Abstract
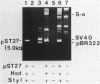
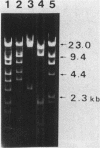
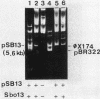
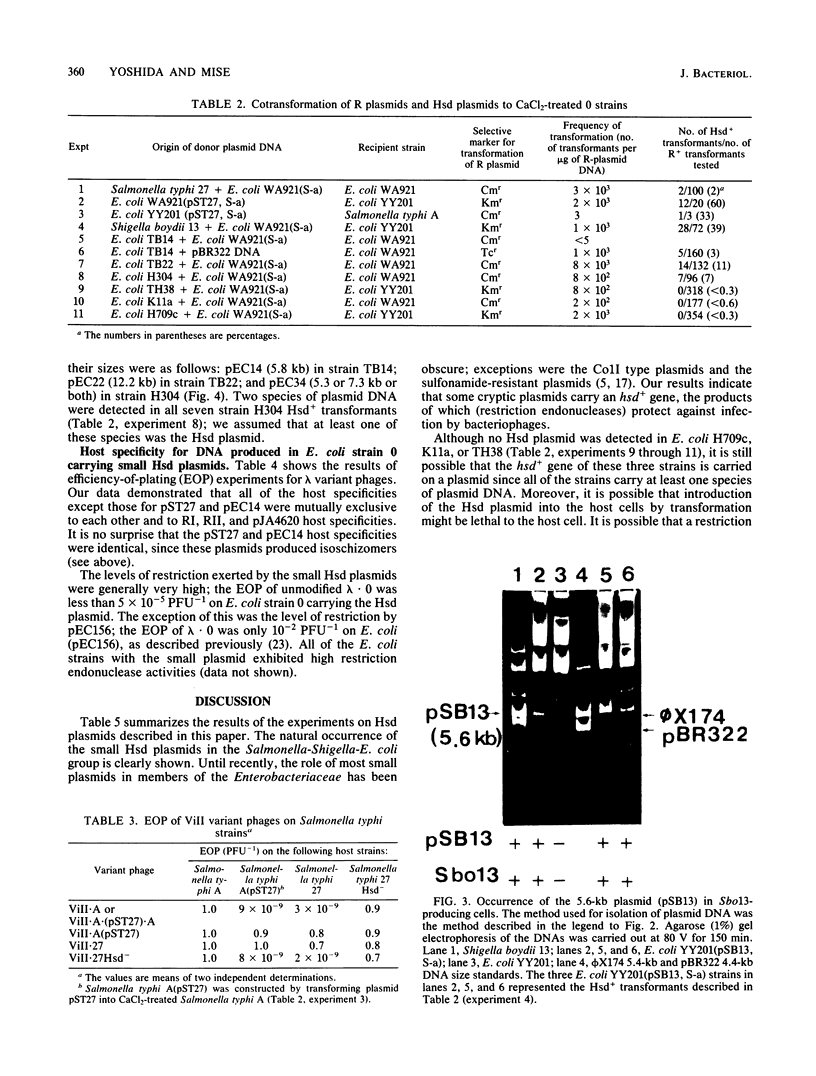
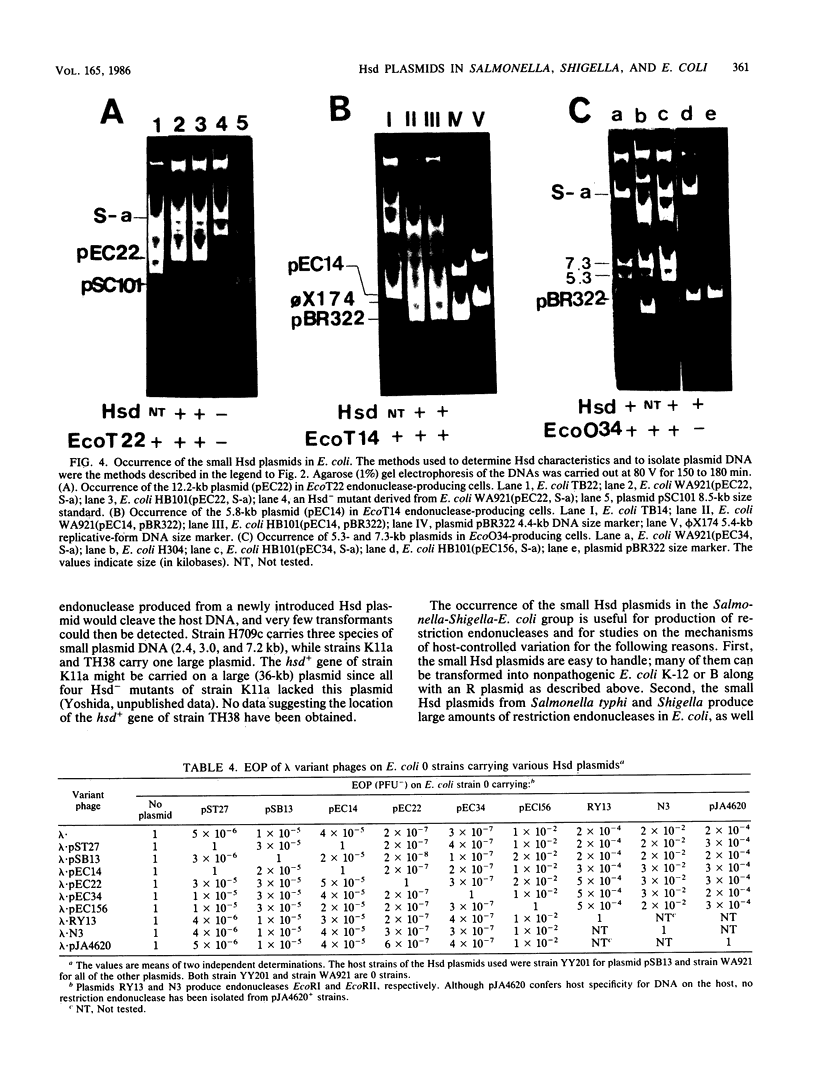
The natural occurrence of small Hsd (host specificity for DNA) plasmids was demonstrated in restriction endonuclease-producing strains of Salmonella typhi, Shigella boydii, and Escherichia coli. The five Hsd plasmids isolated were between 5.0 and 12.2 kilobases long. The copy number of all the Hsd plasmids was high (more than 10 copies per cell). Introduction of these small plasmids into E. coli strain 0 drastically lowered the efficiency of plating of the lambda.0 phages (the efficiency of plating was less than 5 X 10(-5) PFU-1). High restriction endonuclease activities were detected in the Hsd plasmid-positive strains because of the elevated copy numbers of the hsdR+ gene. The advantages of using E. coli strains containing the small Hsd plasmids for purification of type II restriction endonucleases are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON E. S., FRASER A. The influence of the factors determining Vi-type specificity in Salmonella typhi on the adaptation of Vi-phage II. J Gen Microbiol. 1955 Dec;13(3):519–532. doi: 10.1099/00221287-13-3-519. [DOI] [PubMed] [Google Scholar]
- Arai T., Aoki T. New R plasmid-mediated restriction-modification system of deoxyribonucleic acid conferred by group E R plasmids. J Bacteriol. 1977 Apr;130(1):529–531. doi: 10.1128/jb.130.1.529-531.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber W. DNA modification and restriction. Prog Nucleic Acid Res Mol Biol. 1974;14(0):1–37. doi: 10.1016/s0079-6603(08)60204-4. [DOI] [PubMed] [Google Scholar]
- Arber W., Linn S. DNA modification and restriction. Annu Rev Biochem. 1969;38:467–500. doi: 10.1146/annurev.bi.38.070169.002343. [DOI] [PubMed] [Google Scholar]
- BERNSTEIN A., WILSON E. M. AN ANALYSIS OF THE VI-PHAGE TYPING SCHEME FOR SALMONELLA TYPHI. J Gen Microbiol. 1963 Sep;32:349–373. doi: 10.1099/00221287-32-3-349. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bougueleret L., Schwarzstein M., Tsugita A., Zabeau M. Characterization of the genes coding for the Eco RV restriction and modification system of Escherichia coli. Nucleic Acids Res. 1984 Apr 25;12(8):3659–3676. doi: 10.1093/nar/12.8.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broach J. R. Construction of high copy yeast vectors using 2-microns circle sequences. Methods Enzymol. 1983;101:307–325. doi: 10.1016/0076-6879(83)01024-1. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier C., Bernardi G. Localization of E. coli endonuclease I. Biochem Biophys Res Commun. 1965 Sep 8;20(5):555–559. doi: 10.1016/0006-291x(65)90434-1. [DOI] [PubMed] [Google Scholar]
- Glatman L. I., Moroz A. F., Yablokova M. B., Rebentish B. A., Kcholmina G. V. A novel plasmid-mediated DNA restriction-modification system in E. coli. Plasmid. 1980 Nov;4(3):350–351. doi: 10.1016/0147-619x(80)90072-4. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Hardie M. M., Jamieson D., Powell L. M. Genetic map of the opp (Oligopeptide permease) locus of Salmonella typhimurium. J Bacteriol. 1983 Feb;153(2):830–836. doi: 10.1128/jb.153.2.830-836.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Mise K., Arber W. Plaque-forming transducing bacteriophage P1 derivatives and their behaviour in lysogenic conditions. Virology. 1976 Jan;69(1):191–205. doi: 10.1016/0042-6822(76)90206-3. [DOI] [PubMed] [Google Scholar]
- Mise K., Nakajima K. Isolation of restriction enzyme EcoVIII, an isoschizomer of HindIII, produced by Escherichia coli E1585-68. Gene. 1984 Oct;30(1-3):79–85. doi: 10.1016/0378-1119(84)90107-0. [DOI] [PubMed] [Google Scholar]
- Mise K., Nakajima K. Purification of a new restriction endonuclease, StyI, from Escherichia coli carrying the hsd+ miniplasmid. Gene. 1985;33(3):357–361. doi: 10.1016/0378-1119(85)90244-6. [DOI] [PubMed] [Google Scholar]
- Mise K. Recombinants between P22 and P1Cm. Virology. 1976 Jun;71(2):531–545. doi: 10.1016/0042-6822(76)90379-2. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H., Kutter E., Nakanishi M. A restriction map of the bacteriophage T4 genome. Mol Gen Genet. 1980;179(2):421–435. doi: 10.1007/BF00425473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Rowe B. Three new Escherichia coli O antigens O154, O155 and O156, and one new K antigen, K94. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Feb;81(1):59–64. doi: 10.1111/j.1699-0463.1973.tb02187.x. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Restriction and modification enzymes and their recognition sequences. Nucleic Acids Res. 1984;12 (Suppl):r167–r204. doi: 10.1093/nar/12.suppl.r167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanak N., Cramer J. H., Rownd R. H. EcoRI restriction endonuclease map of the composite R plasmid NR1. J Bacteriol. 1976 Jul;127(1):619–636. doi: 10.1128/jb.127.1.619-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Furuse C., Sakaizumi S. Transduction of various R factors by phage P1 in Escherichia coli and by phage P22 in Salmonella typhimurium. J Bacteriol. 1968 Nov;96(5):1791–1795. doi: 10.1128/jb.96.5.1791-1795.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- Yoshimori R., Roulland-Dussoix D., Boyer H. W. R factor-controlled restriction and modification of deoxyribonucleic acid: restriction mutants. J Bacteriol. 1972 Dec;112(3):1275–1279. doi: 10.1128/jb.112.3.1275-1279.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]