Abstract
Most modern eukaryotes diverged from a common ancestor that contained the α-proteobacterial endosymbiont that gave rise to mitochondria. The ‘amitochondriate’ anaerobic protist parasites that have been studied to date, such as Giardia and Trichomonas harbor mitochondrion-related organelles, such as mitosomes or hydrogenosomes. Yet there is one remaining group of mitochondrion-lacking flagellates known as the Preaxostyla that could represent a primitive ‘pre-mitochondrial’ lineage of eukaryotes. To test this hypothesis, we conducted an expressed sequence tag (EST) survey on the preaxostylid flagellate Trimastix pyriformis, a poorly-studied free-living anaerobe. Among the ESTs we detected 19 proteins that, in other eukaryotes, typically function in mitochondria, hydrogenosomes or mitosomes, 12 of which are found exclusively within these organelles. Interestingly, one of the proteins, aconitase, functions in the tricarboxylic acid cycle typical of aerobic mitochondria, whereas others, such as pyruvate:ferredoxin oxidoreductase and [FeFe] hydrogenase, are characteristic of anaerobic hydrogenosomes. Since Trimastix retains genetic evidence of a mitochondriate ancestry, we can now say definitively that all known living eukaryote lineages descend from a common ancestor that had mitochondria.
Introduction
The origin of the eukaryotic cell and mitochondria were major transitions in the evolution of life. However, the mechanisms and the temporal ordering of events underlying these transitions remain poorly understood. There are two main kinds of hypotheses regarding the sequence of events for these transitions. The first kind invokes the origin of the nucleus, cytoskeleton and endomembrane system to yield an amitochondriate eukaryote, followed later by the acquisition of the mitochondrion through endosymbiosis [1]–[3]. The second kind proposes that the mitochondrial endosymbiosis is the key innovation in eukaryogenesis, occurring simultaneously with the formation of the nucleus, or even beforehand [4], [5]. An important difference between these scenarios is that the first predicts that primitively amitochondriate eukaryotes (Archezoa) exist, or once existed but are now extinct, whereas, according to the latter hypotheses, no such organisms ever existed. Among unicellular eukaryotes there are several taxa that lack classical mitochondria (e.g. diplomonads, trichomonads, Entamoeba, pelobionts, Cryptosporidium, chytrid fungi, microsporidia, some ciliates and heterolobosea) and some of these groups were thought to actually be representatives of primitively amitochondriate Archezoa [6]. However, genes of mitochondrial origin have been identified in all of these groups indicating that they contain (or once contained) an organelle homologous to mitochondria [7]–[22]. The nuclear location of most genes encoding mitochondrial proteins is the result of genetic transfer from the endosymbiotic α-proteobacterial ancestor of the organelle. Immunological detection of proteins that function within the mitochondrion, such as those involved in iron-sulphur cluster biogenesis (IscS, IscU) or in protein import and refolding (mt-hsp70, cpn60) enabled the visualization of double-membrane bounded organelles in these “amitochondriates” [12], [15], [18], [20], [23], [24]. These organelles likely share a common evolutionary history with mitochondria implying that these organisms and presumably all modern eukaryotes diverged from an organism that contained a mitochondrion or its homolog [25].
However, there is still one key lineage of amitochondriate protists that has not been investigated: the Preaxostyla. This group is comprised of oxymonads, gut symbionts in animals, and the free-living flagellates of the genus Trimastix [26], [27]. The phylogenetic position of Preaxostyla has not yet been firmly established (compare [28] and [29]), but they are regarded as members of a eukaryotic “supergroup” Excavata [1], [27]. To date, the monophyly of the Excavata has not been proven, and thus it remains possible (although improbable) that Preaxostyla emerge at the base of eukaryotes, a position that would be consistent with a primitively amitochondriate status for this lineage. Double membrane bounded organelles presumed to be related to hydrogenosomes and mitochondria can be found in the cytoplasm of Trimastix [30]–[32]. Typically, no such organelles are observed in oxymonads [33]–[35]. Bloodgood et al. [36] reported large dense cytoplasmic bodies in the oxymonad Pyrsonympha, however, these authors consider them to be neither hydrogenosomes nor other derivatives of mitochondria and they may represent endosymbiotic or engulfed bacteria. Carpenter et al. [37] reported membrane-bounded, rounded, electron-dense bodies present in Saccinobaculus doroaxostylus, but absent in other investigated species of the genus. Some of their micrographs suggest that this body may be bounded by two membranes. We have chosen Trimastix pyriformis, as the representative of Preaxostyla, for an expressed sequence tag (EST) survey to search for genes of mitochondrial origin. The survey revealed 19 genes typical for mitochondria or hydrogenosomes that potentially function in a mitochondrion-related organelle in Trimastix.
Results and Discussion
We constructed a cDNA library from Trimastix pyriformis and sequenced 9615 expressed sequence tags (ESTs) that grouped into 2686 clusters. Using our bioinformatic tool Blastcompare, we found genes that unambiguously code for proteins functioning in mitochondria and related organelles in other organisms. In addition to these genes, we also found genes coding for the typical hydrogenosomal enzymes pyruvate:ferredoxin oxidoreductase (PFO), [FeFe] hydrogenase and two [FeFe] hydrogenase maturases–hydE and hydG. The complete list of putative organellar genes is given in Table 1.
Table 1. Information on the putative organellar genes in Trimastix.
| Product | Nr. of ESTs | Sequence accession numbers | N-terminal extension | Specific for mitochondria and plastids in eukaryotes | Tree fig. |
| Pyruvate:ferredoxin oxidoreductase * $ Pyruvate oxidative decarboxylation | 20 | EU086497 | No | No | S1 |
| [FeFe] hydrogenase * Hydrogen production | 27 | EU086507, EU086508, EU086509 | No | No | 2A |
| hydE Maturation of [FeFe] hydrogenase | 2 | EC836286.1, EC830619.1 | ? | ?† | - |
| hydG Maturation of [FeFe] hydrogenase | 3 | EC839247.1, EC836834.1, EC830944.1 | ? | ?† | - |
| Aconitase TCA cycle enzyme | 11 | EU086483, EU086484 | No | Yes | S2 |
| H-protein of glycine cleavage system central protein in GCS | 1 | EU086492 | Yes | Yes | S7 |
| P1-protein of GCS Glycine dehydrogenase (decarboxylating) subunit 1 | 2 | EU086490 | Yes | Yes | S5 |
| P2-protein of GCS Glycine dehydrogenase (decarboxylating) subunit 2 | 5 | EU086491 | ? | Yes | S6 |
| L-protein of GCS * $ Dihydrolipoyl dehydrogenase | 2 | EU086501, EU086502 | No | Yes | S4 |
| T-protein of GCS Aminomethyltransferase | 6 | EU086485 | Yes | Yes | S3 |
| Lipoyltransferase Lipoylisation of enzymes | 1 | EU086495 | ? | Yes | S8 |
| Pyridine nucleotide transhydrogenase alpha NAD and NADP interconversion | 5 | EC831884, EC832444, EC832298, EC837926, EC836257 | ? | Yes | - |
| Pyridine nucleotide transhydrogenase beta NAD and NADP interconversion | 1 | EU086499 | ? | Yes | S9 |
| TOM40 Protein transport | 3 | EU086500 | ? | Yes | 2B |
| Mitochondrial processing protease - large subunit * Targeting sequence cleavage | 2 | EU086496 | No | Yes | S10 |
| Cpn60 * Protein folding | 1 | EU086489 | Yes | Yes | 2C |
| Mitochondrial carrier 1 Transport of molecules across membrane | 4 | EU086488 | ? | No | S11 |
| Mitochondrial carrier 2 Transport of molecules across membrane | 2 | EU086487 | ? | No | S11 |
| Mitochondrial carrier 3 Transport of molecules across membrane | 8 | EC831184, EC835350, EC835010, EC838819, EC834757, EC836477, EC839962, EC834636 | ? | No | - |
The 5′ end of the cDNA obtained by 5′ RACE,
Genomic sequence determined, ?–N-terminal sequence is incomplete,
data are available for only two eukaryotes.
The codon usages of the genes are similar to the codon usages of other Trimastix genes. Preferred codons have relatively high GC content and most often contain C in the 3rd position. This observation increased our confidence that the genes originated from Trimastix transcripts and do not represent the contamination from bacteria that are present in the culture.
We used rapid amplification of cDNA ends (RACE) to characterize the full-length sequences of some of these genes. For nine genes, the N-terminus of the protein sequence was identified. Four of them contained N-terminal extensions compared to bacteria. They were not recognized as mitochondrial pre-sequences by prediction software TargetP and Mitoprot, but possessed relevant hallmarks of targeting signals–rich in small, hydroxylated, hydrophobic and positively charged amino acids (Table 1, Figure 1).
Figure 1. The N-terminal portion of alignments showing the extensions on Trimastix sequences.
(A) H-, (B) P1-, (C) T-proteins of GCS and (D) cpn60.
The cellular localization of the gene products was not proven experimentally; however, we could tentatively infer their localization based on their phylogenetic relationship to other mitochondrial or hydrogenosomal homologs and, if present, on the putative N-terminal targeting peptides. The putative organellar proteins fell into five functional classes that are reviewed below.
Energy metabolism
We identified key enzymes of anaerobic energy metabolism, PFO and [FeFe] hydrogenase and one protein involved in the energy metabolism of typical aerobic mitochondria, aconitase.
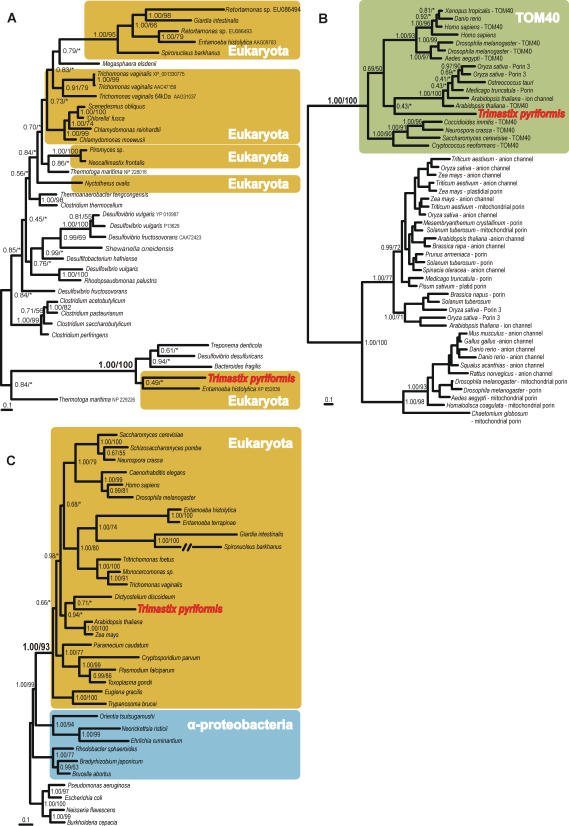
In the majority of aerobic eukaryotes, decarboxylation of pyruvate to form acetyl-CoA is performed by the pyruvate dehydrogenase complex (PDH), which is located in mitochondria. By contrast, in anaerobes, the non-homologous enzyme PFO typically catalyzes this reaction and, in the process, electrons are transferred to a ferredoxin. In the hydrogenosomes of chytrid fungi, pyruvate is degraded by yet another type of enzyme, pyruvate:formate lyase, and no electrons are released. PFO can be located in the cytosol (i.e. Giardia, Entamoeba) [38], [39] or in hydrogenosomes (i.e. Trichomonas) [40]. In our phylogenetic analysis, eukaryotic PFO sequences formed a single, poorly-supported clade (Figure S1), in which the Trimastix sequence emerged as a sister lineage to two cytosolic Entamoeba sequences (1.00 PP/67% BP).
Hydrogenases are widely distributed among eukaryotes and prokaryotes and can be divided into three different classes: [FeFe], [NiFe] and metal-free hydrogenases [41], [42]. They can be cytosolic, membrane bound, periplasmic or organellar and catalyze the coupling of electrons with protons to form hydrogen gas or the reverse reaction. We found three distinct sequences of [FeFe] hydrogenase among the ESTs (Table 1). The sequence EU086507 was completed on the 5′ end and sequences EU086508 and EU086509 on both ends using RACE. The obtained coding sequences differed in both length (292–445 aa) and sequence (56–70 aa differences) but formed a robust clade in the tree (not shown). EU086507 corresponded to the most abundant cluster (22 ESTs) and was used for phylogenetic reconstruction (Figure 2A). The sequence branched robustly outside of most other eukaryotes in a strongly supported (1.00 PP/100% BP) group that also contained three bacterial and one Entamoeba sequence that weakly formed its sister branch. This gene phylogeny strongly indicated that Trimastix acquired its hydrogenase independently from the majority of eukaryotes. The specific relationship of both key enzymes of anaerobic metabolism, PFO and [FeFe] hydrogenase, to homologs from Entamoeba indicates a possible lateral gene transfer of these enzymes between Trimastix and Entamoeba. Two out of three genes required for maturation of [FeFe] hydrogenases were found among the ESTs–hydE and hydG. Although these proteins are regarded as mandatory for the production of the active [FeFe] hydrogenase enzyme in bacteria [42], they have been reported only from two eukaryotes so far, Chlamydomonas [43] and Trichomonas [44], and are absent from the draft genome sequences of Giardia and Entamoeba.
Figure 2. Phylogenetic trees of (A) [FeFe] hydrogenase, (B) TOM40 and (C) cpn60.
Trees were constructed using MrBayes. Numbers on the branches represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstrap support computed with RAxML. Asterisks (*) indicate a bootstrap value of <50%. In (B) brief names of the proteins annotated according to GenBank nomenclature follow the taxon names. The statistical support for non-relevant nodes was not included for simplicity.
Aconitase performs stereo-specific isomerization of citrate to isocitrate and is present both in mitochondria (a part of the TCA cycle) and the cytosol, but the two types are unrelated and form distinct clades in the phylogenetic tree (Figure S2). In Trimastix, both types were detected but the mitochondrial one appears to be more highly expressed because it was found in 11 ESTs, in contrast to the cytosolic type, the partial sequence of which was present in only a single EST (not included in the tree).
Amino acid metabolism
Five proteins comprising a system that decarboxylates a single amino acid were detected among the Trimastix ESTs. They assemble to a complete glycine cleavage system complex (GCS) that is usually located in mitochondria and performs rapid breakdown of glycine molecules to produce methyl-tetrahydrofolate and NADH. The H-protein plays a pivotal role in the GCS complex; its lipoyl group interacts with three GCS proteins: P-protein (glycine dehydrogenase [decarboxylating]), L-protein (dihydrolipoyl dehydrogenase), and T-protein (aminomethyltransferase) [45]. In bacterial systems, the P-protein is composed of two subunits that are encoded by two distinct genes. This is in contrast to eukaryotes where the P-protein is encoded as a single polypeptide. In Trimastix, the transcripts for the two subunits were found in two different clusters, each containing a poly A tail, indicating that they may be transcribed separately. The L-protein (dihydrolipoyl dehydrogenase) is an enzyme shared by four pathways, all of which are mitochondrial. In addition to being part of the GCS, it can function as the E3 subunit of the PDH complex, the oxoglutarate dehydrogenase complex and the branched-chain alpha-keto acid dehydrogenase complex [46]. Because the other subunits of the latter three complexes were missing among the ESTs and PFO appears to have taken the role of PDH, it is probable that the dihydrolipoyl dehydrogenase in Trimastix is involved exclusively in the GCS complex. Curiously, our phylogenetic analyses indicated that the GCS has a mixed evolutionary origin in Trimastix. While both T- and L-protein sequences were robustly embedded within the eukaryotic mitochondrial clade (Figures S3 and S4), the two P-protein subunits were robustly related to α-proteobacteria (Figures S5 and S6) and the H-protein branched weakly with bacteria, although the overall tree topology was only poorly supported (Figure S7). The N-terminus of the H-, P1- and T-proteins included extensions compared to bacterial sequences (Figure 1). It was recently reported that the Trichomonas vaginalis hydrogenosome also harbors some GCS subunits, however it seems that the GCS is incomplete, consisting only of H- and L-proteins, the latter being of apparent prokaryotic origin [47].
Cofactor metabolism
Three proteins involved in metabolism of cofactors were detected–lipoyltransferase and both subunits of pyridine nucleotide transhydrogenase (PNT).
Lipoyltransferase typically performs the first step in lipoylation (covalent binding of lipoic acid) of several enzymes functioning in complexes involved in oxidative and amino acid metabolism [48], [49]. All of these enzyme complexes are present in bacteria, and those in eukaryotes are located in mitochondria and plastids. In the phylogeny of lipoyltransferase (Figure S8), the Trimastix homolog grouped weakly with Dictyostelium (0.58 PP/36% BP) as a deep branch of a moderately supported clade (1.00 PP/79% BP) that consisted mostly of eukaryotes, but included one branch of archaeal and bacterial lipoyltransferases.
PNT is an enzyme exclusively located in the inner membrane of mitochondria or the cytoplasmic membrane of bacteria and transfers hydride ion equivalents between NAD(H) and NADP(H) and, in the process, translocates protons across the inner mitochondrial/cellular membrane [50]. The enzyme is a homodimer and one monomer consists of two domains (α and β) that are expressed as two proteins in Escherichia coli but as a single protein in eukaryotes, e. g. Bos, Eimeria and Entamoeba [50]. The two domains of the Trimastix PNT were found in different EST clusters, each containing a poly A tail, indicating that they are transcribed separately. The sequence available for the α-subunit was too short for a reliable phylogenetic reconstruction (<<100 amino acids). In the phylogenetic tree made from β subunit sequences (Figure S9), Trimastix formed a branch with Entamoeba (1.00 PP/81% BP) at the base of an exclusively eukaryotic clade, however with relatively low support (0.99 PP/51% BP).
Protein import and maturation
Three proteins involved in protein import and maturation were detected suggesting that the Trimastix organelle actively retains the ability to translocate (i.e. import) nuclear-encoded/cytoplasmically translated proteins. The proteins are: mitochondrial translocase of the outer membrane 40 (TOM40), chaperonin 60 (cpn60) and the α-subunit of the mitochondrial processing peptidase (α-MPP). The TOM complex is specific to eukaryotes and evolved early after endosymbiosis [51]. TOM40 is a β-barrel protein that forms the translocation channel in the membrane. It is an essential part of the complex and it seems to be universally distributed among eukaryotes [51], [52]. Our phylogenetic analyses strongly suggest that the Trimastix EST sequence is a TOM40 homolog as it is robustly (1.00 PP/100% BP) embedded in a clade of plant, animal and fungal sequences that, with a few exceptions, have been annotated as TOM40 (Figure 2B). The presence of TOM40 in the anaerobic excavate Trimastix further strengthens the hypothesis that this protein represents an early eukaryotic invention.
MPP is responsible for the processing of N-terminal pre-sequences after proteins have been imported into the mitochondrial matrix. MPP is active usually as a heterodimer of the paralogous alpha and beta subunits [53], the exceptions are Trichomonas and Giardia, in which only beta subunits were found [54]–[56] functioning as a homodimer [56]. The alpha-subunit participates in substrate binding and possibly product release while the catalytic activity responsible for transit peptide cleavage resides in the beta-subunit [53], [57]. All sites required for enzymatic activity [58] are present in the putative α-MPP protein we recovered from Trimastix. Phylogenetic analyses of MPP protein sequences robustly supported clades of the alpha and beta subunits (Figure S10) and the Trimastix sequence was embedded within the α-MPP clade with the Trypanosoma homolog as a sister branch (1.00 PP/79% BP). Eukaryote and α-proteobacterial peptidases shared a most recent common ancestor consistent with the hypothesis that MPP came into eukaryotes with the ancestor of the mitochondrion [57]. The gene duplication leading to the closely related MPP paralogs occurred very early in eukaryote history, probably before the divergence of extant eukaryotes.
Cpn60 is the mitochondrial homolog of GroEL and is involved with the refolding of proteins imported into mitochondria. This molecule is often used as a ‘mitochondrial marker’ because it unambiguously traces its ancestry to α-proteobacteria and is localized not only in mitochondria but also in the hydrogenosome of Trichomonas and the mitosomes of Entamoeba and Giardia [11], [15], [54]. Cpn60 from Trimastix branched within the eukaryote mitochondrial clade with very high statistical support (1.00 PP/93% BP), but without strong affiliation to any particular organism or group (Figure 2C). The various bioinformatic tools we employed did not recognize a mitochondrial targeting signal on the Trimastix cpn60 protein, however it does possess an N-terminal extension relative to bacterial homologs (Fig. 1).
Transport of other molecules
Three members of the mitochondrial carrier family were identified among the Trimastix ESTs (Table 1). This diverse family of proteins facilitates the bidirectional transport of metabolites, nucleotides, amino acids, co-factors, carboxylic acids and inorganic anions, across the inner membrane of the mitochondrion. A few members were also found in the membranes of peroxisomes and plastids [59]–[61]. One member of this family, the ATP/ADP translocator, was detected in the hydrogenosomal inner membrane of Trichomonas, Neocallimastix and in the mitosomal inner membrane of Entamoeba [62]–[64]. The sequences of the three Trimastix homologs were clearly different from each other. The sequence of carrier 3 was the most divergent; moreover, its C-terminus contained a MQGP-rich repetition and showed no sequence similarity to any other eukaryote homolog. The other two carriers were included in the phylogenetic analysis of this protein family (Figure S11). Although support for the backbone tree topology was generally low, it roughly corresponded to substrate specificities. Trimastix carrier 1 showed a weak phylogenetic affiliation to adenine nucleotide (e.g. ATP, NAD) transporters and, in agreement with this, the sequence contained motifs characteristic for this category of carriers representing the binding site of adenine nucleotides [65]–GQ at positions 182/183 and R at position 83 (numbering according to [65]). Carrier 2 did not contain any specific binding site motifs and clustered with pyruvate and folate transporters in the tree.
Conclusions
Among the ESTs we identified 12 proteins that are unique to mitochondrial- or plastid-derived organelles and have never been observed in other cellular compartments of eukaryotes (Table 1). Although we do not have direct evidence of organelle targeting, four of these proteins show clear N-terminal extensions. The proteins known to be specific to the outer (TOM40) or inner (PNTα, PNTβ) membrane of mitochondria are also very unlikely to function in other ‘non-mitochondrial’ membranes in Trimastix and the traditional function assigned to MPP makes sense only if localized in the organellar matrix.
Considering these data, there is little doubt that Trimastix had a mitochondriate past. Furthermore, given the presence of N-terminal extensions on mitochondrion ‘hallmark’ proteins as well as our finding of a component of the protein import system it seems likely that Trimastix contains anaerobic organelles homologous to mitochondria. These in turn likely correspond to the densely-staining double membrane bounded structures described in electron micrographs of these organisms [30]–[32]. As the Preaxostyla (Trimastix and oxymonads) were the last major candidate primitive ‘pre-mitochondrial’ eukaryote group, we can now say definitively that all known extant eukaryote lineages diverged after the mitochondrial symbiosis.
Enzymes typical for anaerobic metabolism and the characteristic enzymes of hydrogenosomes (PFO and [FeFe] hydrogenase) are relatively highly-expressed in Trimastix (each comprised >0.2% of the ESTs). Future localization studies are needed to establish if these enzymes are indeed active in the mitochondrion-derived organelles as in hydrogenosomes or in the cytosol, like in mitosome-containing eukaryotes. The potential presence of a TCA cycle enzyme in the organelle indicates that Trimastix harbors another unique version of anaerobic mitochondrion-like organelles with a unique spectrum of metabolic properties.
Materials and Methods
Cultures and Molecular biology
Trimastix pyriformis (ATCC 50935) was grown at room temperature under anaerobic/microaerophilic conditions in 1 litre tissue culture flasks (tightly sealed) in Sonneborn's Paramecium medium (ATCC 802 medium) pre-inoculated with Stenotrophomonas maltophilia as the sole food source. Cells in exponential growth were harvested by centrifugation at 1200×g for 10 minutes at 4°C. Total RNA was isolated via Tri-reagent (Sigma-Aldrich). Approximately 3mg of total RNA was sent to a commercial vendor (Agencourt Bioscience, Beverly MA, USA), to construct the cDNA library used for EST sequencing.
Both 5′ and 3′ RACE were performed from oligo-capped 1st strand cDNA. PolyA+ RNA was enriched from approximately 1 mg of total RNA with the Poly(A) Purist kit (Ambion, Austin TX) and full-length 1st strand cDNA was prepared with the GeneRacer Kit using SuperScript III RT (Invitrogen, Carlsbad CA). This served as template for both 5′ and 3′ RACE utilizing Taq DNA polymerase (Sigma) with either the GeneRacer 5′ or 3′ primer (GR5′/GR3′ primer) plus a gene specific primer. Often nested reactions were necessary to obtain the desired RACE products: NestedGR5′/NestedGR3′ primer plus a nested gene specific primer, plus 0.5–1.0 µl (of 50 µl) of the primary RACE PCR reaction as template. Products were cloned into TOPO T/A pCR2.1 vector (Invitrogen) and sequenced. For genomic gene sequencing, gDNA was obtained either via Tri-reagent (as a “by product” of RNA isolation) or using the PureGene DNA isolation kit (Gentra Systems, Minneapolis MN). Gene specific primers were used for PCR and products were cloned into TOPO T/A pCR2.1 vector (Invitrogen) and sequenced.
Selected clones from the cDNA library were completely sequenced using vector and gene specific primers.
Comparative BLAST searching of the Trimastix clusters
Four databases were used in ‘subtractive’ BLAST searching to identify putative mitochondrial proteins in the Trimastix ESTs. These included mitochondrial proteome data bases from the human mitoproteome and the yeast Mitop2 database [66], [67] and ‘subtractive’ databases, created by removing proteins matching the mitochondrial proteomes from the whole predicted proteomes of these organisms. The Trimastix clusters were then compared to all four databases, human/yeast non-mitochondrial subtractive and human/yeast mitoproteome, using BLAST [68]. The top scoring hits for each of the subtractive and mitoproteome databases were then compared and the corresponding queries were then sorted into one of four categories: 1) Both human and yeast top hits from the mitoproteome, 2) both human and yeast top hits from the subtractive proteome, 3) human and yeast top hits from different databases, 4) or there were no significant hits in any of the databases. 128 clusters that fell into category 1 or 3 were manually inspected by comparison to the GenBank non-redundant (nr) database using BLAST and ψ-BLAST. For the most ambiguous cases preliminary trees were constructed for the cluster sequence and a selection of its homologs from the nr database. This approach narrowed the selection to 18 clusters coding for 17 unique genes.
Codon usage analysis and removal of probable contaminants of ESTs
Using the INCA2 software (http://www.bioinfo-hr.org/en/research/inca/), the frequencies of codons (codon usages) of the putative mitochondrial/hydrogenosomal genes were compared with the frequency of codons found in 29 other bona fide Trimastix genes that were downloaded from the GenBank nucleotide nr database or assembled from Trimastix EST project.
One transcript for a putative mitochondrial carrier (EC837420) showed considerable differences in codon usage. Since the transcript was present in a single EST clone and the clone did not contain a poly-A tail, we regarded it to be a possible contaminant of the ESTs and removed it from the list of putative organellar proteins.
A transcript encoding the B14 subunit of the mitochondrial electron transport chain complex I (EU086486) also differed in codon usage from other Trimastix genes. This transcript was also present in a single EST, and so we regarded its occurrence in Trimastix as questionable. We carried out several checks to confirm its presence in Trimastix: PCR and nested PCR using exact match gene specific primers on gDNA and cDNA as template, 3′ and 5′ RACE using GeneRacer (Invitrogen, Carlsbad CA) and gene specific primers, and finally hybridization of a DIG-labeled probe prepared using the PCR digoxygenin (DIG) Synthesis Kit (Roche Diagnostics Corp.) to a Southern blot of restriction enzyme digested Trimastix gDNA. As none of these experiments showed positive results, we regard this transcript as a probable rare contaminant of the cDNA library and we removed it from the list of putative organellar proteins.
Construction of phylogenetic trees
Orthologs of the Trimastix genes were downloaded from GenBank, and from the Trichomonas vaginalis (http://www.tigr.org/tdb/e2k1/tvg/) and Entamoeba histolytica (http://www.tigr.org/tdb/e2k1/eha1/) genome projects. Sequences were aligned by ClustalW implemented in BioEdit 7.0.5.3 [69] or using the ProbCons server (http://probcons.stanford.edu/) [70]. Alignments were manually refined in BioEdit 7.0.5.3 and unambiguously aligned positions were subjected to phylogenetic analyses using RAxML [71] and MrBayes 3.1.2. [72]. The PROTMIXWAG model was used in RAxML and the branching support was assessed by 100 bootstrap replicates. Two parallel runs of four chains (temp = 0.5) were run in MrBayes 3.1.2. using the JTT+Γ model with 8 discrete-rate categories. The run was considered as converged after the average standard deviation of split frequencies dropped below 0.01. The profile of tree likelihoods was inspected and the first 25% of the trees were removed from the consensus as the burn-in.
Supporting Information
Phylogenetic tree of PFO. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.58 MB TIF)
Phylogenetic tree of aconitase. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.68 MB TIF)
Phylogenetic tree of T-protein of GCS. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.69 MB TIF)
Phylogenetic tree of L-protein of GCS. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.52 MB TIF)
Phylogenetic tree of P1-protein of GCS. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.63 MB TIF)
Phylogenetic tree of P2-protein of GCS. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.71 MB TIF)
Phylogenetic tree of H-protein of GCS. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.56 MB TIF)
Phylogenetic tree of lipoyltransferase. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.62 MB TIF)
Phylogenetic tree of β subunit of pyridine nucleotide transhydrogenase. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.54 MB TIF)
Phylogenetic tree of mitochondrial processing peptidase. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.48 MB TIF)
Phylogenetic tree of mitochondrial carrier protein family. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.48 MB TIF)
Acknowledgments
The authors would like to thank Dr. Miklós Müller and an anonymous reviewer for helpful comments on the manuscript and Jacqueline de Mestral for technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Trimastix pyriformis EST data were generated as part of AJRs involvement in the Genome Atlantic/Genome Canada sponsored Protist EST Program Project. This work and partial salary support for VH, JDS and SDT came from an operating grant MOP-62809 from the Canadian Institutes for Health Research (CIHR) awarded to AJR. VH was also supported by a fellowship from the Canadian Institute for Advanced Research (CIFAR). AS was supported by a postdoctoral fellowship from the Canadian Institutes of Health Research (CIHR). AJR is supported by the E.W.R. Steacie Memorial Fellowship from the Natural Sciences and Engineering Research Council of Canada and the CIFAR Program in Integrated Microbial Diversity.
References
- 1.Cavalier-Smith T. The phagotrophic origin of eukaryotes and phylogenetic classification of protozoa. Int J Syst Evol Microbiol. 2002;52:297–354. doi: 10.1099/00207713-52-2-297. [DOI] [PubMed] [Google Scholar]
- 2.Margulis L, Dolan MF, Guerrero R. The chimeric eukaryote: origin of the nucleus from the karyomastigont in amitochondriate protists. Proc Natl Acad Sci U S A. 2000;97:6954–6959. doi: 10.1073/pnas.97.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreira D, Lopez-Garcia P. Symbiosis between methanogenic archaea and delta-proteobacteria as the origin of eukaryotes: The syntrophic hypothesis. J Mol Evol. 1998;47:517–530. doi: 10.1007/pl00006408. [DOI] [PubMed] [Google Scholar]
- 4.Martin W, Müller M. The hydrogen hypothesis for the first eukaryote. Nature. 1998;392:37–41. doi: 10.1038/32096. [DOI] [PubMed] [Google Scholar]
- 5.Karlin S, Brocchieri L, Mrazek J, Campbell AM, Spormann AM. A chimeric prokaryotic ancestry of mitochondria and primitive eukaryotes. Proc Natl Acad Sci U S A. 1999;96:9190–9195. doi: 10.1073/pnas.96.16.9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavalier-Smith T. Endosymbiotic origin of the mitochondrial envelope. In: Schwemmler W, Schenk HEA, editors. Endocytobiology II. Berlin: de Gruyter; 1983. pp. 265–279. [Google Scholar]
- 7.Clark CG, Roger AJ. Direct evidence for secondary loss of mitochondria in Entamoeba histolytica. Proc Natl Acad Sci U S A. 1995;92:6518–6521. doi: 10.1073/pnas.92.14.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horner DS, Hirt RP, Kilvington S, Lloyd D, Embley TM. Molecular data suggest an early acquisition of the mitochondrion endosymbiont. P Roy Soc Lond B Bio. 1996;263:1053–1059. doi: 10.1098/rspb.1996.0155. [DOI] [PubMed] [Google Scholar]
- 9.Bui ET, Bradley PJ, Johnson PJ. A common evolutionary origin for mitochondria and hydrogenosomes. Proc Natl Acad Sci U S A. 1996;93:9651–9656. doi: 10.1073/pnas.93.18.9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Germot A, Philippe H, Le Guyader H. Presence of a mitochondrial-type 70-kDa heat shock protein in Trichomonas vaginalis suggests a very early mitochondrial endosymbiosis in eukaryotes. Proc Natl Acad Sci U S A. 1996;93:14614–14617. doi: 10.1073/pnas.93.25.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roger AJ, Clark CG, Doolittle WF. A possible mitochondrial gene in the early-branching amitochondriate protist Trichomonas vaginalis. Proc Natl Acad Sci U S A. 1996;93:14618–14622. doi: 10.1073/pnas.93.25.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bozner P. Immunological detection and subcellular localization of HSP70 and HSP60 homologs in Trichomonas vaginalis. J Parasitol. 1997;83:224–229. [PubMed] [Google Scholar]
- 13.Hirt RP, Healy B, Vossbrinck CR, Canning EU, Embley TM. A mitochondrial Hsp70 orthologue in Vairimorpha necatrix: Molecular evidence that microsporidia once contained mitochondria. Curr Biol. 1997;7:995–998. doi: 10.1016/s0960-9822(06)00420-9. [DOI] [PubMed] [Google Scholar]
- 14.Roger AJ, Svard SG, Tovar J, Clark CG, Smith MW, et al. A mitochondrial-like chaperonin 60 gene in Giardia lamblia: evidence that diplomonads once harbored an endosymbiont related to the progenitor of mitochondria. Proc Natl Acad Sci U S A. 1998;95:229–234. doi: 10.1073/pnas.95.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tovar J, Fischer A, Clark CG. The mitosome, a novel organelle related to mitochondria in the amitochondrial parasite Entamoeba histolytica. Mol Microbiol. 1999;32:1013–1021. doi: 10.1046/j.1365-2958.1999.01414.x. [DOI] [PubMed] [Google Scholar]
- 16.Tachezy J, Sánchez LB, Müller M. Mitochondrial type iron-sulfur cluster assembly in the amitochondriate eukaryotes Trichomonas vaginalis and Giardia intestinalis, as indicated by the phylogeny of IscS. Mol Biol Evol. 2001;18:1919–1928. doi: 10.1093/oxfordjournals.molbev.a003732. [DOI] [PubMed] [Google Scholar]
- 17.Horner DS, Embley TM. Chaperonin 60 phylogeny provides further evidence for secondary loss of mitochondria among putative early-branching eukaryotes. Mol Biol Evol. 2001;18:1970–1975. doi: 10.1093/oxfordjournals.molbev.a003737. [DOI] [PubMed] [Google Scholar]
- 18.Williams BAP, Hirt RP, Lucocq JM, Embley TM. A mitochondrial remnant in the microsporidian Trachipleistophora hominis. Nature. 2002;418:865–869. doi: 10.1038/nature00949. [DOI] [PubMed] [Google Scholar]
- 19.LaGier MJ, Tachezy J, Stejskal F, Kutišová K, Keithly JS. Mitochondrial-type iron-sulfur cluster biosynthesis genes (IscS and IscU) in the apicomplexan Cryptosporidium parvum. Microbiology. 2003;149:3519–3530. doi: 10.1099/mic.0.26365-0. [DOI] [PubMed] [Google Scholar]
- 20.van der Giezen M, Birdsey GM, Horner DS, Lucocq J, Dyal PL, et al. Fungal hydrogenosomes contain mitochondrial heat-shock proteins. Mol Biol Evol. 2003;20:1051–1061. doi: 10.1093/molbev/msg103. [DOI] [PubMed] [Google Scholar]
- 21.Hrdý I, Hirt RP, Doležal P, Bardoňová L, Foster PG, et al. Trichomonas hydrogenosomes contain the NADH dehydrogenase module of mitochondrial complex I. Nature. 2004;432:618–622. doi: 10.1038/nature03149. [DOI] [PubMed] [Google Scholar]
- 22.Dyall SD, Yan WH, Delgadillo-Correa MG, Lunceford A, Loo JA, et al. Non-mitochondrial complex I proteins in a hydrogenosomal oxidoreductase complex. Nature. 2004;431:1103–1107. doi: 10.1038/nature02990. [DOI] [PubMed] [Google Scholar]
- 23.Tovar J, Leon-Avila G, Sánchez LB, Šuťák R, Tachezy J, et al. Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation. Nature. 2003;426:172–176. doi: 10.1038/nature01945. [DOI] [PubMed] [Google Scholar]
- 24.Riordan CE, Ault JG, Langreth SG, Keithly JS. Cryptosporidium parvum Cpn60 targets a relict organelle. Curr Genet. 2003;44:138–147. doi: 10.1007/s00294-003-0432-1. [DOI] [PubMed] [Google Scholar]
- 25.Embley TM. Multiple secondary origins of the anaerobic lifestyle in eukaryotes. Phil Trans R Soc B. 2006;361:1055–1067. doi: 10.1098/rstb.2006.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dacks JB, Silberman JD, Simpson AGB, Moriya S, Kudo T, et al. Oxymonads are closely related to the excavate taxon Trimastix. Mol Biol Evol. 2001;18:1034–1044. doi: 10.1093/oxfordjournals.molbev.a003875. [DOI] [PubMed] [Google Scholar]
- 27.Simpson AGB. Cytoskeletal organization, phylogenetic affinities and systematics in the contentious taxon Excavata (Eukaryota). Int J Syst Evol Microbiol. 2003;53:1759–1777. doi: 10.1099/ijs.0.02578-0. [DOI] [PubMed] [Google Scholar]
- 28.Hampl V, Horner DS, Dyal P, Kulda J, Flegr J, et al. Inference of the phylogenetic position of oxymonads based on nine genes: Support for Metamonada and Excavata. Mol Biol Evol. 2005;22:2508–2518. doi: 10.1093/molbev/msi245. [DOI] [PubMed] [Google Scholar]
- 29.Simpson AGB, Inagaki Y, Roger AJ. Comprehensive multigene phylogenies of excavate protists reveal the evolutionary positions of “primitive” eukaryotes. Mol Biol Evol. 2006;23:615–625. doi: 10.1093/molbev/msj068. [DOI] [PubMed] [Google Scholar]
- 30.Brugerolle G, Patterson D. Ultrastructure of Trimastix convexa Hollande, an amitochondriate anaerobic flagellate with a previously undescribed organization. Europ J Protistol. 1997;33:121–130. [Google Scholar]
- 31.O'Kelly CJ, Farmer MA, Nerad TA. Ultrastructure of Trimastix pyriformis (Klebs) Bernard et al.: similarities of Trimastix species with retortamonad and jakobid flagellates. Protist. 1999;150:149–162. doi: 10.1016/S1434-4610(99)70018-0. [DOI] [PubMed] [Google Scholar]
- 32.Simpson AGB, Bernard C, Patterson DJ. The ultrastructure of Trimastix marina Kent, 1880 (eukaryota), an excavate flagellate. Europ J Protistol. 2000;36:229–251. [Google Scholar]
- 33.Kulda J, Nohýnková E. Flagellates of the human intestine and of intestines of other species. In: Kreier JP, editor. Parasitic protozoa. New York: Academic Press; 1978. pp. 1–138. [Google Scholar]
- 34.Simpson AGB, Radek R, Dacks JB, O'Kelly CJ. How oxymonads lost their groove: An ultrastructural comparison of Monocercomonoides and excavate taxa. J Euk Microbiol. 2002;49:239–248. doi: 10.1111/j.1550-7408.2002.tb00529.x. [DOI] [PubMed] [Google Scholar]
- 35.Leander BS, Keeling PJ. Symbiotic innovation in the oxymonad Streblomastix strix. J Euk Microbiol. 2004;51:291–300. doi: 10.1111/j.1550-7408.2004.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 36.Bloodgood RA, Miller KR, Fitzharris TP, McIntosh JR. The Ultrastructure of Pyrsonympha and Its Associated Microorganisms. J Morph. 1974;143:77–105. doi: 10.1002/jmor.1051430104. [DOI] [PubMed] [Google Scholar]
- 37.Carpenter KJ, Waller RF, Keeling PJ. Surface Morphology of Saccinobaculus (Oxymonadida): Implications for Character Evolution and Function in Oxymonads. Protist. 2007 doi: 10.1016/j.protis.2007.09.002. In press: doi:10.1016/j.protis.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Reeves RE, Warren LG, Susskind B, Loi HS. An energy-conserving pyruvate-to-acetate pathway in Entamoeba histolytica. Pyruvate synthase and a new acetate thiokinase. J Biol Chem. 1977;252:726–731. [PubMed] [Google Scholar]
- 39.Townson SM, Upcroft JA, Upcroft P. Characterisation and purification of pyruvate:ferredoxin oxidoreductase from Giardia duodenalis. Mol Biochem Parasitol. 1996;79:183–193. doi: 10.1016/0166-6851(96)02661-8. [DOI] [PubMed] [Google Scholar]
- 40.Müller M. The Hydrogenosome. J Gen Microbiol. 1993;139:2879–2889. doi: 10.1099/00221287-139-12-2879. [DOI] [PubMed] [Google Scholar]
- 41.Vignais PM, Billoud B, Meyer J. Classification and phylogeny of hydrogenases. Fems Microbiol Rev. 2001;25:455–501. doi: 10.1111/j.1574-6976.2001.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 42.Meyer J. [FeFe] hydrogenases and their evolution: a genomic perspective. Cell Mol Life Sci. 2007;64:1063–1084. doi: 10.1007/s00018-007-6477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Posewitz MC, King PW, Smolinski SL, Zhang L, Seibert M, Ghirardi ML. Discovery of two novel radical S-adenosylmethionine proteins required for the assembly of an active [Fe] hydrogenase. J Biol Chem. 2004;279:25711–25720. doi: 10.1074/jbc.M403206200. [DOI] [PubMed] [Google Scholar]
- 44.Putz S, Doležal P, Gelius-Dietrich G, Boháčová L, Tachezy J, Henze K. Fe-hydrogenase maturases in the hydrogenosomes of Trichomonas vaginalis. Eukaryot Cell. 2006;5:579–586. doi: 10.1128/EC.5.3.579-586.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Douce R, Bourguignon J, Neuburger M, Rebeille F. The glycine decarboxylase system: a fascinating complex. Trends Plant Sci. 2001;6:167–176. doi: 10.1016/s1360-1385(01)01892-1. [DOI] [PubMed] [Google Scholar]
- 46.Carothers DJ, Pons G, Patel MS. Dihydrolipoamide dehydrogenase: functional similarities and divergent evolution of the pyridine nucleotide-disulfide oxidoreductases. Arch Biochem Biophys. 1989;268:409–425. doi: 10.1016/0003-9861(89)90309-3. [DOI] [PubMed] [Google Scholar]
- 47.Mukherjee M, Brown MT, McArthur AG, Johnson PJ. Proteins of the glycine decarboxylase complex in the hydrogenosome of Trichomonas vaginalis. Eukaryot Cell. 2006;5:2062–2071. doi: 10.1128/EC.00205-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kruger N, Oppermann FB, Lorenzl H, Steinbuchel A. Biochemical and molecular characterization of the Clostridium magnum acetoin dehydrogenase enzyme system. J Bacteriol. 1994;176:3614–3630. doi: 10.1128/jb.176.12.3614-3630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jordan SW, Cronan JE., Jr A new metabolic link. The acyl carrier protein of lipid synthesis donates lipoic acid to the pyruvate dehydrogenase complex in Escherichia coli and mitochondria. J Biol Chem. 1997;272:17903–17906. doi: 10.1074/jbc.272.29.17903. [DOI] [PubMed] [Google Scholar]
- 50.Olausson T, Fjellstrom O, Meuller J, Rydstrom J. Molecular biology of nicotinamide nucleotide transhydrogenase–a unique proton pump. Biochim Biophys Acta. 1995;1231:1–19. doi: 10.1016/0005-2728(95)00058-q. [DOI] [PubMed] [Google Scholar]
- 51.Doležal P, Likic V, Tachezy J, Lithgow T. Evolution of the molecular machines for protein import into mitochondria. Science. 2006;313:314–318. doi: 10.1126/science.1127895. [DOI] [PubMed] [Google Scholar]
- 52.Macasev D, Whelan J, Newbigin E, Silva-Filho MC, Mulhern TD, et al. Tom22', an 8-kDa trans-site receptor in plants and protozoans, is a conserved feature of the TOM complex that appeared early in the evolution of eukaryotes. Mol Biol Evol. 2004;21:1557–1564. doi: 10.1093/molbev/msh166. [DOI] [PubMed] [Google Scholar]
- 53.Luciano P, Geoffroy S, Brandt A, Hernandez JF, Geli V. Functional cooperation of the mitochondrial processing peptidase subunits. J Mol Biol. 1997;272:213–225. doi: 10.1006/jmbi.1997.1231. [DOI] [PubMed] [Google Scholar]
- 54.Regoes A, Zourmpanou D, Leon-Avila G, van der Giezen M, Tovar J, et al. Protein import, replication, and inheritance of a vestigial mitochondrion. J Biol Chem. 2005;280:30557–30563. doi: 10.1074/jbc.M500787200. [DOI] [PubMed] [Google Scholar]
- 55.Doležal P, Šmíd O, Rada P, Zubáčová Z, Bursac D, et al. Giardia mitosomes and trichomonad hydrogenosomes share a common mode of protein targeting. Proc Natl Acad Sci U S A. 2005;102:10924–10929. doi: 10.1073/pnas.0500349102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown MT, Goldstone HM, Bastida-Corcuera F, gadillo-Correa MG, McArthur AG, et al. A functionally divergent hydrogenosomal peptidase with protomitochondrial ancestry. Mol Microbiol. 2007;64:1154–1163. doi: 10.1111/j.1365-2958.2007.05719.x. [DOI] [PubMed] [Google Scholar]
- 57.Braun HP, Schmitz UK. The mitochondrial processing peptidase. Int J Biochem Cell Biol. 1997;29:1043–1045. doi: 10.1016/s1357-2725(97)00032-0. [DOI] [PubMed] [Google Scholar]
- 58.Nagao Y, Kitada S, Kojima K, Toh H, Kuhara S, et al. Glycine-rich region of mitochondrial processing peptidase alpha-subunit is essential for binding and cleavage of the precursor proteins. J Biol Chem. 2000;275:34552–34556. doi: 10.1074/jbc.M003110200. [DOI] [PubMed] [Google Scholar]
- 59.Palmieri L, Rottensteiner H, Girzalsky W, Scarcia P, Palmieri F, et al. Identification and functional reconstitution of the yeast peroxisomal adenine nucleotide transporter. EMBO J. 2001;20:5049–5059. doi: 10.1093/emboj/20.18.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bedhomme M, Hoffmann M, McCarthy EA, Gambonnet B, Moran RG, et al. Folate metabolism in plants: an Arabidopsis homolog of the mammalian mitochondrial folate transporter mediates folate import into chloroplasts. J Biol Chem. 2005;280:34823–34831. doi: 10.1074/jbc.M506045200. [DOI] [PubMed] [Google Scholar]
- 61.Satre M, Mattei S, Aubry L, Gaudet P, Pelosi L, et al. Mitochondrial carrier family: Repertoire and peculiarities of the cellular slime mould Dictyostelium discoideum. Biochimie. 2007;89:1058–1069. doi: 10.1016/j.biochi.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 62.Dyall SD, Koehler CM, Delgadillo-Correa MG, Bradley PJ, Plumper E, et al. Presence of a member of the mitochondrial carrier family in hydrogenosomes: Conservation of membrane-targeting pathways between hydrogenosomes and mitochondria. Mol Cell Biol. 2000;20:2488–2497. doi: 10.1128/mcb.20.7.2488-2497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Giezen M, Slotboom DJ, Horner DS, Dyal PL, Harding M, et al. Conserved properties of hydrogenosomal and mitochondrial ADP/ATP carriers: a common origin for both organelles. EMBO J. 2002;21:572–579. doi: 10.1093/emboj/21.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chan KW, Slotboom DJ, Cox S, Embley TM, Fabre O, et al. A novel ADP/ATP transporter in the mitosome of the microaerophilic human parasite Entamoeba histolytica. Curr Biol. 2005;15:737–742. doi: 10.1016/j.cub.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 65.Kunji ER, Robinson AJ. The conserved substrate binding site of mitochondrial carriers. Biochim Biophys Acta. 2006;1757:1237–1248. doi: 10.1016/j.bbabio.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 66.Taylor SW, Fahy E, Zhang B, Glenn GM, Warnock DE, et al. Characterization of the human heart mitochondrial proteome. Nat Biotechnol. 2003;21:281–286. doi: 10.1038/nbt793. [DOI] [PubMed] [Google Scholar]
- 67.Andreoli C, Prokisch H, Hortnagel K, Mueller JC, Munsterkotter M, et al. MitoP2, an integrated database on mitochondrial proteins in yeast and man. Nucleic Acids Res. 2004;32:D459–D462. doi: 10.1093/nar/gkh137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999:95–98. [Google Scholar]
- 70.Do CB, Mahabhashyam MS, Brudno M, Batzoglou S. ProbCons: Probabilistic consistency-based multiple sequence alignment. Genome Res. 2005;15:330–340. doi: 10.1101/gr.2821705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 72.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic tree of PFO. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.58 MB TIF)
Phylogenetic tree of aconitase. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.68 MB TIF)
Phylogenetic tree of T-protein of GCS. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.69 MB TIF)
Phylogenetic tree of L-protein of GCS. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.52 MB TIF)
Phylogenetic tree of P1-protein of GCS. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.63 MB TIF)
Phylogenetic tree of P2-protein of GCS. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.71 MB TIF)
Phylogenetic tree of H-protein of GCS. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.56 MB TIF)
Phylogenetic tree of lipoyltransferase. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.62 MB TIF)
Phylogenetic tree of β subunit of pyridine nucleotide transhydrogenase. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.54 MB TIF)
Phylogenetic tree of mitochondrial processing peptidase. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.48 MB TIF)
Phylogenetic tree of mitochondrial carrier protein family. Tree was constructed by Bayesian method. Numbers at the nodes represent statistical support expressed in Bayesian posterior probabilities/maximum likelihood bootstraps computed in RaxML. * Indicates bootstrap value below 50%.
(0.48 MB TIF)