Abstract
Voltage-gated Ca2+ channels (VGCC) play a key role in many physiological functions by their high selectivity for Ca2+ over other divalent and monovalent cations in physiological situations. Divalent/monovalent selection is shared by all VGCC and is satisfactorily explained by the existence, within the pore, of a set of four conserved glutamate/aspartate residues (EEEE locus) coordinating Ca2+ ions. This locus however does not explain either the choice of Ca2+ among other divalent cations or the specific conductances encountered in the different VGCC. Our systematic analysis of high- and low-threshold VGCC currents in the presence of Ca2+ and Ba2+ reveals highly specific selectivity profiles. Sequence analysis, molecular modeling, and mutational studies identify a set of nonconserved charged residues responsible for these profiles. In HVA (high voltage activated) channels, mutations of this set modify divalent cation selectivity and channel conductance without change in divalent/monovalent selection, activation, inactivation, and kinetics properties. The CaV2.1 selectivity profile is transferred to CaV2.3 when exchanging their residues at this location. Numerical simulations suggest modification in an external Ca2+ binding site in the channel pore directly involved in the choice of Ca2+, among other divalent physiological cations, as the main permeant cation for VGCC. In LVA (low voltage activated) channels, this locus (called DCS for divalent cation selectivity) also influences divalent cation selection, but our results suggest the existence of additional determinants to fully recapitulate all the differences encountered among LVA channels. These data therefore attribute to the DCS a unique role in the specific shaping of the Ca2+ influx between the different HVA channels.
INTRODUCTION
Low (LVA = CaV3.1-3) and high (HVA = CaV1.1-4, CaV2.1-3) threshold voltage-gated Ca2+ channels, by allowing selective, rapid, and large Ca2+ influx into cells, are key actors of the Ca2+ homeostasis (Isom et al., 1994; Catterall, 2000) and thus play a fundamental role in cell physiology by activating a myriad of Ca2+-driven processes. Although channel-specific gating, localization, regulation, and protein partners finely tune this Ca influx and thus directly participate in the precise channel physiological or pathophysiological roles, the key feature that makes these channels so important is undoubtedly their capacity to select Ca2+ ions among other physiological monovalent and divalent cations. The channel preference for Ca2+ versus the monovalent Na+ has been the subject of a number of studies (Sather et al., 1994; Varadi et al., 1999; Cibulsky and Sather, 2003; Sather and McCleskey, 2003) mostly performed on the prototypal HVA CaV1.2 channels with only sparse data available on other channel types (Serrano et al., 2000; Talavera et al., 2001; Sather and McCleskey, 2003).
The mechanism of Ca2+ ion selection and permeation relies on the pore-forming CaVα subunit. CaVα subunits are composed of four domains, each containing six transmembrane segments and a pore-forming loop (P loop), arranged around a pseudo-symmetrical axis forming a central pore. Theoretical analysis and experimental finding, performed on CaV1.2 channels, have suggested that the basis of selection and high transfer rate of Ca2+ ions relies on direct interactions of two ions with specific residues in the pore (Hess and Tsien, 1984; Varadi et al., 1999; Sather and McCleskey, 2003). Molecular studies identified a single site, the EEEE (EEDD in LVA channels) locus, constituted by a ring of four glutamates (2E + 2D in LVA channels) located at equivalent position in each of the P loop of the four domains (* in Fig. 2), and responsible for the multi-ionic nature of the Ca2+ channel pore (Heinemann et al., 1992; Kim et al., 1993; Tang et al., 1993; Yang et al., 1993; Ellinor et al., 1995; Cibulsky and Sather, 2000). The binding of one or two Ca2+ ions in the EEEE locus can explain the apparent antagonism between high permeability and high selectivity typical of these Ca2+ channels (Sather and McCleskey, 2003). However several studies indicate VGCC-specific selectivities for divalent cations with Ca/Ba permeability ratios varying from 0.3 to 1.2 for CaV1.2 and CaV2.3 for example (Bourinet et al., 1996), suggesting that the choice of Ca2+, crucial for cell physiology, cannot be explained by this conserved EEEE locus. Other motifs known to contribute to channel permeation (Feng et al., 2001) are conserved among HVA channels and thus are not good candidates to explain channel-specific properties. Furthermore, mutation of the two Asp present at the EEDD locus of all LVA channels to Glu, as in HVA, cannot bestow HVA-like selectivity profiles (Talavera et al., 2001). Clearly, additional mechanisms and/or loci may exist to fully explain Ca2+ selection.
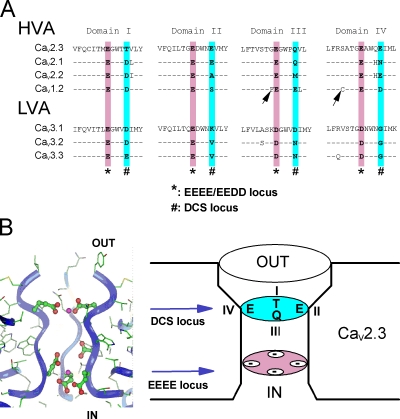
Figure 2.
Molecular modeling of the DCS and EEEE loci in the channel pore. (A) Sequence alignment of the P loops of CaV1.2, CaV2.1, and CaV2.3 HVA channels and CaV3.1, CaV3.2, and CaV3.3 LVA channels. * and # boxes, position of EEEE and DCS loci in each domain of the CaVα subunit. Note the nonconservation of the DCS charged amino acids. Arrows underline the non-DCS amino acids mutated in Fig S4: S1611 and G1323 in CaV2.3. (B) A molecular model of the C-terminal end of the four P loops of CaV2.3 channel. Left, radial projection of the channel with one of the domains omitted for the clearness of the picture. Right, schematic representation of the EEEE and DCS loci of CaV2.3 channel. Ribbons represent the backbone of the peptidic chains. Ca2+ ions are shown as magenta balls. Negatively charged side chains of the channel are shown by ball-and-stick representation (see Materials and methods for details).
We have performed the first analysis of the relative permeability of Ba2+ and Ca2+ ions of three LVA and four HVA VGCCs expressed in Xenopus oocytes. Specific permeation profiles are detected for each channel by characterizing the anomalous mole fraction effect (AMFE). Inspection of the amino acid sequences of these channels in the pore region reveals a novel and channel-specific locus composed of nonconserved charged residues. In HVA channels, modifications of this site strongly influence the Ba2+/Ca2+ permeability ratio without modifying the monovalent/divalent selection and the sensitivity to Cd2+ block. Our results, completed with numerical simulation and molecular modeling, suggest that this set of amino acids (thereafter called DCS [divalent cation selection]), is located in the upper halve of the channel and points toward the pore, where it may form a second Ca2+ binding site. In LVA channels, mutation of the DCS also influences channel selectivity, although additional sites clearly exit. DCS is present in all VGCC and is crucial for the definition of their specific permeability profile, and thus for Ca2+ entry into cells.
MATERIALS AND METHODS
Molecular Biology
Mutations in rat brain CaV2.1 and CaV2.3 were introduced by PCR (GenBank/EMBL/DDBJ accession nos. M64372 and L15453, respectively). Mutations T264D, T264A, T264G, E612S, E612A, E612G, G1323F, Q1328E, Q1328A, Q1328G, S1611C, E1619N, E1619A, E1619G, E1619D, and QE1618-1619HN were introduced by PCR mutagenesis into the rbEII sequence (CaV2.3, GenBank/EMBL/DDBJ accession no. 15453). PCR fragments were then subcloned into a modified version of the pBluescript cloning vector, using native (NotI, XbaI, Bst98I, ApaI, HindIII, XhoI) or created (Mlu(1007), KpnI(polylinker)) restriction sites, and fully sequenced. Full-length cDNA was constructed by ligating appropriate fragments. Mutations are written explicitly in the text. The same approach has been followed to construct CaV2.1(TEQE) (rbAI, M64373). In this case, an additional mutation (histidine at position −1, referred to the DCS locus, into glutamine) was also made to preserve the pore structure of CaV2.3 (see Fig. 2). The reverse mutation (Q to H) was made on the CaV2.3 (DEQN). Finally, CaV2.3, CaV2.1, CaV1.2 (M67515), CaVβ2a (M80545), and CaVα2-δ (M86621) were subcloned into the pMT2 expression vector for oocyte injection and expression.
Electrophysiology
Isolated Xenopus laevis oocytes were injected with a mixture of plasmids encoding the α2-δ + β2 and each of the mutant CaV2.1 or CaV2.3 Ca2+ channel subunits at a total DNA concentration of 0.2 μg/μl. LVA CaV3.1, CaV3.2, and CaV3.3 subunits were injected alone. Total whole-cell currents were recorded under two-electrode (resistance: 0.5–1 MΩ in 3 M KCl ) voltage-clamp (geneclamp 500; Axon Instruments Inc.) using solutions of increasing Ca2+ mole fraction (in mM: BaOH/CaOH, 10/0, 9/1, 8/2, 6/4, 4/6, 2/8, 0/10; TEAOH, 20; CsOH, 2; NMDG, 50; HEPES, 10, pH set to 7.2 using methane sulfonic acid). Voltage ramps from −80 to +80 mV (at 0.4 V/s) or −100 mV to +60 mV (at 0.4 to 2.6 V/s) were applied every 15 s on HVA or LVA VGCC–expressing oocytes, respectively. The speed of the ramp was chosen after experimental testing to minimize the error in the current–voltage curve when channel inactivation was fast (CaV3.1, CaV3.2). Oocytes were injected with 40 nl of a 100 mM BAPTA solution to block contaminating Ca2+-activated Cl current. In Fig. 5, the extent of AMFE was quantified by dividing the minimum of the current–mole fraction curve by the current recorded at 10 mM Ca2+. This allowed us to visualize the presence of AMFE (values <1) and the extent of the Ca/Ba current ratio on the same graph. This is not valid for channels without AMFE and with Ca2+ current larger than Ba currents, a case not present in HVA channels. The correlation between the number of charges and the extent of AMFE was tested using the Spearman correlation test at the 0.05 level.
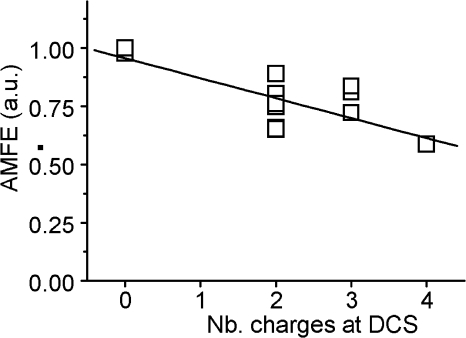
Figure 5.
AMFE amplitude is correlated with the number of negative charges at the DCS. Scatterplot showing the amplitude of AMFE (calculated as the minimum of the current–mole fraction curve divided by the current in 10 mM Ca2+) as a function of the number of negative charges for all the HVA mutations presented in this article.
Single-channel recordings were obtained in cell-attached patch with a bath solution composed of (in mM) 100 KCl, 2 MgCl2, 10 HEPES, 10 EGTA; pH set to 7.4 using KOH. The pipettes (10– 20 MΩ) were filled with (in mM) 100 BaCl2, 10 HEPES; pH set to 7.2 using NaOH. Currents were filtered at 2 kHz and digitized at 10 kHz. Channel conductances were calculated from linear regressions of single channel amplitudes obtained at different pipette voltages and determined using idealized event histograms (Clampfit, version 9.0, Axon Instruments Inc.). All values are presented as mean ± SEM. Bay-K 8644 (1 μM) was added to the pipette solution for the recordings obtained on the CaV1.2 channels.
Molecular Modeling
The helices P and S6 being located in the hydrophobic interior of the structure have highly apolar sequences and are connected by a variable hydrophilic link. This motif was identified in each of four Ca2+ channel domains and aligned with the sequence of KvAT by using CLUSTALW program (Higgins et al., 1996). The sequence alignment was then manually corrected whenever necessary based on the available crystal structure of the K+ channel (Jiang et al., 2003). The structure of the K+ channel provides several constraints that we used to model the 3D structure of Ca2+ channel. The C-terminal ends of four P helices come in direct contact in the tetramer center. This arrangement requires a perpendicular exit from the α-helical C-terminal cap (Efimov, 1993) induced by the lefthanded α-helical conformation of Thr196. In the alignment that was used for the modeling, the hydrophobic region of the Ca2+ channel corresponding to the P helix is frequently followed by glycine (methionine in domain I). Glycine following the α-helix in the lefthanded α-helical conformation is the typical case of the perpendicular exit from the α-helix (Efimov, 1993). Therefore, these glycines were aligned with Thr196 of KvAT. The next five residues represent a pore-lining region (pink bar in Fig. S2 B, available at http://www.jgp.org/cgi/content/full/jgp.200709771/DC1). In the alignment, the residues of the EEEE and DCS loci of the Ca2+ channel are within the pore-lining region. Furthermore, an aromatic Tyr199 of the K+ pore-lining region that faces the hydrophobic interior of the structure (marked by an arrow in Fig. S2 B) is aligned with the conserved aromatic tryptophans of the Ca2+ channel. The following loop between the pore-forming region and α-helix S6 moves away from the pore and can be variable (broken line on Fig. S2 B). Therefore, in the sequence alignment these regions are hydrophilic and of different length. The initial model containing helices P and S6 was built by using the HOMOLOGY module of InsightII program (Dayring et al., 1986) and the alignment shown on Fig. S2 B. Then the pore-lining region of the Ca2+ channel was modeled manually taking into consideration the following constraints: (a) all four loops have the same backbone conformation, (b) the side chains of the EEEE and DCS loci are directed toward the Ca2+ ions, as suggested by effects of the mutations, and (c) the aromatic tryptophans occupy the same hydrophobic pocket of the helices P and S6 as the aromatic Tyr199 in the K+ channel. The conformational analysis showed that to meet all these conditions the pore-lining region of the Ca2+ channel requires a conformation that is different from one of the K+ channel. In our model (Fig. 2 B), this region has a conformation of the polyproline helix instead of an alternation of lefthanded and righthanded α-helical conformations observed in the K+ channel. Two Ca2+ ions were introduced in the EEEE and DCS locus, and side chains of the locus were manually adjusted to coordinate the cations. The final structure was energy minimized using InsightII program (Dayring et al., 1986). The energy minimization and molecular dynamics calculations of our model suggested that the side chains of the EEEE locus coordinating Ca2+ are packed tighter than the ones of the DCS locus and this can be explained by the higher affinity of the EEEE site. The superposition of the energy-minimized Ca2+ channel model with the structure of K+ channel shows that the subunits of Ca2+ channel move away from the center of the pore to allow the side chains of EEEE locus to accommodate the pore.
Numerical Simulation
The two binding-site model for calcium channels was essentially derived from (Begenisich and Cahalan, 1980; Hess and Tsien, 1984; Campbell et al., 1988; Kurata et al., 1999) with minor changes in the energy profiles to account for the specific permeation properties of CaV2.3. The energy profile of LVA channels was obtained from that of CaV2.3 by slight adjustment of the binding energy of the internal well (the EEEE/EEDD locus) to provide current traces compatible with CaV3.1 currents. These two profiles served then as references and all subsequent computations were made by varying only the binding energy of the first extracellular well (DCS locus) that was supposed to be modified by the mutations. The three energy peaks (P1, P2, P3) and the two wells (DCS and EEE) were regularly spaced within the membrane at similar electrical distances (Rosenberg and Chen, 1991). The membrane potential (Vm) is supposed to fall across the narrow region of the pore. The rate constants for the transitions between the different channel states were calculated by Eyring rate theory as rate = kT/h exp(−ΔG), where ΔG is the sum of the chemical energy, given by the energy differences between the peak and the well, the electric energy, given by the movement of the cation through the electrical field (zVm/d; d electrical distance), and an electrostatic repulsive force due to occupancy of two binding sites by two cations (zizj ln(F)) (see Begenisich and Cahalan, 1980; Hess and Tsien, 1984; Campbell et al., 1988; Kurata et al., 1999). The energy profiles for Ca2+ and Ba2+ were (in RT units) 4, −19.8, −7.5, −17.5, 5 and 4, −17.4, 1, −15.5, 5.2 for LVA and 4, −19.5, −7.5, −18.1, 1 and 4, −14.5, 1, −14.7, 2 for HVA channels. Extracellular and intracellular total divalent cation concentrations were set to 10 mM and 0.1 μM, respectively. Computation of steady-state ionic currents at different Ca2+ mole fractions and decreasing DCS locus energy (−20 to −12 RT units) was made by using the matrix inversion method to solve the nine differential equations of the occupancy probabilities for the nine possible states of the model. ICa/IBa was computed as the ratio of the currents calculated in 10 mM pure Ca2+ and Ba2+, respectively. The program was written using Matlab (The Mathworks Inc.).
Online Supplemental Material
The online supplemental material is available at http://www.jgp.org/cgi/content/full/jgp.200709771/DC1. Tables S1–S3 show voltage-dependent properties of CaV2.1 and CaV2.3 mutant channels. Fig. S1 shows comparative analysis of ramp versus step voltage to record AMFE. Fig. S2 is a compilation of the selective permeation profile and amino acid alignment of the pore region of CaV2.3 and Kv channels. Fig. S3 shows activation and inactivation curves of CaV2.1 and CaV2.3 mutant channels. Fig. S4 shows the effect of non-DCS mutations on AMFE.
RESULTS
One of the major theoretical outcomes of the two Ca2+ binding-site model for Ca2+ channel permeation is the presence of AMFE, where, in the presence of mixtures of Ba2+ and Ca2+ ions at a total constant concentration, the measured peak current–mole fraction curve is non-monotonic and goes through a minimum (Hess and Tsien, 1984). AMFE was first described for L-type VGCC (Almers et al., 1984; Hess and Tsien, 1984). AMFE was also later reported for P/Q-type CaV2.1 channels (Mangoni et al., 1997) and expected to be very weak, from theoretical considerations, in Cav3.1 channels (Serrano et al., 2000). However, a complete comparative analysis of the Ba/Ca selectivity and AMFE of all LVA and HVA channels has never been done.
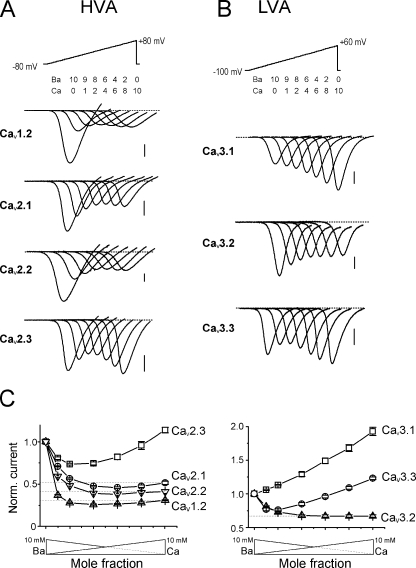
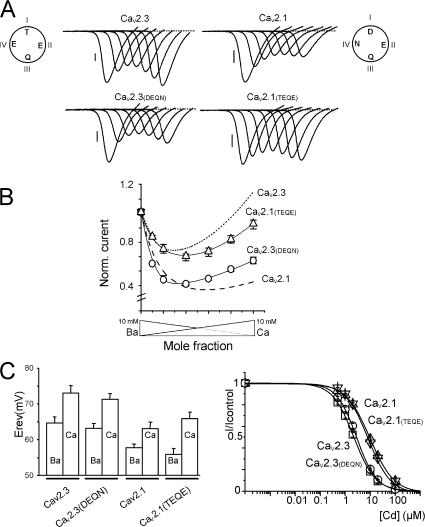
Current–voltage curves were recorded on Xenopus oocytes expressing different LVA and HVA VGCC under extracellular ionic conditions that allowed the detection of AMFE and the characterization of the Ba/Ca permeability. As shown on the current traces recorded during voltage ramps in the presence of 10/0, 9/1, 8/2, 6/4, 4/6, 2/8, and 0/10 Ba/Ca mole fractions (total cation concentration kept constant at 10 mM), L-type (CaV1.2), P/Q-type (CaV2.1), N-type (CaV2.2), but also R-type (CaV2.3) HVA Ca2+ channels displayed a marked AMFE, with a characteristic (and statistically significant) minimum peak current value for low Ca2+ mole fractions (current traces and peak current–Ba/Ca mole fraction curves are shown in Fig. 1, A and C). These channels nevertheless displayed different relative amplitudes of their Ca2+ and Ba2+ currents at 10 mM (ICa/IBa), ranging from 0.31 ± 0.01 (n = 23) for CaV1.2, 0.41 ± 0.02 (n = 8) for CaV2.2, and 0.51 ± 0.02 (n = 17) for CaV2.1 to 1.12 ± 0.03 (n = 13) for CaV2.3 (Fig. 1 C), suggesting specific permeation profiles despite conserved EEEE locus. Similar results were also found when current–voltage curves were obtained from voltage steps instead of voltage ramps, demonstrating that these differences were not due to differences in Ca2+-dependent inactivation among the different VGCC (Fig. S1, available at http://www.jgp.org/cgi/content/full/jgp.200709771/DC1).
Figure 1.
Low and high voltage–activated Ca2+ channels have specific Ca2+/Ba2+ selectivities. (A and B) Representative current traces recorded in oocytes expressing HVA CaV1.2, CaV2.1, CaV2.2, or CaV2.3 and α2-δ + β2 Ca2+ channel subunits (A) or LVA CaV3.1, CaV3.2, or CaV3.3 Ca2+ channels (B) using solutions of different Ca2+ mole fractions (Ba2+/Ca2+: 10/0, 9/1, 8/2, 6/4, 4/6, 2/8, and 0/10 mM). Whole-cell currents were recorded during voltage ramps from −80 to +80 mV (A) or from −100 to +60 mV (B) at a speed of 0.4 to 2.6 V/s applied every 15 s. Bars, 100 nA. (C) Normalized current/mole fraction curves obtained from recordings similar to those in A or B by plotting the peak current of the IV curve against the Ca/Ba mole fraction. Note the different ICa/IBa ratios and the presence/absence of AMFE in each case. Left, averaged curves obtained from oocytes expressing CaV1.2 (▵), CaV2.1 (○), CaV2.2 (▿), or CaV2.3 (□) HVA VGCC. Right, curves obtained from oocytes expressing CaV3.1 (□), CaV3.2 (▵) or CaV3.3 (○) LVA VGCC.
Surprisingly, when LVA T-type CaV3.1, CaV3.2, or CaV3.3 VGCC were expressed in oocytes and submitted to the same experimental protocol (Fig. 1, B and C), AMFE was only recorded for CaV3.3, while peak current decreased, or increased, monotonically with increasing Ca2+ mole fractions for CaV3.2 and CaV3.1 channels, respectively. These new results were completely unexpected since these three channels display the same sequence at their EEDD locus. Moreover, all three channels display a specific Ba/Ca permeability with either larger (ICa/IBa = 1.91 ± 0.06, n = 10, for CaV3.1), smaller (ICa/IBa = 0.67 ± 0.02, n = 8, for CaV3.2), or similar (1.23 ± 0.02, n = 5, for CaV3.3) Ca2+ currents relative to Ba2+ currents.
When compiled together, these data unambiguously underlined channel-specific permeation profiles in the two channel families (see Fig. S2 A). Such a specificity cannot be explained by the only high-affinity Ca2+ binding site identified, which is perfectly conserved in these two families (the EEEE and EEDD loci in HVA and LVA channels, respectively, see * box in Fig. 2 A) (Cibulsky and Sather, 2000; Sather and McCleskey, 2003). Inspection of the aligned amino acid sequences (Fig. 2 A) of the pore-forming P loops of HVA and LVA channels reveals the existence of a single set of nonconserved negatively charged residues located at a homologous position in each of the four domains of the CaVα subunits, four residues upstream of the well-characterized EEEE/EEDD locus (# box in Fig. 2 A). This DCS locus comprises amino acids DSED, DEQN, DAME, and TEQE in the channel domains I–IV, respectively, in CaV1.2, CaV2.1, CaV2.2, and CaV2.3 and amino acids DKDG, DVNG, and EVNG in CaV3.1, CaV3.2, and CaV3.3 channels.
The lack of structural data on the pore of the VGCC imposed the use of molecular modeling to visualize the possible arrangement of the four CaV2.3 P loops at the EEEE and DCS loci. This exercise has been done using voltage-gated K+ channel as template (Doyle et al., 1998; Jiang et al., 2003) with major constraints that take into account the fundamental differences that exist between the K+ and Ca2+ channel pores; see Fig. 2 B and Fig. S2 B (Hille, 2001; Lipkind and Fozzard, 2001). The DCS locus is located on the extracellular side of the EEEE locus but likely within the membrane electric field as suggested (Hess and Tsien, 1984) and with interloci distances of ∼8 Å. The carboxyl oxygens of the DCS locus can point toward the channel lumen, allowing their participation in the coordination of incoming divalent cations. Thus, from theoretical and experimental consideration on AMFE, the DCS locus constitutes, in several respects, a good candidate for a specific role in divalent cation selection.
Although all residues at this locus may not play an equivalent role, as is the case at the EEEE locus (Kim et al., 1993; Mikala et al., 1993; Tang et al., 1993; Yang et al., 1993; Parent and Gopalakrishnan, 1995; Cloues et al., 2000), our first hypothesis was that the total number of negatively charged residues at this location was critical for the binding of Ca2+ ions.
To test this hypothesis, 19 mutations were engineered in the DCS locus of CaV2.3, where the number of negatively charged residues was either increased (DSEE, DSED, and DEEE) or decreased (TSQN, GGGG, and AAAA). The resulting six mutants, labeled CaV2.3(DSEE), CaV2.3(DSED), CaV2.3(DEEE), CaV2.3(TSQN), CaV2.3 (GGGG), and CaV2.3(AAAA), respectively (Fig. 3), were tested for their effect on channel selectivity. Currents recorded from Xenopus oocytes injected with the CaV2.3 (DSED) mutant cDNA showed a marked minimum for Ba/Ca mixtures of low Ca2+ mole fractions, and pure Ca2+ currents were clearly smaller than pure Ba2+ currents (ICa/IBa = 0.60 ± 0.05, n = 16, circle, Fig. 3, A and B). This type of behavior was typical of CaV1.2 channels, which also possessed three negative charges at the DCS locus. Similar results were also found with the CaV2.3(DSEE) mutant (ICa/IBa = 0.65 ± 0.02, n = 20; unpublished data). The CaV2.3(TSQN) mutant (square in Fig. 3, A and B), with no negative charges at the DCS, also had a smaller ICa/IBa ratio (0.44 ± 0.02, n = 11), but in this case the AMFE completely disappeared and the total current became a monotonic function of the Ca2+ mole fraction, without any marked minimum. Similar results were also found when the four residues of the DCS locus were mutated to alanine or glycine (CaV2.3(AAAA) or CaV2.3(GGGG) in Fig. 3), revealing important modifications in the energy profile of the channel pore (Hess and Tsien, 1984) clearly dependent upon the net charge carried by the residues at the DCS. Interestingly, the CaV2.3(DEEE) mutant with four charged residues at the DCS locus had an intermediate behavior, with a clear minimum in the current–mole fraction curve and an ICa/IBa ratio of 1.02 ± 0.05 (n = 15) between that of CaV1.2 and CaV2.3 (Fig. 3 B; see Discussion).
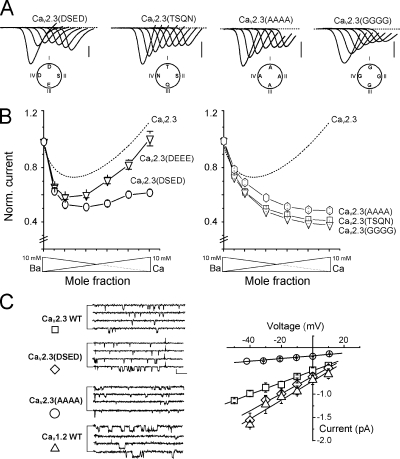
Figure 3.
Mutations at the DCS locus suppress anomalous mole fraction of HVA. The four amino acids of the CaV2.3 DCS locus were mutated by adding or removing negatively charged residues. (A) Typical current traces recorded during voltage ramps under different ionic conditions (see Fig. 1) applied to oocytes expressing CaV2.3 channels with their DCS loci mutated in DSED (one negative charge added, CaV2.3(DSED)), DEEE (two negative charges added, CaV2.3(DEEE)), TSQN, AAAA, or GGGG (two negative charges removed, CaV2.3(TSQN), CaV2.3(AAAA) or CaV2.3 (GGGG)). (B) Current–mole fraction curves obtained for the above mutations. Right, effect of adding negative charges (CaV2.3(DSED), ○; CaV2.3 (DEEE), ▿). Left, effect of removing negative charges (CaV2.3(TSQN), □; CaV2.3 (AAAA), hexagone; CaV2.3 (GGGG), ▿). Note that the removal of the negative charges suppressed AMFE in all three cases. The dotted line represents the curve obtained with the WT CaV2.3 in Fig. 1. (C) Single-channel conductances of CaV2.3, CaV2.3(DSED), CaV2.3(AAAA), and CaV1.2 VGCC. Left, current traces in 100 mM BaCl2 (pipette potential = +10 mV, except CaV2.3(AAAA) −20 mV). Right, current–voltage curves with superimposed linear regressions (13 ± 1 pS, 21 ± 1 pS, 4 ± 1 pS, and 22 ± 1 pS for CaV2.3, CaV2.3(DSED), CaV2.3(AAAA), and CaV1.2, respectively). Bar, 0.5 pA, 25 ms.
As a putative structural determinant of VGCC selectivity filter, mutations in the DCS locus are expected to modify permeation and single-channel conductance. In cell-attached patches performed in 100 mM Ba2+, the single channel conductance of CaV2.3(DSED) (21 ± 1 pS, n = 9; Fig. 3 C) and CaV2.3(DSEE) (22 ± 1 pS, n = 7; not depicted) channels were clearly larger than the wild-type CaV2.3 (13 ± 1 pS, n = 8) and closely matched that of CaV1.2 channels (22 ± 2 pS, n = 8; Fig. 3 C) without any obvious changes in the channel open time (not depicted but see WT and mutated CaV2.3 current traces in Fig. 3 C). The CaV2.3(AAAA) single channel amplitude was on the other hand much smaller with only 4 ± 1 pS (n = 7). Our macroscopic recordings confirmed that, for the six mutated channels studied here, as for the others described below, the changes in selectivity occurred with no other modification in the biophysical properties of the channel, including current–voltage and steady-state inactivation curves (Fig. S3, A and B, and Tables S2 and S3). The kinetics of inactivation, estimated by the fractional current recorded at the end of a 2.5-s-long pulse (to +10 mV) in Ba2+ and in Ca2+, were also not modified by these mutations (Fig. S3 C). Moreover, Cd2+ block of Ba2+ currents and current reversal potentials in 10 mM Ba2+ and 10 mM Ca2+ were only marginally affected (Table S1), demonstrating that mutations at the DCS have localized effects that did not spread through the protein via global perturbation of the channel gating, the pore structure, or the EEEE locus.
To challenge the idea that the DCS was involved in the specificity of the divalent cation selectivity profiles of HVA Ca2+ channels, we decided to switch the DCS of two related channels (CaV2.1 and CaV2.3) and analyze the selectivity profiles of the resulting channels.
Currents recorded from oocytes expressing CaV2.3 (DEQN) mutated channels (with CaV2.1 DCS implemented in CaV2.3) displayed a strong AMFE, with a clear minimum recorded for small Ca2+ mole fractions and ICa/IBa ratio close to that of CaV2.1 channels (0.66 ± 0.02, n = 11). Conversely, currents recorded from oocytes expressing CaV2.1(TEQE) Ca2+ channels (with CaV2.3 DCS introduced in CaV2.1) displayed AMFE and IC/IBa ratio similar to those of CaV2.3 channels (ICa/IBa = 0.92 ± 0.02, n = 30, Fig. 4, A and B). Again, these modifications occurred without changes in the divalent over monovalent selectivity (Ca2+ or Ba2+ current reversal potentials were not modified, Fig. 4 C), and in the channel affinity for Cd2+, the small differences in the EC50 for Cd2+ block between CaV2.3 and CaV2.1 being preserved (Fig. 4 C). Thus, mutations at the DCS locus only affected divalent selectivity without affecting the EEEE locus and EEEE-related properties (divalent/monovalent selectivity and Cd2+ binding site). These data suggested that DCS forms a distinct locus involved in the multi ionic nature of the channel selectivity filter (Kuo and Hess, 1993a,b).
Figure 4.
DCS locus controls the channel-specific cation selectivity profile. (A and B) A CaV2.3 channel with an engineered CaV2.1 DCS locus (CaV2.3(DEQN), square) displays AMFE and has an ICa/IBa ratio similar to CaV2.1. Conversely, a Ca2.1 channel with a CaV2.3 DCS locus (CaV2.1(TEQE), ▵) behaves like CaV2.3. (A) Current traces and (B) current–mole fraction curves. Dashed lines, current/mole fraction curves of parent channels, CaV2.3 and CaV2.1, determined in Fig. 1. (C) The current reversal potentials, recorded in 10 mM Ca2+ or 10 mM Ba2+ (left), and the Cd2+ block of Ba2+ current (right) for CaV2.3 (□) and CaV2.1(▿) channels are not changed by the mutations (CaV2.3(DEQN), ○; and CaV2.1(TEQE), ▵), suggesting the EEEE locus is not modified.
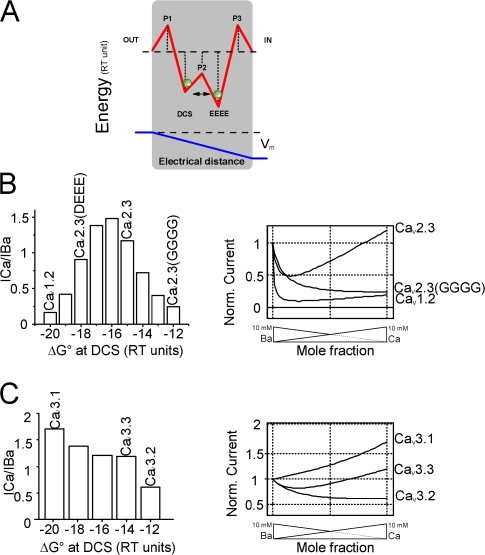
To summarize the effects of these different HVA Ca2+ channel mutations on Ba2+/Ca2+ selection, we have plotted the extent of AMFE (in arbitrary units, see methods) as a function of the number of negative charges at the DCS. AMFE values close to 1 indicate no mole fraction, while smaller values indicate the presence of stronger AMFE with larger Ca/Ba current ratios. As shown in Fig. 5, a clear and statistically significant relation between charges and AMFE exists (using the Spearman correlation test at a 0.05 level that makes no assumption on the nature of the relation). Although care should be taken in interpreting such a graph because AMFE and ICa/IBa ratios clearly do not vary linearly with ion binding energy (see Fig. 7); this nevertheless confirms the implication of the DCS locus on HVA channels selectivity.
Figure 7.
Numerical computation predicts mutant behavior. (A) Schematic representation of the energy profile used for computer modeling of the three barriers–two sites channel model. Barriers (P1, P2, P3) and wells (DCS and EEEE) are spaced equally (see Materials and methods). Membrane voltage drops across the narrow portion of the channel. (B, left) Simulated ICa/IBa ratios calculated for decreasing free energies at the external DCS locus of the CaV2.3 channel. The bell-shaped relation obtained for the DCS locus reached a maximum when DCS ΔG° equals the energy of the internal site. (B, right) Simulated current–mole fraction curves with three out of the four decreasing DCS ΔG° depicted on the left. These curves were labeled CaV1.2, CaV2.3(DEEE), and CaV2.3(GGGG), after comparison with original traces shown in Figs. 1 and 3, and suspected variations in free energy at DCS locus due to mutation. (C) Simulated ICa/IBa ratios calculated for decreasing free energies at the external DCS locus of the CaV3.1 channel. Energy profile of CaV3.1 was first adapted from CaV2.3 by adjusting the ΔG° at the EEEE locus considering that this locus in LVA channels (EEDD) has lower affinity for Ca2+. Simulated current–mole fraction curves were then obtained by decreasing ΔG° at the DCS locus. Traces were labeled after comparison with original traces shown in Fig. 1 and suspected variations in free energy at DCS for the purpose of discussion.
It is worth noting that the conservation of the EEEE locus among HVA-VGCC does not exclude a crucial role in divalent selection but rather conservation of this role in the VGCC family. To test this idea, we mutated a nonconserved glycine residue on CaV2.3 that has been shown to slightly decrease Ca2+ binding at the EEEE locus and perturb channel permeation (Williamson and Sather, 1999) and analyzed the effects of this mutation on AMFE and Ca/Ba selectivity. The mutant CaV2.3 (G1323F) channels displayed a decrease in AMFE and in relative Ca2+ currents (ICa/IBa) in close proportion with the reported decrease in the binding energy for divalent cation (Williamson and Sather, 1999) at the EEEE locus (ICa/IBa = 1.12 ± 0.03, n = 13 and 0.70 ± 0.05, n = 10 for CaV2.3 and CaV2.3(G1323F), respectively, Fig. S4, A and B), confirming the role of the EEEE locus in profiling channel permeation. On the other hand, mutation of a more distant nonconserved residue outside these two loci (S1611C) had no effect either on CaV2.3 AMFE or on the relative Ca2+ currents (ICa/IBa = 1.12 ± 0.03, n = 13 and 1.06 ± 0.05, n = 6 for CaV2.3 and CaV2.3 (S1611C), respectively, Fig. S4, A and B). This mutation had also no effect when made on the CaV2.3(DSED) mutant channels with ICa/IBa = 0.60 ± 0.05, n = 16 and ICa/IBa = 0.60 ± 0.08, n = 9 for CaV2.3(DSED) and CaV2.3(DSED)(S1611C).
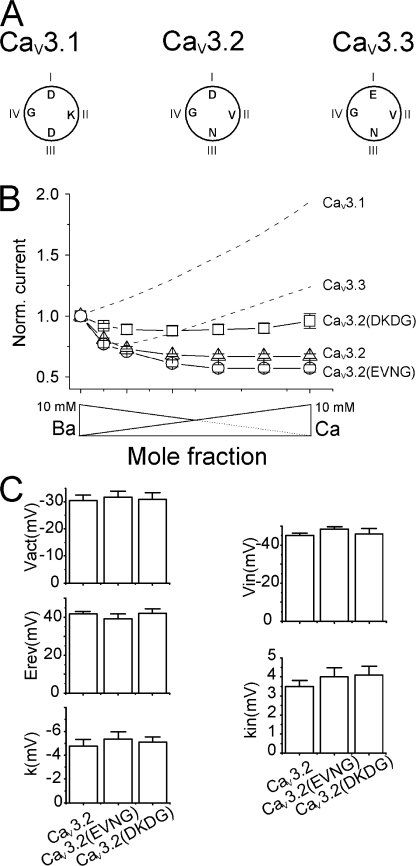
Having in mind the large differences in the current– mole fraction curves between the different LVA channels, we have mutated the DCS locus of CaV3.2 (DCS: DVNG, see Fig. 6 A) to match that of either CaV3.1 or CaV3.3 (DCS locus: DKDG or EVNG, respectively), giving rise to the two mutated CaV3.2 channels: CaV3.2 (DKDG) and CaV3.2(EVNG). Current–mole fraction curves obtained with these three channels are displayed in Fig. 6 B, and clearly showed that the mutation of the CaV3.2 DCS to EVNG had no marked effect on AMFE and on the relative Ca2+ currents, while mutation to DKDG clearly modified these two parameters with the appearance of a small AMFE, absent in the parent CaV3.2 channels (curves of CaV3.1 and CaV3.3 channels are in dashed lines for comparison). As was the case for HVA channels, these changes appeared without any modification in the current–voltage and steady-state inactivation curves of the channels, the potential of half-activation, the potential for half-inactivation, the slope of the two curves, and the reversal potential remaining unchanged (Fig. 6 C). The kinetics of the currents was also not modified (unpublished data). These data suggest that the modifications recorded with the CaV3.2(DKDG) mutant are not due to perturbations of the LVA EEDD locus, known to change reversal potential and current kinetics (Talavera et al., 2001, 2003).
Figure 6.
Mutations at the DCS locus modify selectivity profiles of LVA without changes in gating. (A) Schematic representation of the amino acids present at the CaV3.1, CaV3.2, and CaV3.3 DCS loci. (B) Current–mole fraction curves obtained for the WT (▵) and two mutant CaV3.2 LVA channels mimicking the DCS loci of CaV3.1 (CaV3.2(DKDG), □) and CaV3.3 (CaV3.2(EVNG), ○). Ba/Ca current ratio was significantly changed for the CaV3.2(DKDG) mutant that also displayed a small AMFE as opposed to WT CaV3.2. (C) Voltage-dependent parameters of the current–voltage curves and inactivation curves of the CaV3.2, CaV3.2(EVNG), and CaV3.2(DKDG) LVA channels. These two mutations were without noticeable effects on these parameters.
Altogether, these results suggest that in HVA channels, the differences in divalent cation permeation recorded between voltage-gated Ca2+ channels were mainly due to variations in the structure of the DCS and EEEE loci, while in LVA channels, although these two sites are also implicated, they may not be the only determinants.
DISCUSSION
This first detailed analysis of the divalent cation selectivity of LVA and HVA VGCC identified a ring of nonconserved negative charges in the upper part of the pore directly involved in the selection of Ca2+ by Ca2+ channels. Modifications of the pattern of charged residues at this site affect both AMFE and relative Ca2+ current in HVA and LVA VGCC.
Previous work on CaV1.2 (Ellinor et al., 1995; Cibulsky and Sather, 2000; Sather and McCleskey, 2003) strongly suggested that high-affinity Ca2+ binding in the VGCC pore involved a single site, the EEEE locus. Although a role for the EEEE locus in divalent selection makes no doubt, its implication in the specificity of the selection observed here, via a global perturbation of the channel structure by DCS mutations, seems very unlikely for several reasons. First, the conservation of the pattern of charged residues at the EEEE/EEDD locus on HVA/LVA channels excludes this locus as a major determinant for channel-specific properties. Second, properties known to be strongly modified by mutation at the EEEE locus (Ba2+ or Ca2+ current reversal potentials, Cd2+ inhibition) are not changed by similar mutation at the DCS. Third, DCS mutations have no side effects on the voltage-dependent properties or on the current kinetics. On the other hand, we can speculate that quadruple mutations at internal EEEE locus (Ellinor et al., 1995; Cibulsky and Sather, 2000) may destabilize the less-conserved DCS locus and reduce Ca2+ binding at this site. Of course, our results on the CaV2.3(G1323F) mutant demonstrate that the EEEE locus participates at the global shaping of these permeation profiles, but not at their specificity encountered in the different VGCC.
We propose that the number of charged residues present at the VGCC DCS locus is critical for the binding of Ca2+ ions. Three negative charges are present in the CaV1.2 and CaV2.3(DSED) channels that display the highest Ba2+ conductance and the lowest ICa/IBa ratio. With only two negative charges, CaV2.1(DCS=DEQN), CaV2.2 (DCS=DAME), and CaV2.3 (DCS=TEQE) have lower Ba2+ conductances but higher ICa/IBa ratios. The differences in the permeation profile that remain between these three channels may rely (a) on the noncharged residues present at this site (i.e., glutamines are known to be able to participate in Ca2+ binding; Kim et al., 1993), (b) on nonequivalent positioning of these two charges within the four channel domains and/or (c) on other neighboring amino acids that could finely shape the structure of the DCS. Although the characterization of the precise role of these three factors will require further experiments, it is worth noting that the effects of the mutation of glutamate at the EEEE or EEDD loci in HVA and LVA channels are known to be strongly dependent on their location (Mikala et al., 1993; Talavera et al., 2001; Sather and McCleskey, 2003).
Different theoretical models have been proposed (Hess and Tsien, 1984; Miller, 1999; Roux, 1999; Sather and McCleskey, 2003) to account for the specific permeation properties of VGCC including (a) the selection of Ca over Na despite identical size and unfavorable concentration, (b) high Ca fluxes through open channels, and (c) high affinity block of monovalent currents by Ca2+. In our case, we thought modeling the Ca2+ channel as a multi-ion single file pore with two Ca2+ binding sites and three energy barriers (Hess and Tsien, 1984) was particularly appropriate to understand the role of rings of negative charges in the channel lumen (Fig. 7 A). AMFE was computed using rate transitions calculated from Eyring rate theory with energy profiles for Ca2+ and Ba2+ derived from previous works (Hess and Tsien, 1984; Campbell et al., 1988). Current/mole fraction curves were obtained by sequentially decreasing the binding energy (ΔG°) of the external locus. For large ΔG° at the DCS (as expected for CaV1.2 channels), the ICa/IBa ratio was around 0.3 and total current showed AMFE with a strong reduction for small Ca2+ mole fractions (Fig. 7 B). When ΔG° was progressively decreased, keeping all other parameters constant, ICa/IBa reached a maximum at ∼1.5 (close to wild-type CaV2.3 channels) and then progressively returned to lower values. For these values, however, the current decreased monotonically and did not display AMFE (Fig. 7 B and Hess and Tsien, 1984) similar to recordings obtained from the CaV2.3(TSQN) CaV2.3 (AAAA) or the CaV2.3 (GGGG) channels without negative charges at the DCS. Whether one or multiple Ca2+ ions remain bound to the EEEE locus in these mutants devoid of AMFE is an open question that requires further experiments.
Our simulation also suggests that CaV2.3 (DEEE) mutant, with strong AMFE but ICa/IBa ratio close to the parent CaV2.3 channel, despite two additional negative charges at the DCS locus may result from DCS with a smaller ΔG° than CaV1.2 (Fig. 7 B). This can be explained by an increase in the electrostatic repulsion generated by the two additional carboxylates present on the DCS locus (Wu et al., 2000) leading to looser cation coordination. Mimicking the decrease in the ΔG° of the EEEE locus for the CaV2.3(G1323F) mutant (by 1 RT unit, see Williamson and Sather, 1999) also resulted in computed current–mole fraction curves (Fig. S4 B) close to those obtained experimentally in terms of relative Ca2+ currents and amplitude of AMFE. In an interesting alternative, the DCS locus could also constitute one of the low affinity sites proposed by Dang and McCleskey (1998) to be necessary to overcome the large energy barriers flanking a single high affinity binding site. However, further experimental testing is required to more definitively validate one of these models.
In the case of LVA VGCC, simulation with decreasing ΔG° at the DCS (Fig. 7 C) and comparison with experimental results (Fig. 1) suggested the following sequence of ΔG° at DCS CaV3.1 > CaV3.3 > CaV3.2. Calculation of single channel currents using these differences in ΔG° gives a higher single channel conductance for CaV3.3 than for CaV3.1 or CaV3.2 (unpublished data), a situation that is recorded experimentally (Perez Reyes et al., 1998; Cribbs et al., 1998; Lee et al., 1999). However, although the DCS locus is undoubtedly involved in shaping the specific selectivity of LVA channels (see CaV3.2(DKDG) mutant), we were not able to completely restore the properties of the different CaV3 channels by mutation of the DCS. The existence of non-EEEE/EEDD structural determinants involved in the differences in the selectivity recorded between HVA and LVA and among LVA channels have been postulated by Talavera et al. (2001). Our results demonstrate that DCS is one of them and clearly suggest the existence of other elements, at least for LVA channels. The strong conservation of the EEDD locus and the P loop sequences in the different members of the LVA channel family locate these putative determinants implicated in LVA permeation at a more external position.
The presence of two sets of negative charges in the channel pore to regulate channel selectivity is a general scheme that has been conserved among different Ca2+ channels. A homologous external locus is also found in KV (MacKinnon and Yellen, 1990; Crouzy et al., 2001) and NaV channels (Hille, 2001) with important roles in permeation and pharmacology. From a physiological point of view, DCS clearly and directly participates in the generation of large Ca2+ influx (see Fig. 5). Its nonconservation among HVA channels plays a role in the definition of the channel-specific conductance, and thus could participate in the adaptation of each channel to its particular function: large and global Ca2+ influx for excitation–contraction coupling driven by CaV1.2, or smaller and more local Ca2+ changes required for CaV2.1-triggered synaptic transmission, for example. Our preliminary data, on CaV2.1/CaV2.3 and CaV3.1/CaV3.2 channels also suggest an implication in the differential block of these channels by external magnesium (unpublished data).
In conclusion, these results demonstrate the unexpected existence of an external locus that may form part of a structural context necessary for the pore of HVA and LVA Ca2+ channels to display high Ca2+ influx and specific divalent cation selectivity. Whether this site is also important for other pore-dependent properties such as toxin or drug binding is now under investigation.
Supplemental Material
Acknowledgments
The authors would like to thank Dr. M. Bellis for help with Matlab, Dr. T.P. Snutch (University of British Columbia, Vancouver, British Columbia, Canada) for providing the CaV1.2, CaV2.1, and CaV2.3 Ca2+ channel cDNA, Drs. E. Perez-Reyes (University of Virginia, Charlottesville, VA) and A. Monteil (IGH CNRS UMR 5203, Montpellier, France) for providing human CaV3.1, CaV3.2, and CaV3.3 cDNA, Drs. R. Dolmetsch, I. Findlay, I.A. Lefevre, R. MacKinnon, and G. Vassort for critical reading of the manuscript, and C. Delattre, J.M. Donnay, and J.C. Mazur for technical help.
This work was supported by CNRS, INSERM, Association Française contre les Myopathies, Association pour la Recherche contre le Cancer, Fondation pour la Recherche sur le Cerveau et Fondation Simone et Cino del Duca, and ANR N°Blan_06_01- 148568.
Olaf S. Andersen served as editor.
Abbreviations used in this paper: AMFE, anomalous mole fraction effect; DCS, divalent cation selectivity; HVA, high voltage–activated; LVA, low voltage–activated; VGCC, voltage-gated Ca2+ channels.
References
- Almers, W., E.W. McCleskey, and P.T. Palade. 1984. A non-selective cation conductance in frog muscle membrane blocked by micromolar external calcium ions. J. Physiol. 353:565–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich, T.B., and M.D. Cahalan. 1980. Sodium channel permeation in squid axons. I: Reversal potential experiments. J. Physiol. 307:217–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourinet, E., Zamponi, G.W., Stea, A., Soong, T.W., Lewis, B.A., Jones, L.P., Yue, D.T., and Snutch, T.P. 1996. The α1E calcium channel exhibits permeation properties similar to low-voltage-activated calcium channels. J. Neurosci. 16:4983–4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, D.L., R.L. Rasmusson, and H.C. Strauss. 1988. Theoretical study of the voltage and concentration dependence of the anomalous mole fraction effect in single calcium channels. New insights into the characterization of multi-ion channels. Biophys. J. 54:945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall, W.A. 2000. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16:521–555. [DOI] [PubMed] [Google Scholar]
- Cibulsky, S.M., and W.A. Sather. 2000. The EEEE locus is the sole high-affinity Ca2+ binding structure in the pore of a voltage-gated Ca2+ channel: block by Ca2+ entering from the intracellular pore entrance. J. Gen. Physiol. 116:349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibulsky, S.M., and W.A. Sather. 2003. Control of ion conduction in L-type Ca2+ channels by the concerted action of S5-6 regions. Biophys. J. 84:1709–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloues, R.K., S.M. Cibulsky, and W.A. Sather. 2000. Ion interactions in the high-affinity binding locus of a voltage-gated Ca2+ channel. J. Gen. Physiol. 116:569–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs, L.L., J.H. Lee, J. Yang, J. Satin, Y. Zhang, A. Daud, J. Barclay, M.P. Williamson, M. Fox, M. Rees, and E. Perez Reyes. 1998. Cloning and characterization of alpha1H from human heart, a member of the T-type Ca2+ channel gene family. Circ. Res. 83:103–109. [DOI] [PubMed] [Google Scholar]
- Crouzy, S., S. Berneche, and B. Roux. 2001. Extracellular blockade of K+ channels by TEA: results from molecular dynamics simulations of the KcsA channel. J. Gen. Physiol. 118:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang, T.X., and E.W. McCleskey. 1998. Ion channel selectivity through stepwise changes in binding affinity. J. Gen. Physiol. 111:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayring, H.E., A. Tramonato, S.R. Sprang, and R.J. Fletterick. 1986. Interactive program for visualization and modeling of proteins, nucleic acids and small molecules. J. Mol. Graph. 4:82–87. [Google Scholar]
- Doyle, D.A., C.J. Morais, R.A. Pfuetzner, A. Kuo, J.M. Gulbis, S.L. Cohen, B.T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- Efimov, A.V. 1993. Standard structures in proteins. Prog. Biophys. Mol. Biol. 60:201–239. [DOI] [PubMed] [Google Scholar]
- Ellinor, P.T., J. Yang, W.A. Sather, J.F. Zhang, and R.W. Tsien. 1995. Ca2+ channel selectivity at a single locus for high-affinity Ca2+ interactions. Neuron. 15:1121–1132. [DOI] [PubMed] [Google Scholar]
- Feng, Z.P., J. Hamid, C. Doering, S.E. Jarvis, G.M. Bosey, E. Bourinet, T.P. Snutch, and G.W. Zamponi. 2001. Amino acid residues outside of the pore region contribute to N-type calcium channel permeation. J. Biol. Chem. 276:5726–5730. [DOI] [PubMed] [Google Scholar]
- Heinemann, S.H., H. Terlau, W. Stuhmer, K. Imoto, and S. Numa. 1992. Calcium channel characteristics conferred on the sodium channel by single mutations. Nature. 356:441–443. [DOI] [PubMed] [Google Scholar]
- Hess, P., and R.W. Tsien. 1984. Mechanism of ion permeation through calcium channels. Nature. 309:453–456. [DOI] [PubMed] [Google Scholar]
- Higgins, D.G., J.D. Thompson, and T.J. Gibson. 1996. Using CLUSTAL for multiple sequence alignments. Methods Enzymol. 266:383–402. [DOI] [PubMed] [Google Scholar]
- Hille, B. 2001. Ion channels of Excitable Membranes. Third edition. Sinauer Associates Inc., Sunderland, MA. 814 pp.
- Isom, L.L., K.S. De Jongh, and W.A. Catterall. 1994. Auxiliary subunits of voltage-gated ion channels. Neuron. 12:1183–1194. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, V. Ruta, M. Cadene, B.T. Chait, and R. MacKinnon. 2003. X-ray structure of a voltage-dependent K+ channel. Nature. 423:33–41. [DOI] [PubMed] [Google Scholar]
- Kim, M.S., T. Morii, L.X. Sun, K. Imoto, and Y. Mori. 1993. Structural determinants of ion selectivity in brain calcium channel. FEBS Lett. 318:145–148. [DOI] [PubMed] [Google Scholar]
- Kuo, C.C., and P. Hess. 1993. a. Characterization of the high-affinity Ca2+ binding sites in the L-type Ca2+ channel pore in rat phaeochromocytoma cells. J. Physiol. 466:657–682. [PMC free article] [PubMed] [Google Scholar]
- Kuo, C.C., and P. Hess. 1993. b. Ion permeation through the L-type Ca2+ channel in rat phaeochromocytoma cells: two sets of ion binding sites in the pore. J. Physiol. 466:629–655. [PMC free article] [PubMed] [Google Scholar]
- Kurata, Y., R. Sato, I. Hisatome, and S. Imanishi. 1999. Mechanisms of cation permeation in cardiac sodium channel: description by dynamic pore model. Biophys. J. 77:1885–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.H., A.N. Daud, L.L. Cribbs, A.E. Lacerda, A. Pereverzev, U. Klockner, T. Schneider, and E. Perez-Reyes. 1999. Cloning and expression of a novel member of the low voltage-activated T- type calcium channel family. J. Neurosci. 19:1912–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkind, G.M., and H.A. Fozzard. 2001. Modeling of the outer vestibule and selectivity filter of the L-type Ca2+ channel. Biochemistry. 40:6786–6794. [DOI] [PubMed] [Google Scholar]
- MacKinnon, R., and G. Yellen. 1990. Mutations affecting TEA blockade and ion permeation in voltage-activated K+ channels. Science. 250:276–279. [DOI] [PubMed] [Google Scholar]
- Mangoni, M.E., T. Cens, C. Dalle, J. Nargeot, and P. Charnet. 1997. Characterisation of α1A Ba2+, Sr2+ and Ca2+ currents recorded with ancillary β1-4 subunits. Receptors Channels. 5:1–14. [PubMed] [Google Scholar]
- Mikala, G., A. Bahinski, A. Yatani, S. Tang, and A. Schwartz. 1993. Differential contribution by conserved glutamate residues to an ion-selectivity site in the L-type Ca2+ channel pore. FEBS Lett. 335:265–269. [DOI] [PubMed] [Google Scholar]
- Miller, C. 1999. Ionic hopping defended. J. Gen. Physiol. 113:783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent, L., and M. Gopalakrishnan. 1995. Glutamate substitution in repeat IV alters divalent and monovalent cation permeation in the heart Ca2+ channel. Biophys. J. 69:1801–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Reyes, E., L.L. Cribbs, A. Daud, A.E. Lacerda, J. Barclay, M.P. Williamson, M. Fox, M. Rees, and J.H. Lee. 1998. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 391:896–900. [DOI] [PubMed] [Google Scholar]
- Rosenberg, R.L., and X.H. Chen. 1991. Characterization and localization of two ion-binding sites within the pore of cardiac L-type calcium channels. J. Gen. Physiol. 97:1207–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux, B. 1999. Theories of ion permeation: a chaser. J. Gen. Physiol. 114:605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather, W.A., and E.W. McCleskey. 2003. Permeation and selectivity in calcium channels. Annu. Rev. Physiol. 65:133–159. [DOI] [PubMed] [Google Scholar]
- Sather, W.A., J. Yang, and R.W. Tsien. 1994. Structural basis of ion channel permeation and selectivity. Curr. Opin. Neurobiol. 4:313–323. [DOI] [PubMed] [Google Scholar]
- Serrano, J.R., S.R. Dashti, E. Perez-Reyes, and S.W. Jones. 2000. Mg2+ block unmasks Ca2+/Ba2+ selectivity of α1G T-type calcium channels. Biophys. J. 79:3052–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera, K., Staes, M., Janssens, A., Klugbauer, N., Droogmans, G., Hofmann, F., and Nilius, B. 2001. Aspartate residues of the EEDD pore locus control selectivity and permeation of the T-type Ca2+ channel α1G. J. Biol. Chem. 276:45628–45635. [DOI] [PubMed] [Google Scholar]
- Talavera, K., A. Janssens, N. Klugbauer, G. Droogmans, and B. Nilius. 2003. Pore structure influences gating properties of the T-type Ca2+ channel α1G. J. Gen. Physiol. 121:529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, S., G. Mikala, A. Bahinski, A. Yatani, G. Varadi, and A. Schwartz. 1993. Molecular localization of ion selectivity sites within the pore of a human L-type cardiac calcium channel. J. Biol. Chem. 268:13026–13029. [PubMed] [Google Scholar]
- Varadi, G., M. Strobeck, S. Koch, L. Caglioti, C. Zucchi, and G. Palyi. 1999. Molecular elements of ion permeation and selectivity within calcium channels. Crit. Rev. Biochem. Mol. Biol. 34:181–214. [DOI] [PubMed] [Google Scholar]
- Williamson, A.V., and W.A. Sather. 1999. Nonglutamate pore residues in ion selection and conduction in voltage-gated Ca2+ channels. Biophys. J. 77:2575–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X.S., H.D. Edwards, and W.A. Sather. 2000. Side chain orientation in the selectivity filter of a voltage-gated Ca2+ channel. J. Biol. Chem. 275:31778–31785. [DOI] [PubMed] [Google Scholar]
- Yang, J., P.T. Ellinor, W.A. Sather, J.F. Zhang, and R.W. Tsien. 1993. Molecular determinants of Ca2+ selectivity and ion permeation in L-type Ca2+ channels. Nature. 366:158–161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.