Abstract
The hybrid plasmid pNov1 readily acquired genetic information from the chromosome of wild-type, but not rec-2, cells. Most of the recombination had taken place 1 h after entrance of the plasmid into the cell, as judged by transformation of rec-2 by lysates made from wild-type cells exposed to pNov1. Measurement of physical transfer from radioactively labeled cellular DNA to plasmids recombining in wild-type cells failed, since there was little more radioactivity in plasmids from such cells than from labeled rec-2 recipients, in which no recombination took place. EcoRI digestion of pNov1 divided the DNA into a 1.7-kilobase-pair fragment containing the novobiocin resistance marker and a 13-kilobase-pair fragment containing all of the original vector and considerable portions homologous to the chromosome. Transformation by the large fragment alone resulted in a plasmid the size of the original pNov1. Our hypothesis to explain the data is that genetic transfer from chromosome to plasmid took place by a copy choice mechanism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnhart B. J., Cox S. H. Radiation-sensitive and radiation-resistant mutants of Haemophilus influenzae. J Bacteriol. 1968 Jul;96(1):280–282. doi: 10.1128/jb.96.1.280-282.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chak K. F., de Lencastre H., Liu H. M., Piggot P. J. Facile in vivo transfer of mutations between the Bacillus subtilis chromosome and a plasmid harbouring homologous DNA. J Gen Microbiol. 1982 Nov;128(11):2813–2816. doi: 10.1099/00221287-128-11-2813. [DOI] [PubMed] [Google Scholar]
- Elwell L. P., De Graaff J., Seibert D., Falkow S. Plasmid-linked ampicillin resistance in haempohilus influenza type b. Infect Immun. 1975 Aug;12(2):404–410. doi: 10.1128/iai.12.2.404-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc J. E., Setlow J. K. Postreplication repair of ultraviolet damage in Haemophilus influenzae. J Bacteriol. 1972 Jun;110(3):930–934. doi: 10.1128/jb.110.3.930-934.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez P., Espinosa M., Stassi D. L., Lacks S. A. Facilitation of plasmid transfer in Streptococcus pneumoniae by chromosomal homology. J Bacteriol. 1982 May;150(2):692–701. doi: 10.1128/jb.150.2.692-701.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K., McCarthy D., Clayton N. L. Transformation of Haemophilus influenzae by plasmid RSF0885. J Bacteriol. 1981 Dec;148(3):812–816. doi: 10.1128/jb.148.3.812-816.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K. Mechanism of bacterial transformation and transfection. Prog Nucleic Acid Res Mol Biol. 1974;14(0):39–100. doi: 10.1016/s0079-6603(08)60205-6. [DOI] [PubMed] [Google Scholar]
- Notani N., Goodgal S. H. On the nature of recombinants formed during transformation in Hemophilus influenzae. J Gen Physiol. 1966 Jul;49(6):197–209. doi: 10.1085/jgp.49.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Allison D. P., Beattie K. L. Relationship between prophage induction and transformation in Haemophilus influenzae. J Bacteriol. 1973 Jul;115(1):153–161. doi: 10.1128/jb.115.1.153-161.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Beattie K. L., Kimball R. F. A complex of recombination and repair genes in Haemophilus influenzae. J Mol Biol. 1972 Jul 21;68(2):361–378. doi: 10.1016/0022-2836(72)90218-5. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., McCarthy D., Clayton N. L. Novobiocin resistance marker in Haemophilus influenzae that is not expressed on a plasmid. J Bacteriol. 1982 Sep;151(3):1358–1362. doi: 10.1128/jb.151.3.1358-1362.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
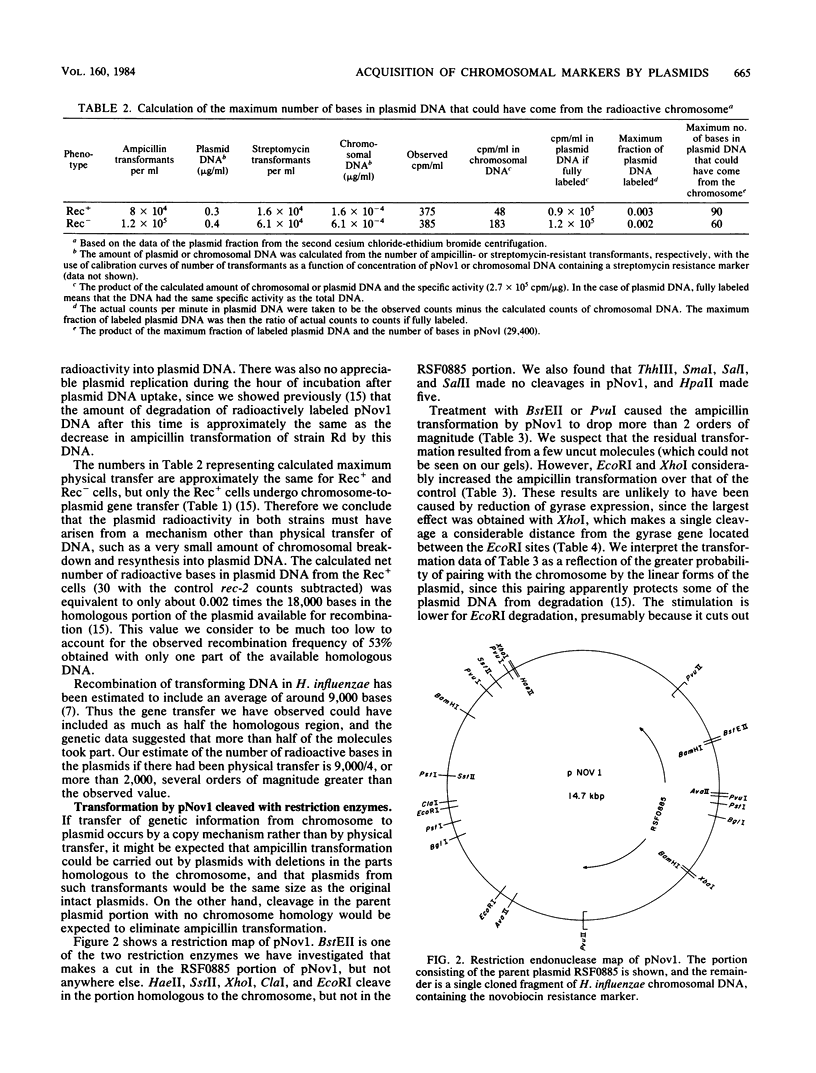
- Setlow J. K., Notani N. K., McCarthy D., Clayton N. L. Transformation of Haemophilus influenzae by plasmid RSF0885 containing a cloned segment of chromosomal deoxyribonucleic acid. J Bacteriol. 1981 Dec;148(3):804–811. doi: 10.1128/jb.148.3.804-811.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Spikes D., Ledbetter M. Loss of plasmids containing cloned inserts coding for novobiocin resistance or novobiocin sensitivity in Haemophilus influenzae. J Bacteriol. 1984 Jun;158(3):872–877. doi: 10.1128/jb.158.3.872-877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart W. L., Herriott R. M. Fate of recipient deoxyribonucleic acid during transformation in Haemophilus influenzae. J Bacteriol. 1968 Nov;96(5):1718–1724. doi: 10.1128/jb.96.5.1718-1724.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]