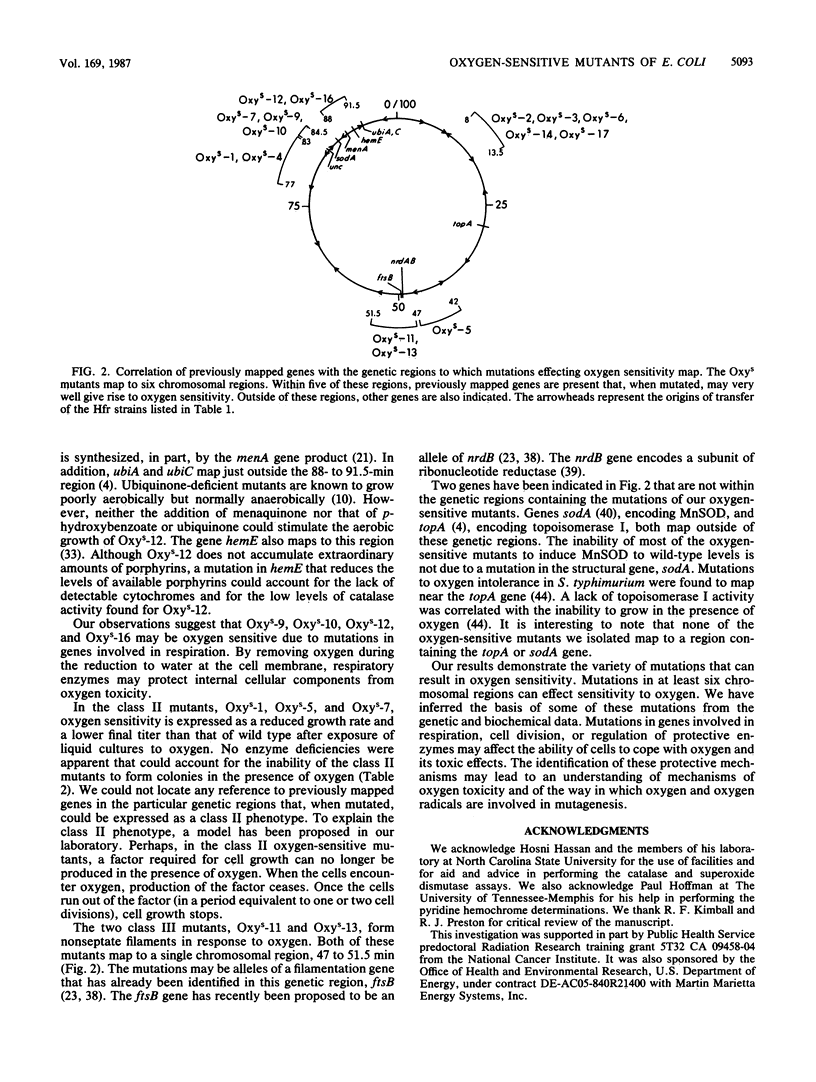
Fifteen oxygen-sensitive (Oxys) mutants of Escherichia coli were isolated after exposure to UV light. The mutants did not form macroscopic colonies when plated aerobically. They did form macroscopic colonies anaerobically. Oxygen, introduced during log phase, inhibited the growth of liquid cultures. The degree of inhibition was used to separate the mutants into three classes. Class I mutants did not grow after exposure to oxygen. Class II mutants were able to grow, but at a reduced rate and to a reduced final titer, when compared with the wild-type parent. Class III mutants formed filaments in response to oxygen. Genetic experiments indicated that the mutations map to six different chromosomal regions. The results of enzymatic assays indicated that 7 of the 10 class I mutants have low levels of catalase, peroxidase, superoxide dismutase, and respiratory enzymes when compared with the wild-type parent. Mutations in five of the seven class I mutants which have the low enzyme activities mapped within the region 8 to 13.5 min. P1 transduction data indicated that mutations in three of these five mutants, Oxys-6, Oxys-14, and Oxys-17, mapped to 8.4 min. The correlation of low enzyme levels and mapping data suggests that a single gene may regulate several enzymes in response to oxygen. The remaining three class I mutants had wild-type levels of catalase, peroxidase, and superoxide dismutase, but decreased respiratory activity. The class II and III mutants had enzyme activities similar to those of the wild-type parent. Our results demonstrate that mutations in at least six genes can be expressed as oxygen sensitivity. Some of these genes may be involved in respiration or cell division or may regulate the expression of several enzymes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Carrasco A., Crow W., Gill J. S. Cytoplasmic membrane fraction that promotes septation in an Escherichia coli lon mutant. J Bacteriol. 1981 Aug;147(2):326–332. doi: 10.1128/jb.147.2.326-332.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliabadi Z., Warren F., Mya S., Foster J. W. Oxygen-regulated stimulons of Salmonella typhimurium identified by Mu d(Ap lac) operon fusions. J Bacteriol. 1986 Mar;165(3):780–786. doi: 10.1128/jb.165.3.780-786.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot N., Weissbach L., Werth J., Weissbach H. Enzymatic reduction of protein-bound methionine sulfoxide. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2155–2158. doi: 10.1073/pnas.78.4.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyninckx W. J., Mason H. S., Morse S. A. Are physiological oxygen concentrations mutagenic? Nature. 1978 Aug 10;274(5671):606–607. doi: 10.1038/274606a0. [DOI] [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Downie J. A. Isolation and characterization of mutants of Escherichia coli K-12 affected in oxidative phosphorylation of quinone biosynthesis. Methods Enzymol. 1979;56:106–117. doi: 10.1016/0076-6879(79)56013-3. [DOI] [PubMed] [Google Scholar]
- Demple B., Halbrook J. Inducible repair of oxidative DNA damage in Escherichia coli. Nature. 1983 Aug 4;304(5925):466–468. doi: 10.1038/304466a0. [DOI] [PubMed] [Google Scholar]
- Downie J. A., Gibson F., Cox G. B. Membrane adenosine triphosphatases of prokaryotic cells. Annu Rev Biochem. 1979;48:103–131. doi: 10.1146/annurev.bi.48.070179.000535. [DOI] [PubMed] [Google Scholar]
- Farr S. B., D'Ari R., Touati D. Oxygen-dependent mutagenesis in Escherichia coli lacking superoxide dismutase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8268–8272. doi: 10.1073/pnas.83.21.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr S. B., Natvig D. O., Kogoma T. Toxicity and mutagenicity of plumbagin and the induction of a possible new DNA repair pathway in Escherichia coli. J Bacteriol. 1985 Dec;164(3):1309–1316. doi: 10.1128/jb.164.3.1309-1316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn W. O., Gerschman R., Gilbert D. L., Terwilliger D. E., Cothran F. V. MUTAGENIC EFFECTS OF HIGH OXYGEN TENSIONS ON ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1957 Dec 15;43(12):1027–1032. doi: 10.1073/pnas.43.12.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gifford G. D. Mutation of an auxotrophic strain of Escherichia coli by high pressure oxygen. Biochem Biophys Res Commun. 1968 Oct 24;33(2):294–298. doi: 10.1016/0006-291x(68)90783-3. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Superoxide, hydrogen peroxide, and oxygen tolerance of oxygen-sensitive mutants of Escherichia coli. Rev Infect Dis. 1979 Mar-Apr;1(2):357–369. doi: 10.1093/clinids/1.2.357. [DOI] [PubMed] [Google Scholar]
- Ingledew W. J., Poole R. K. The respiratory chains of Escherichia coli. Microbiol Rev. 1984 Sep;48(3):222–271. doi: 10.1128/mr.48.3.222-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. W., Redfearn E. R. Electron transport in Azotobacter vinelandii. Biochim Biophys Acta. 1966 Mar 7;113(3):467–481. doi: 10.1016/s0926-6593(66)80005-x. [DOI] [PubMed] [Google Scholar]
- Kren B., Fuchs J. A. Characterization of the ftsB gene as an allele of the nrdB gene in Escherichia coli. J Bacteriol. 1987 Jan;169(1):14–18. doi: 10.1128/jb.169.1.14-18.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin D. E., Hollstein M., Christman M. F., Schwiers E. A., Ames B. N. A new Salmonella tester strain (TA102) with A X T base pairs at the site of mutation detects oxidative mutagens. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7445–7449. doi: 10.1073/pnas.79.23.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Moody C. S., Hassan H. M. Mutagenicity of oxygen free radicals. Proc Natl Acad Sci U S A. 1982 May;79(9):2855–2859. doi: 10.1073/pnas.79.9.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. G. Fifth Stenhous-Williams memorial lecture. Oxygen and the obligate anaerobe. J Appl Bacteriol. 1976 Jun;40(3):229–244. doi: 10.1111/j.1365-2672.1976.tb04171.x. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. Proteins induced by aerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):344–350. doi: 10.1128/jb.154.1.344-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. Proteins induced by anaerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):336–343. doi: 10.1128/jb.154.1.336-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector M. P., Aliabadi Z., Gonzalez T., Foster J. W. Global control in Salmonella typhimurium: two-dimensional electrophoretic analysis of starvation-, anaerobiosis-, and heat shock-inducible proteins. J Bacteriol. 1986 Oct;168(1):420–424. doi: 10.1128/jb.168.1.420-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Chartrand P., Proschek R., Desrochers M., Tardif D., Lapointe C. Uroporphyrin-accumulating mutant of Escherichia coli K-12. J Bacteriol. 1975 Dec;124(3):1205–1212. doi: 10.1128/jb.124.3.1205-1212.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschner P. E., Verest J. G., Woldringh C. L. Genetic and morphological characterization of ftsB and nrdB mutants of Escherichia coli. J Bacteriol. 1987 Jan;169(1):19–25. doi: 10.1128/jb.169.1.19-25.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Touati D. Cloning and mapping of the manganese superoxide dismutase gene (sodA) of Escherichia coli K-12. J Bacteriol. 1983 Sep;155(3):1078–1087. doi: 10.1128/jb.155.3.1078-1087.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman J. W., Clark D. P. Anaerobically induced genes of Escherichia coli. J Bacteriol. 1986 Jul;167(1):362–367. doi: 10.1128/jb.167.1.362-367.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Droffner M. L. Mechanisms determining aerobic or anaerobic growth in the facultative anaerobe Salmonella typhimurium. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2077–2081. doi: 10.1073/pnas.82.7.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost F. J., Jr, Fridovich I. Superoxide and hydrogen peroxide in oxygen damage. Arch Biochem Biophys. 1976 Aug;175(2):514–519. doi: 10.1016/0003-9861(76)90539-7. [DOI] [PubMed] [Google Scholar]


