Abstract
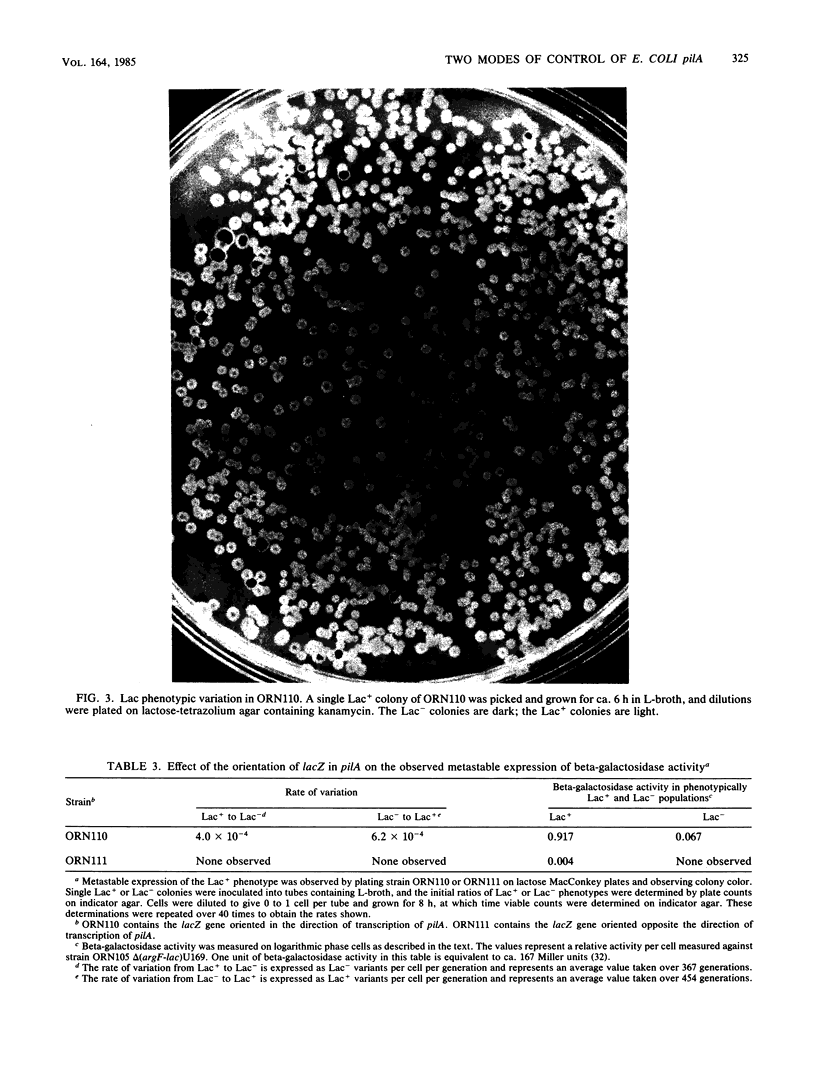
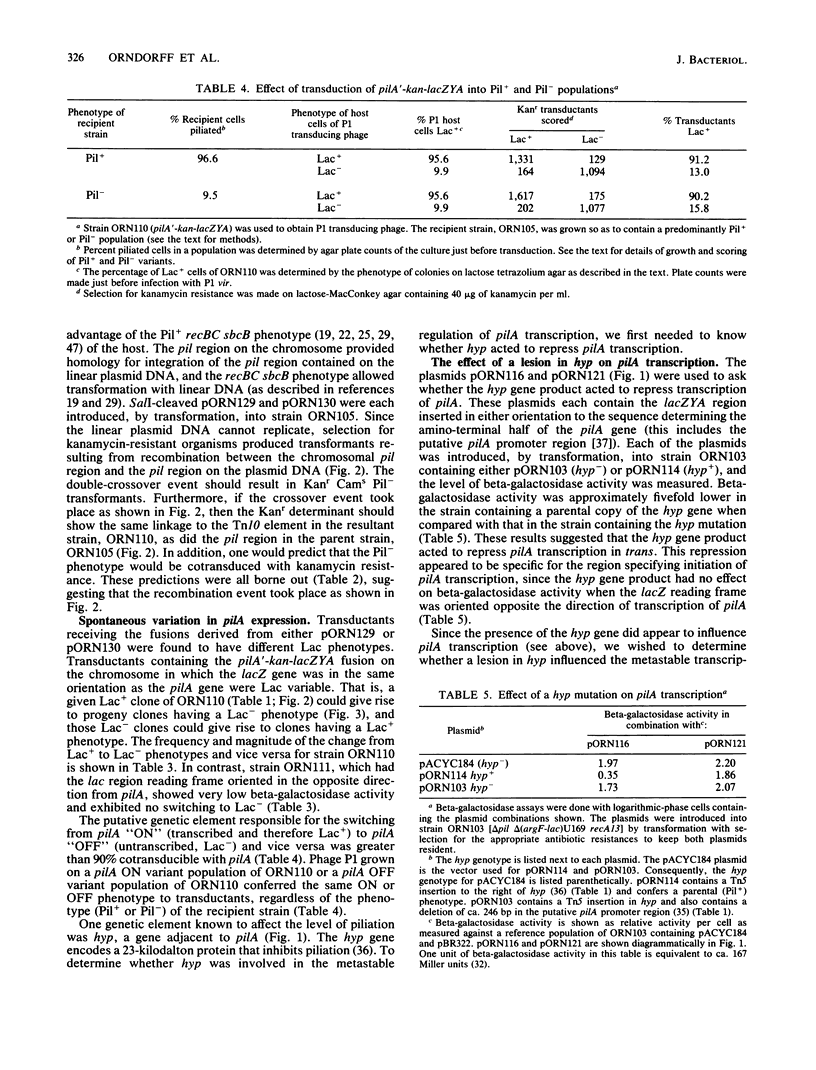
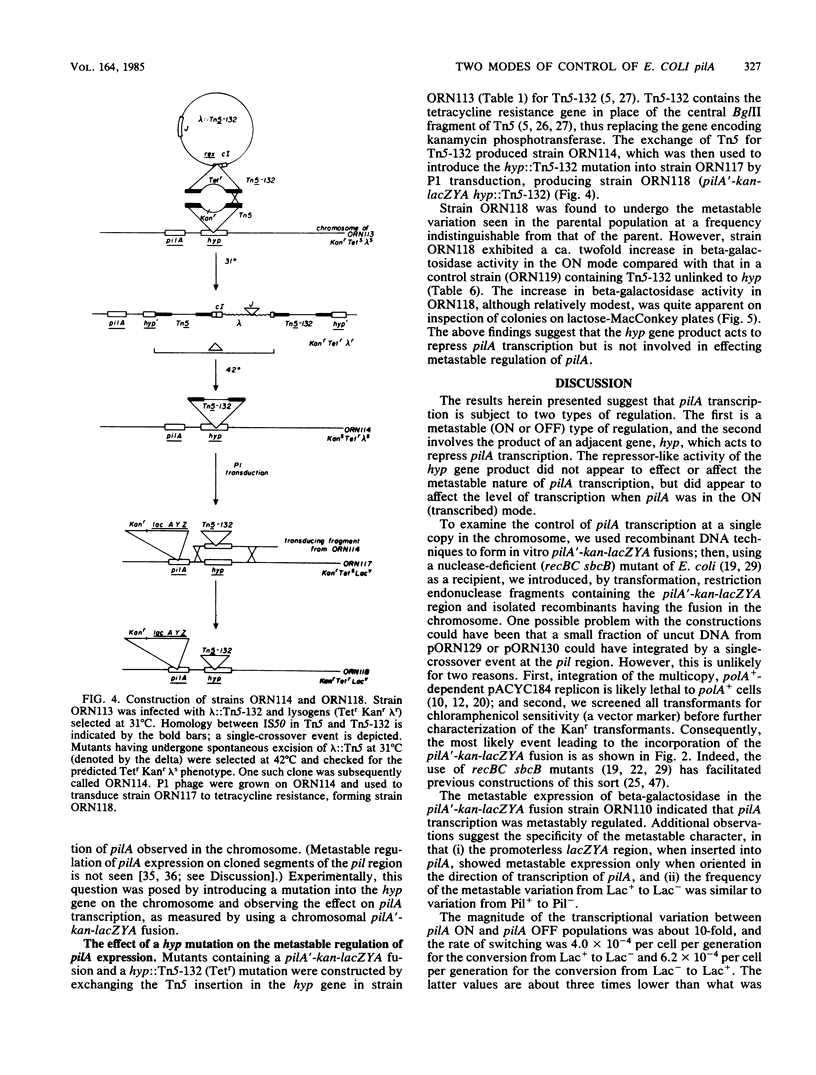
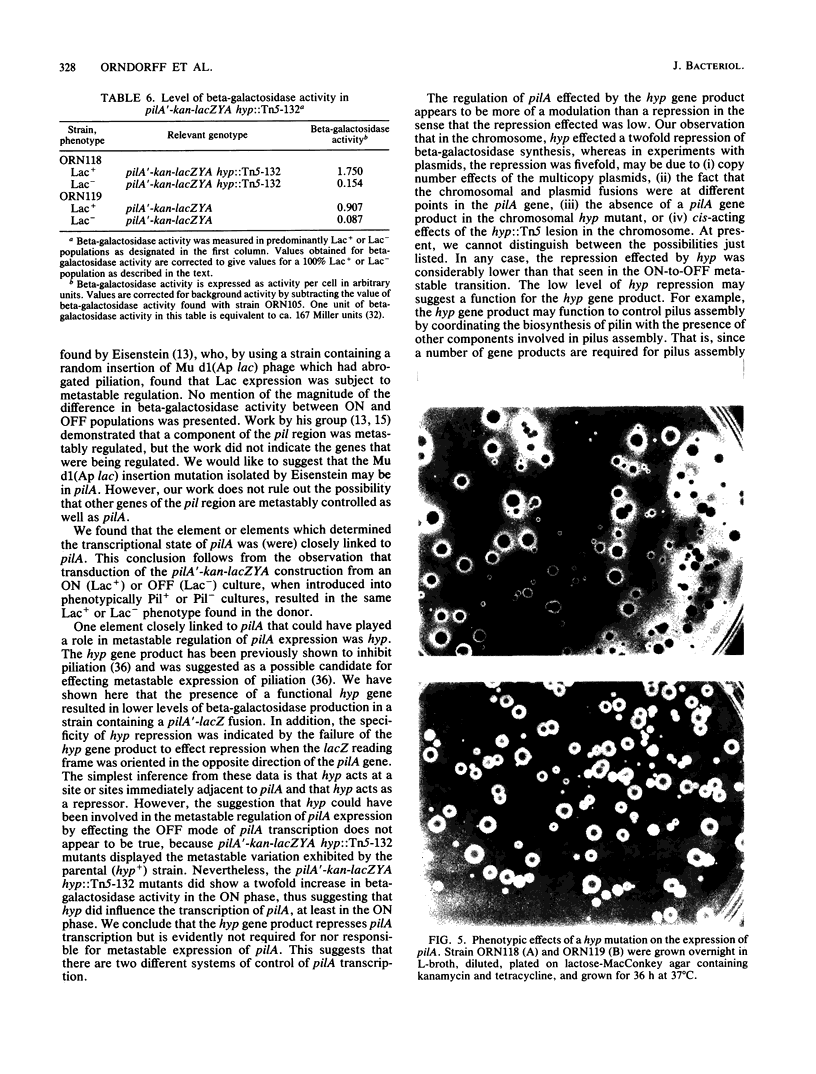
Type 1 piliation in Escherichia coli is subject to metastable regulation at the transcriptional level (B. I. Eisenstein, Science 214:337-339, 1981). However, the genes controlling in this fashion are not known. We present evidence that the pilA gene, encoding the structural subunit of type 1 pili, is subject to metastable transcriptional regulation. A pilA'-lacZ fusion, constructed in vitro on a recombinant plasmid, was used in conjunction with a recBC sbcB mutant of E. coli K-12 to introduce the fusion into the chromosomal region encoding Pil. This fusion was found to be subject to metastable transcriptional control. The rate of switching from the Lac+ to the Lac- phenotype was 4 X 10(-4) per cell per generation and 6.2 X 10(-4) in the opposite direction. A ca. 10-fold difference in beta-galactosidase activity was observed between phenotypically "ON" (Lac+) and "OFF" (Lac-) populations. P1 transduction experiments showed that the element determining the ON or OFF phenotype was tightly linked to pilA. In addition to the metastable regulation of pilA, a second type of transcriptional regulation was effected by the product of a gene, hyp, adjacent to pilA. By using a recombinant plasmid containing just a pilA'-lacZ fusion and the putative pilA promoter, we found that a lesion in hyp conferred a beta-galactosidase activity about fivefold higher than that of a strain possessing the parental hyp gene. Mutants constructed to have a pilA'-lacZ fusion and a hyp::Tn5-132 mutation in the chromosome exhibited a frequency of switching from Lac+ to Lac- and vice versa indistinguishable from that of the parental strain. However, in the ON mode, hyp::Tn5-132 mutants showed a twofold-higher beta-galactosidase activity. Thus, hyp does not appear to affect metastable variation but does affect the level of transcription of the pilA gene in the ON (transcribed) mode.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerswald E. A., Ludwig G., Schaller H. Structural analysis of Tn5. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):107–113. doi: 10.1101/sqb.1981.045.01.019. [DOI] [PubMed] [Google Scholar]
- BRINTON C. C., Jr Non-flagellar appendages of bacteria. Nature. 1959 Mar 21;183(4664):782–786. doi: 10.1038/183782a0. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E., Ludwig G., Auerswald E. A., Reiss B., Schaller H. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene. 1982 Oct;19(3):327–336. doi: 10.1016/0378-1119(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Berg D. E., Egner C., Hirschel B. J., Howard J., Johnsrud L., Jorgensen R. A., Tlsty T. D. Insertion, excision, and inversion of Tn5. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):115–123. doi: 10.1101/sqb.1981.045.01.020. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. P., Anderson E. S., Campbell I. Fimbriae and adhesive properties in Salmonellae. J Pathol Bacteriol. 1966 Jul;92(1):107–138. doi: 10.1002/path.1700920113. [DOI] [PubMed] [Google Scholar]
- Durkacz B. W., Sherratt D. J. Segregation kinetics of colicinogenic factor col E1 from a bacterial population temperature sensitive for DNA polymerase I. Mol Gen Genet. 1973;121(1):71–75. doi: 10.1007/BF00353694. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science. 1981 Oct 16;214(4518):337–339. doi: 10.1126/science.6116279. [DOI] [PubMed] [Google Scholar]
- Fader R. C., Avots-Avotins A. E., Davis C. P. Evidence for pili-mediated adherence of Klebsiella pneumoniae to rat bladder epithelial cells in vitro. Infect Immun. 1979 Aug;25(2):729–737. doi: 10.1128/iai.25.2.729-737.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag C. S., Abraham J. M., Clements J. R., Eisenstein B. I. Genetic analysis of the phase variation control of expression of type 1 fimbriae in Escherichia coli. J Bacteriol. 1985 May;162(2):668–675. doi: 10.1128/jb.162.2.668-675.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag C. S., Eisenstein B. I. Genetic mapping and transcriptional orientation of the fimD gene. J Bacteriol. 1983 Dec;156(3):1052–1058. doi: 10.1128/jb.156.3.1052-1058.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingeras T. R., Myers P. A., Olson J. A., Hanberg F. A., Roberts R. J. A new specific endonuclease present in Xanthomonas holcicola, Xanthomonas papavericola and Brevibacterium luteum. J Mol Biol. 1978 Jan 5;118(1):113–122. doi: 10.1016/0022-2836(78)90247-4. [DOI] [PubMed] [Google Scholar]
- Giphart-Gassler M., Plasterk R. H., van de Putte P. G inversion in bacteriophage Mu: a novel way of gene splicing. Nature. 1982 May 27;297(5864):339–342. doi: 10.1038/297339a0. [DOI] [PubMed] [Google Scholar]
- Goldmark P. J., Linn S. Purification and properties of the recBC DNase of Escherichia coli K-12. J Biol Chem. 1972 Mar 25;247(6):1849–1860. [PubMed] [Google Scholar]
- Greener A., Hill C. W. Identification of a novel genetic element in Escherichia coli K-12. J Bacteriol. 1980 Oct;144(1):312–321. doi: 10.1128/jb.144.1.312-321.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., Falkow S., Freter R., Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983 Apr;40(1):265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii Z., Clark A. J. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- Iwahi T., Abe Y., Nakao M., Imada A., Tsuchiya K. Role of type 1 fimbriae in the pathogenesis of ascending urinary tract infection induced by escherichia coli in mice. Infect Immun. 1983 Mar;39(3):1307–1315. doi: 10.1128/iai.39.3.1307-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasin M., Schimmel P. Deletion of an essential gene in Escherichia coli by site-specific recombination with linear DNA fragments. J Bacteriol. 1984 Aug;159(2):783–786. doi: 10.1128/jb.159.2.783-786.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. A., Berg D. E., Allet B., Reznikoff W. S. Restriction enzyme cleavage map of Tn10, a transposon which encodes tetracycline resistance. J Bacteriol. 1979 Jan;137(1):681–685. doi: 10.1128/jb.137.1.681-685.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Templin A., Clark A. J. Genetic recombination in Escherichia coli: the role of exonuclease I. Proc Natl Acad Sci U S A. 1971 Apr;68(4):824–827. doi: 10.1073/pnas.68.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old D. C., Duguid J. P. Selective outgrowth of fimbriate bacteria in static liquid medium. J Bacteriol. 1970 Aug;103(2):447–456. doi: 10.1128/jb.103.2.447-456.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old D. C., Scott S. S. Hemagglutinins and fimbriae of Providencia spp. J Bacteriol. 1981 Apr;146(1):404–408. doi: 10.1128/jb.146.1.404-408.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orndorff P. E., Falkow S. Identification and characterization of a gene product that regulates type 1 piliation in Escherichia coli. J Bacteriol. 1984 Oct;160(1):61–66. doi: 10.1128/jb.160.1.61-66.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orndorff P. E., Falkow S. Nucleotide sequence of pilA, the gene encoding the structural component of type 1 pili in Escherichia coli. J Bacteriol. 1985 Apr;162(1):454–457. doi: 10.1128/jb.162.1.454-457.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orndorff P. E., Falkow S. Organization and expression of genes responsible for type 1 piliation in Escherichia coli. J Bacteriol. 1984 Aug;159(2):736–744. doi: 10.1128/jb.159.2.736-744.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasterk R. H., Brinkman A., van de Putte P. DNA inversions in the chromosome of Escherichia coli and in bacteriophage Mu: relationship to other site-specific recombination systems. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5355–5358. doi: 10.1073/pnas.80.17.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Staskawicz B. J., Panopoulos N. J., Peters S., Honma M. A host-dependent hybrid plasmid suitable as a suicidal carrier for transposable elements. Plasmid. 1981 Nov;6(3):325–331. doi: 10.1016/0147-619x(81)90040-8. [DOI] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Swaney L. M., Liu Y. P., Ippen-Ihler K., Brinton C. C., Jr Genetic complementation analysis of Escherichia coli type 1 somatic pilus mutants. J Bacteriol. 1977 Apr;130(1):506–511. doi: 10.1128/jb.130.1.506-511.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney L. M., Liu Y. P., To C. M., To C. C., Ippen-Ihler K., Brinton C. C., Jr Isolation and characterization of Escherichia coli phase variants and mutants deficient in type 1 pilus production. J Bacteriol. 1977 Apr;130(1):495–505. doi: 10.1128/jb.130.1.495-505.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieg J., Simon M. Analysis of the nucleotide sequence of an invertible controlling element. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4196–4200. doi: 10.1073/pnas.77.7.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]




