Abstract
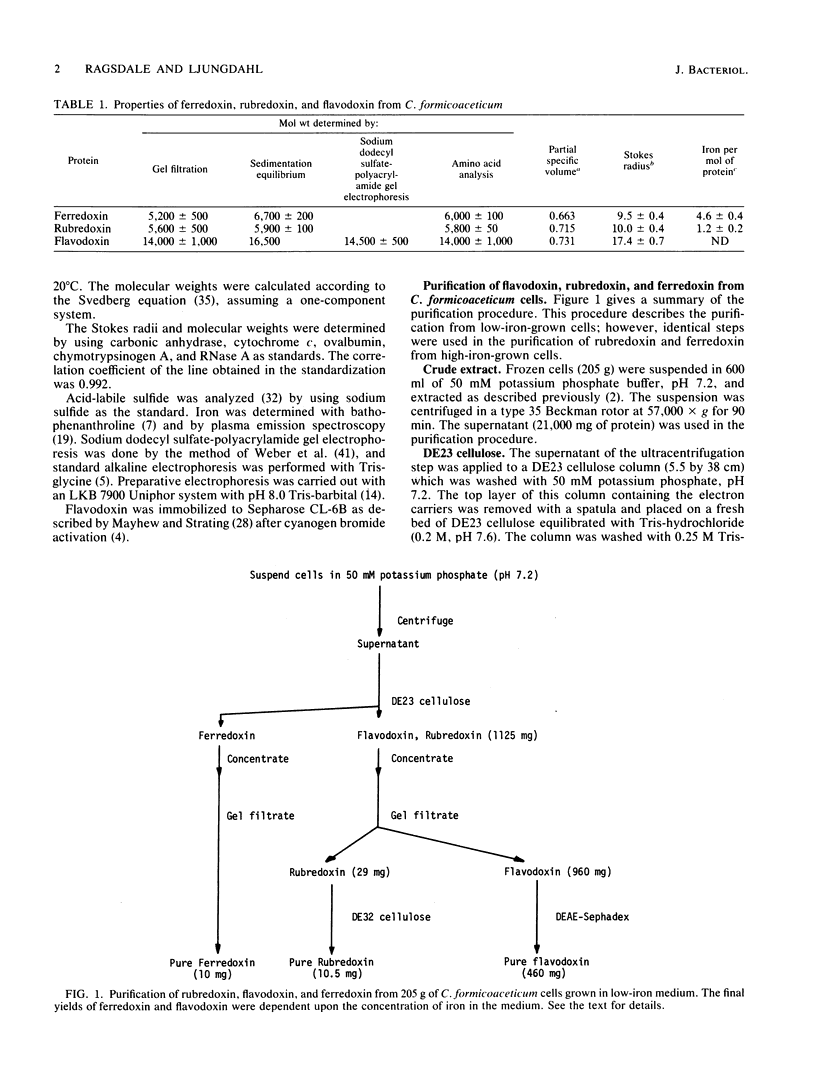
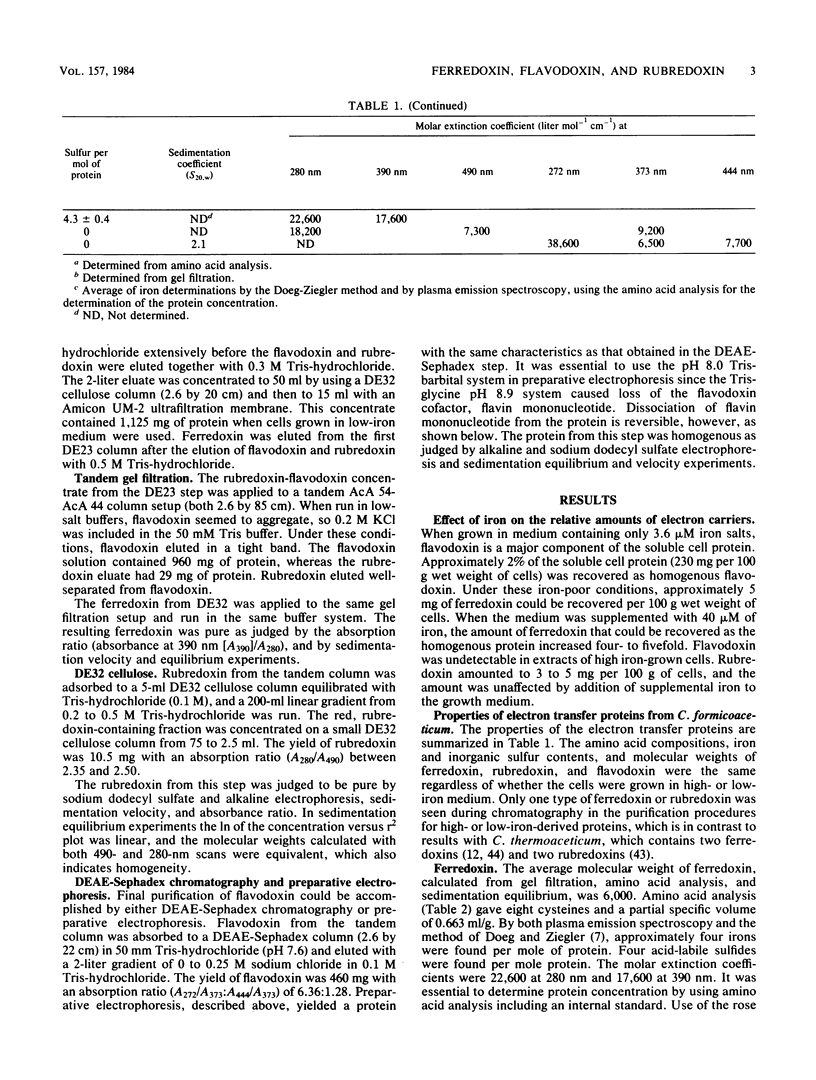
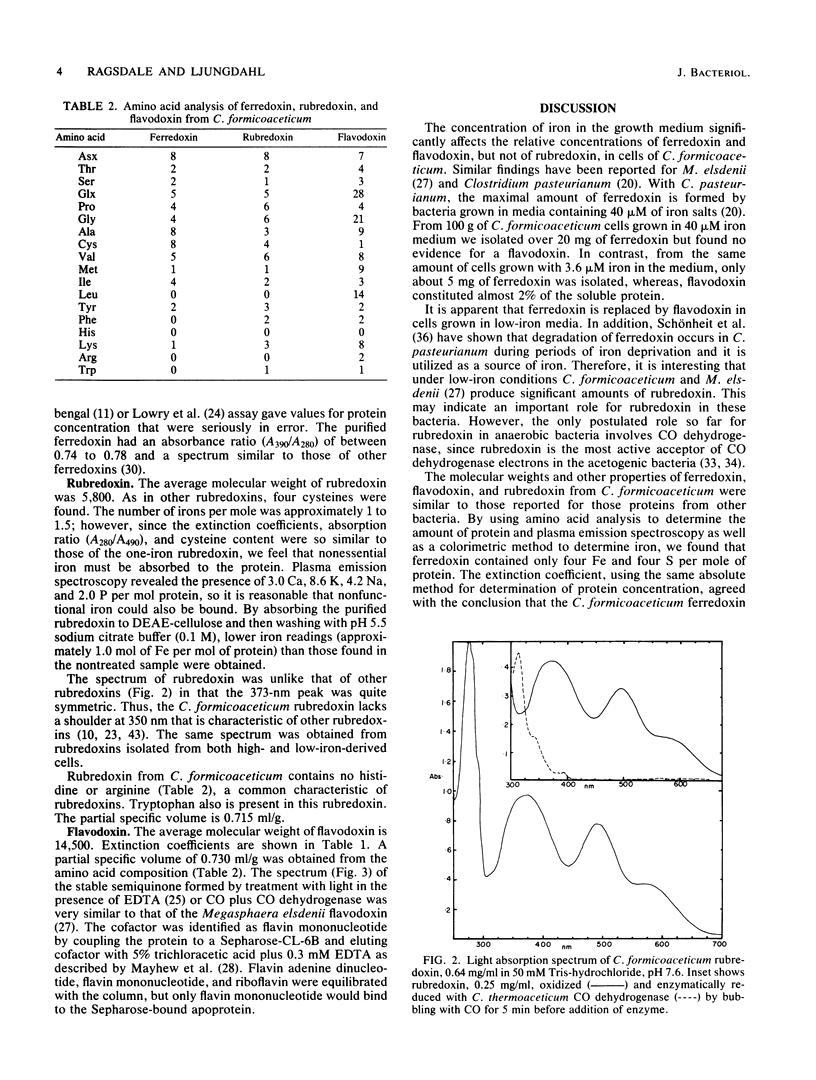
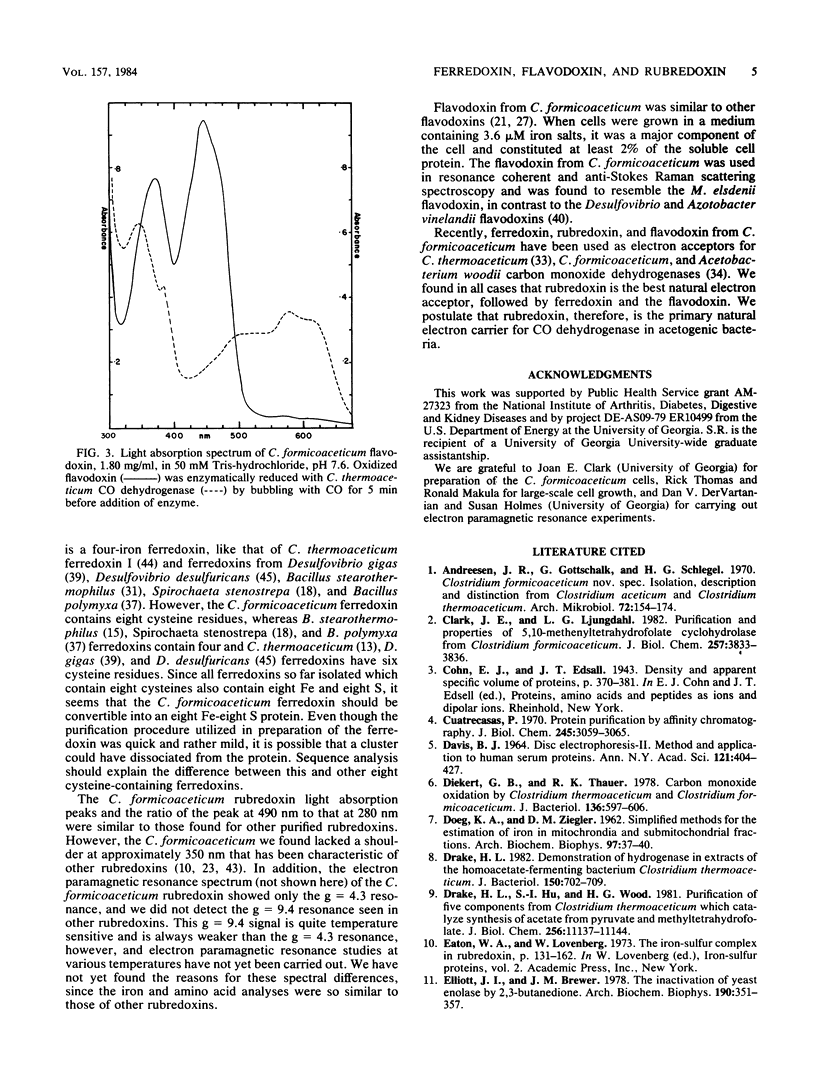
Ferredoxin, flavodoxin, and rubredoxin were purified to homogeneity from Clostridium formicoaceticum and characterized. Variation of the iron concentration of the growth medium caused substantial changes in the concentrations of ferredoxin and flavodoxin but not of rubredoxin. The ferredoxin has a molecular weight of 6,000 and is a four iron-four sulfur protein with eight cysteine residues. The spectrum is similar to that of other ferredoxins. The molar extinction coefficients are 22.6 X 10(3) and 17.6 X 10(3) at 280 and 390 nm, respectively. From 100 g wet weight of cells grown with 3.6 microM iron and with 40 microM iron, 5 and 20 mg offerredoxin were isolated, respectively. The molecular weight of rubredoxin is 5,800 and it contains one iron and four cysteines. The UV-visible absorption spectrum is dissimilar to those of other rubredoxins in that the 373 nm absorption peak is quite symmetric, lacking the characteristic 350-nm shoulder found in other rubredoxins. The flavodoxin is a 14,500-molecular-weight protein which contains 1 mol of flavin mononucleotide per mol of protein. It forms a stable, blue semiquinone upon light irradiation in the presence of EDTA or during enzymatic reduction. When cells were grown in low-iron medium, flavodoxin constituted at least 2% of the soluble cell protein; however, it was not detected in extracts of cells grown in high-iron medium. The rubredoxin and ferredoxin expressed during growth in low-iron and high-iron media are identical as judged by iron, inorganic sulfide, and amino acid analysis, as well as light absorption spectroscopy.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen J. R., Gottschalk G., Schlegel H. G. Clostridium formicoaceticum nov. spec. isolation, description and distinction from C. aceticum and C. thermoaceticum. Arch Mikrobiol. 1970;72(2):154–174. doi: 10.1007/BF00409521. [DOI] [PubMed] [Google Scholar]
- Clark J. E., Ljungdahl L. G. Purification and properties of 5,10-methenyltetrahydrofolate cyclohydrolase from Clostridium formicoaceticum. J Biol Chem. 1982 Apr 10;257(7):3833–3836. [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DOEG K. A., ZIEGLER D. M. Simplified methods for the estimation of iron in mitochondria and submitochondrial fractions. Arch Biochem Biophys. 1962 Apr;97:37–40. doi: 10.1016/0003-9861(62)90041-3. [DOI] [PubMed] [Google Scholar]
- Diekert G. B., Thauer R. K. Carbon monoxide oxidation by Clostridium thermoaceticum and Clostridium formicoaceticum. J Bacteriol. 1978 Nov;136(2):597–606. doi: 10.1128/jb.136.2.597-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake H. L. Demonstration of hydrogenase in extracts of the homoacetate-fermenting bacterium Clostridium thermoaceticum. J Bacteriol. 1982 May;150(2):702–709. doi: 10.1128/jb.150.2.702-709.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake H. L., Hu S. I., Wood H. G. Purification of five components from Clostridium thermoaceticum which catalyze synthesis of acetate from pyruvate and methyltetrahydrofolate. Properties of phosphotransacetylase. J Biol Chem. 1981 Nov 10;256(21):11137–11144. [PubMed] [Google Scholar]
- Elliott J. I., Brewer J. M. The inactivation of yeast enolase by 2,3-butanedione. Arch Biochem Biophys. 1978 Sep;190(1):351–357. doi: 10.1016/0003-9861(78)90285-0. [DOI] [PubMed] [Google Scholar]
- Elliott J. I., Ljungdahl L. G. Isolation and characterization of an Fe,-S8 ferredoxin (ferredoxin II) from Clostridium thermoaceticum. J Bacteriol. 1982 Jul;151(1):328–333. doi: 10.1128/jb.151.1.328-333.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. I., Yang S. S., Ljungdahl L. G., Travis J., Reilly C. F. Complete amino acid sequence of the 4Fe-4S, thermostable ferredoxin from Clostridium thermoaceticum. Biochemistry. 1982 Jul 6;21(14):3294–3298. doi: 10.1021/bi00257a007. [DOI] [PubMed] [Google Scholar]
- Hase T., Ohmiya N., Matsubara H., Mullinger R. N., Rao K. K., Hall D. O. Amino acid sequence of a four-iron-four-sulphur ferredoxin isolated from Bacillus stearothermophilus. Biochem J. 1976 Oct 1;159(1):55–63. doi: 10.1042/bj1590055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. I., Drake H. L., Wood H. G. Synthesis of acetyl coenzyme A from carbon monoxide, methyltetrahydrofolate, and coenzyme A by enzymes from Clostridium thermoaceticum. J Bacteriol. 1982 Feb;149(2):440–448. doi: 10.1128/jb.149.2.440-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. W., Canale-Parola E. Properties of rubredoxin and ferredoxin isolated from spirochetes. Arch Mikrobiol. 1973;89(4):341–353. doi: 10.1007/BF00408901. [DOI] [PubMed] [Google Scholar]
- Knight E., Jr, Hardy R. W. Flavodoxin. Chemical and biological properties. J Biol Chem. 1967 Apr 10;242(7):1370–1374. [PubMed] [Google Scholar]
- Knight E., Jr, Hardy R. W. Isolation and characteristics of flavodoxin from nitrogen-fixing Clostridium pasteurianum. J Biol Chem. 1966 Jun 25;241(12):2752–2756. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lovenberg W., Sobel B. E. Rubredoxin: a new electron transfer protein from Clostridium pasteurianum. Proc Natl Acad Sci U S A. 1965 Jul;54(1):193–199. doi: 10.1073/pnas.54.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey V., Palmer G. On the existence of spectrally distinct classes of flavoprotein semiquinones. A new method for the quantitative production of flavoprotein semiquinones. Biochemistry. 1966 Oct;5(10):3181–3189. doi: 10.1021/bi00874a016. [DOI] [PubMed] [Google Scholar]
- Matsubara H., Sasaki R. M. High recovery of tryptophan from acid hydrolysates of proteins. Biochem Biophys Res Commun. 1969 Apr 29;35(2):175–181. doi: 10.1016/0006-291x(69)90263-0. [DOI] [PubMed] [Google Scholar]
- Mayhew S. G., Massey V. Purification and characterization of flavodoxin from Peptostreptococcus elsdenii. J Biol Chem. 1969 Feb 10;244(3):794–802. [PubMed] [Google Scholar]
- Mayhew S. G., Strating M. J. Properties of immobilized flavodoxin from Peptostreptococcus elsdenii. An affinity ligand for the purification of riboflavin 5'-phosphate (FMN) and its analogues. Eur J Biochem. 1975 Nov 15;59(2):539–544. doi: 10.1111/j.1432-1033.1975.tb02480.x. [DOI] [PubMed] [Google Scholar]
- Moore M. R., O'Brien W. E., Ljungdahl L. G. Purification and characterization of nicotinamide adenine dinucleotide-dependent methylenetetrahydrofolate dehydrogenase from Clostridium formicoaceticum. J Biol Chem. 1974 Aug 25;249(16):5250–5253. [PubMed] [Google Scholar]
- Mullinger R. N., Cammack R., Rao K. K., Hall D. O., Dickson D. P., Johnson C. E., Rush J. D., Simopoulos A. Physicochemical characterization of the four-iron-four-sulphide ferredoxin from Bacillus stearothermophilus. Biochem J. 1975 Oct;151(1):75–83. doi: 10.1042/bj1510075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz J. C. Analysis of acid-labile sulfide and sulfhydryl groups. Methods Enzymol. 1978;53:275–277. doi: 10.1016/s0076-6879(78)53033-4. [DOI] [PubMed] [Google Scholar]
- Ragsdale S. W., Clark J. E., Ljungdahl L. G., Lundie L. L., Drake H. L. Properties of purified carbon monoxide dehydrogenase from Clostridium thermoaceticum, a nickel, iron-sulfur protein. J Biol Chem. 1983 Feb 25;258(4):2364–2369. [PubMed] [Google Scholar]
- Ragsdale S. W., Ljungdahl L. G., DerVartanian D. V. Isolation of carbon monoxide dehydrogenase from Acetobacterium woodii and comparison of its properties with those of the Clostridium thermoaceticum enzyme. J Bacteriol. 1983 Sep;155(3):1224–1237. doi: 10.1128/jb.155.3.1224-1237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönheit P., Brandis A., Thauer R. K. Ferredoxin degradation in growing Clostridium pasteurianum during periods of iron deprivation. Arch Microbiol. 1979 Jan 16;120(1):73–76. doi: 10.1007/BF00413277. [DOI] [PubMed] [Google Scholar]
- Strombaugh N. A., Burris R. H., Orme-Johnson W. H. Ferredoxins from Bacillus polymyxa. Low potential iron-sulfur proteins which appear to contain single four iron, four sulfur centers accepting a single electron on reduction. J Biol Chem. 1973 Nov 25;248(22):7951–7956. [PubMed] [Google Scholar]
- Thauer R. K. CO(2)-reduction to formate by NADPH. The initial step in the total synthesis of acetate from CO(2) in Clostridium thermoaceticum. FEBS Lett. 1972 Oct 15;27(1):111–115. doi: 10.1016/0014-5793(72)80421-6. [DOI] [PubMed] [Google Scholar]
- Travis J., Newman D. J., LeGall J., Peck H. D., Jr The amino acid sequence of ferredoxin from the sulfate reducing bacterium, Desulfovibrio gigas. Biochem Biophys Res Commun. 1971 Oct 15;45(2):452–458. doi: 10.1016/0006-291x(71)90840-0. [DOI] [PubMed] [Google Scholar]
- Visser A. J., Vervoort J., O'Kane D. J., Lee J., Carreira L. A. Raman spectra of flavin bound in flavodoxins and in other flavoproteins. Evidence for structural variations in the flavin-binding region. Eur J Biochem. 1983 Apr 5;131(3):639–645. doi: 10.1111/j.1432-1033.1983.tb07311.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto I., Saiki T., Liu S. M., Ljungdahl L. G. Purification and properties of NADP-dependent formate dehydrogenase from Clostridium thermoaceticum, a tungsten-selenium-iron protein. J Biol Chem. 1983 Feb 10;258(3):1826–1832. [PubMed] [Google Scholar]
- Yang S. S., Ljungdahl L. G., Dervartanian D. V., Watt G. D. Isolation and characterization of two rubredoxins from Clostridium thermoaceticum. Biochim Biophys Acta. 1980 Mar 7;590(1):24–33. doi: 10.1016/0005-2728(80)90143-7. [DOI] [PubMed] [Google Scholar]
- Yang S. S., Ljungdahl L. G., LeGall J. A four-iron, four-sulfide ferredoxin with high thermostability from Clostridium thermoaceticum. J Bacteriol. 1977 Jun;130(3):1084–1090. doi: 10.1128/jb.130.3.1084-1090.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta J. A., Mason R., Postgate J. R. A four-iron ferredoxin from Desulfovibrio desulfuricans. Biochem J. 1973 Aug;133(4):851–854. doi: 10.1042/bj1330851. [DOI] [PMC free article] [PubMed] [Google Scholar]


