Abstract
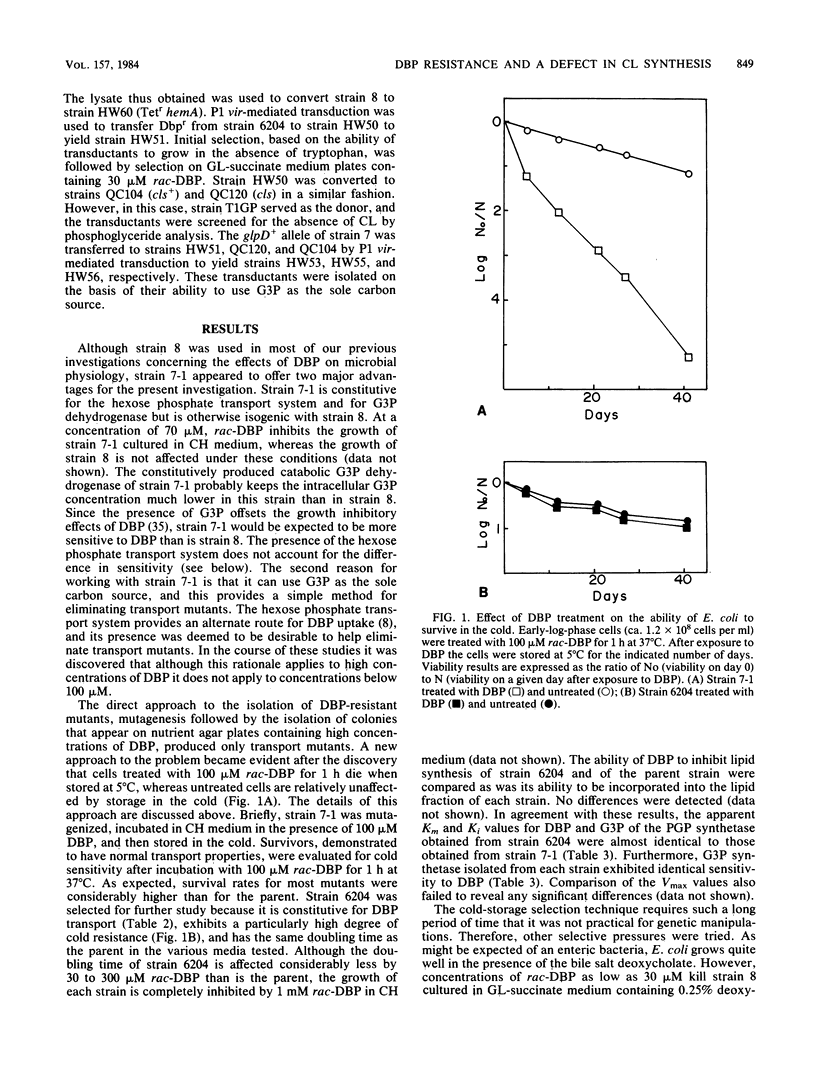
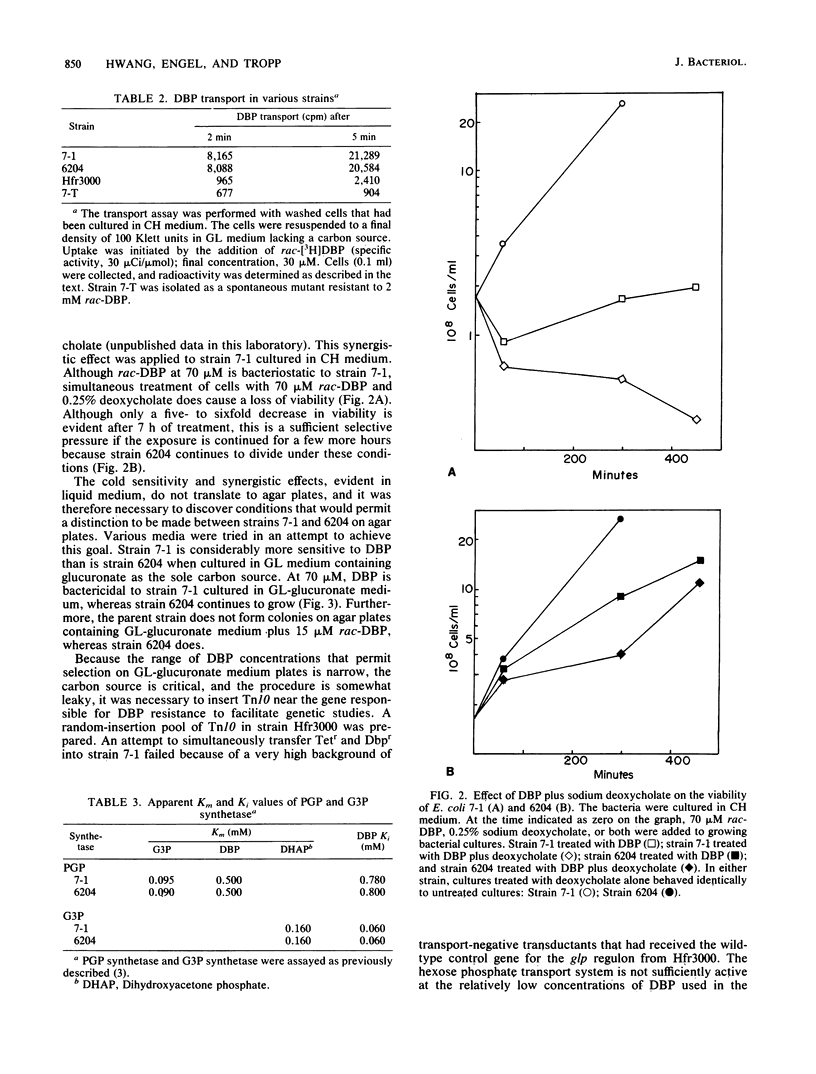
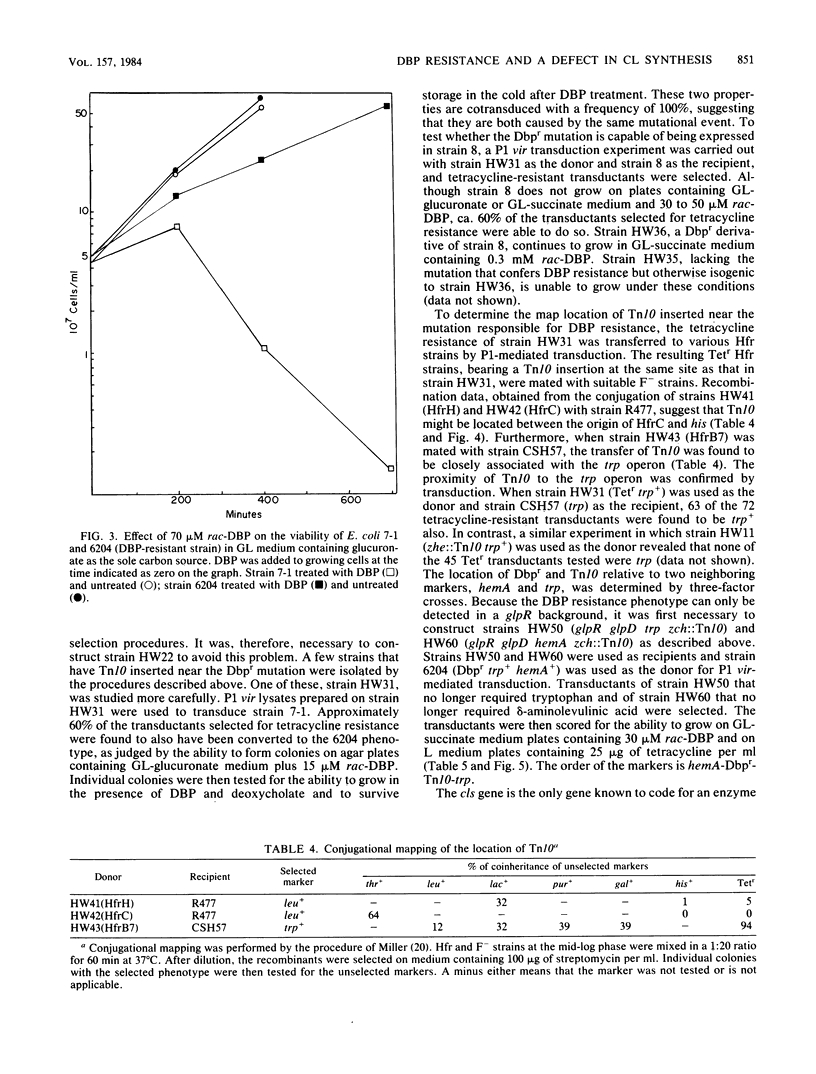
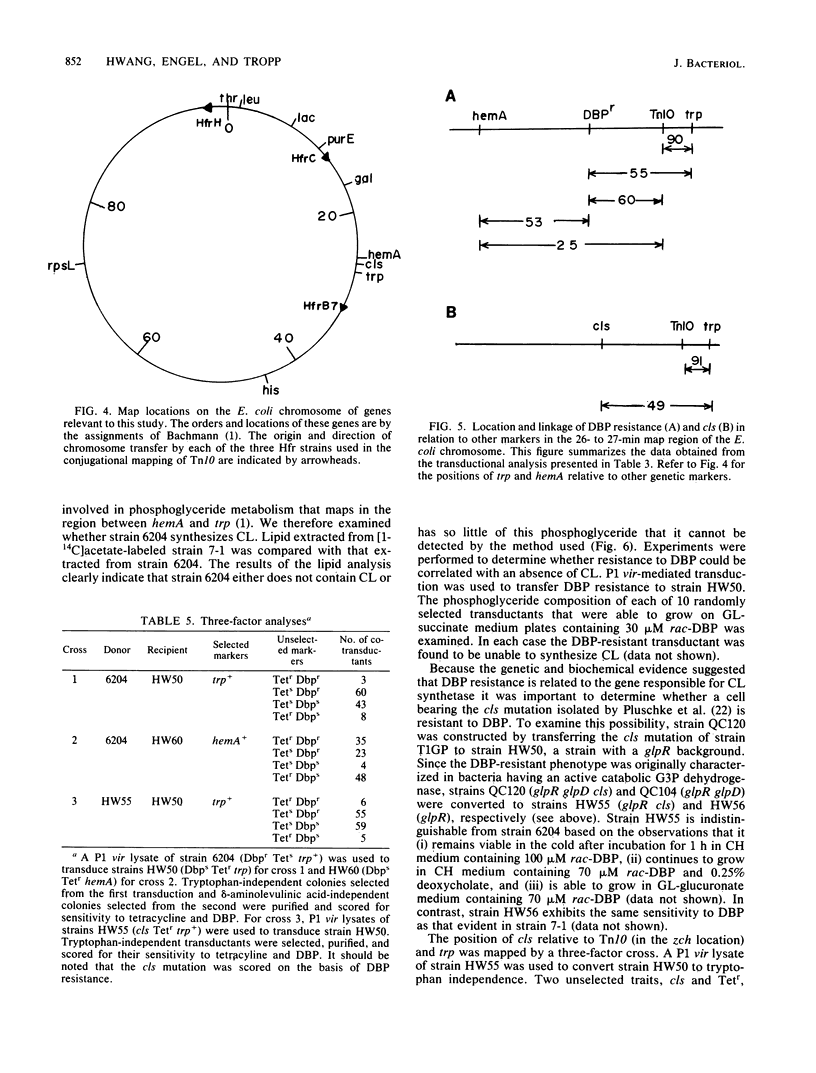
Escherichia coli treated for 1 h with 100 microM rac-3,4-dihydroxybutyl 1-phosphonate (DBP), a glycerol-3-phosphate analog, die when sorted at 5 degrees C, whereas the viability of untreated cells is relatively unaffected. This observation formed the basis of a selection procedure that was used to isolate mutants that are partially resistant to DBP. One such mutant, strain 6204, is constitutive for DBP transport, exhibits a particularly high degree of cold resistance, has the same doubling time as the parent, and is similar to the parent strain in terms of incorporation of DBP into the lipid fraction. Glycerol-3-phosphate and phosphatidylglycerol phosphate synthetases obtained from strain 6204 and its parent were identical in terms of DBP recognition. The parent strain is killed when incubated in the presence of a combination of 70 microM rac-DBP and 0.25% deoxycholate, whereas strain 6204 continues to grow, albeit more slowly, in the presence of this combination. Strain 6204 can be distinguished from the parent strain on agar plates (low phosphate minimal medium with glucuronate as the sole carbon source) containing 15 microM rac-DBP. The insertion of Tn10 near the 6204 mutation has facilitated genetic manipulations. All phenotypic effects attributed to strain 6204 appear to be due to a single mutation. Genetic analysis indicates that Tn10, inserted near the gene responsible for DBP resistance, maps in the vicinity of 27 min. Three-factor crosses reveal a gene order of hemA-Dbpr-Tn10(zch)-trp. The only gene for phosphoglyceride metabolism known to map in this region is the gene associated with cardiolipin synthetase, cls. Genetic results suggest that the mutation responsible for DBP resistance maps in or very near cls. Analysis of the lipids isolated from untreated strain 6204 (and from each of the transductants prepared by P1 vir-mediated transfer of DBP resistance of wild-type strains) reveals that cardiolipin synthesis is defective. These results strongly suggest that the mutation responsible for DBP resistance has its primary effect on cardiolipin synthesis. To further test this hypothesis, strains with an authentic cls mutation were constructed and examined for resistance to DBP. These strains had growth properties that were identical with those of strain 6204. Wild-type strains and mutants defective in cardiolipin synthesis were treated with DBP and 20 mM magnesium or calcium chloride. Simultaneous treatment of either cell type with DBP and divalent cation not only failed to stimulate growth but, quite the contrary, had a marked synergistic growth inhibitory effect.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay P. K., Wu H. C. Biosynthesis of the covalently linked diglyceride in murein lipoprotein of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5318–5322. doi: 10.1073/pnas.74.12.5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P. J., Nunn W. D., Tyhach R. J., Goldstein S. L., Engel R., Tropp B. E. Investigations concerning the mode of action of 3,4-dihydroxybutyl-1-phosphonate on Escherichia coli. J Biol Chem. 1975 Mar 10;250(5):1633–1639. [PubMed] [Google Scholar]
- Cozzarelli N. R., Freedberg W. B., Lin E. C. Genetic control of L-alpha-glycerophosphate system in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):371–387. doi: 10.1016/0022-2836(68)90415-4. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr Molecular biology of bacterial membrane lipids. Annu Rev Biochem. 1978;47:163–189. doi: 10.1146/annurev.bi.47.070178.001115. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Goldstein S. L., Braksmayer D., Tropp B. E., Engel R. Isosteres of natural phosphates. 2. Synthesis of the monosodium salt of 4-hydroxy-3-oxobutyl-1-phosphonic acid, an isostere of dihydroxyacetone phosphate. J Med Chem. 1974 Mar;17(3):363–364. doi: 10.1021/jm00249a026. [DOI] [PubMed] [Google Scholar]
- Guth A., Engel R., Tropp B. E. Uptake of glycerol 3-phosphate and some of its analogs by the hexose phosphate transport system of Escherichia coli. J Bacteriol. 1980 Jul;143(1):538–539. doi: 10.1128/jb.143.1.538-539.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrot E., Kennedy E. P. Phospholipid composition and membrane function in phosphatidylserine decarboxylase mutants of Escherichia coli. J Biol Chem. 1978 Nov 25;253(22):8213–8220. [PubMed] [Google Scholar]
- Hirota Y., Suzuki H., Nishimura Y., Yasuda S. On the process of cellular division in Escherichia coli: a mutant of E. coli lacking a murein-lipoprotein. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1417–1420. doi: 10.1073/pnas.74.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg C. B., Kennedy E. P. Mechanism of the enzymatic synthesis of cardiolipin in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Mar;69(3):648–651. doi: 10.1073/pnas.69.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. P. Osmotic regulation and the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1092–1095. doi: 10.1073/pnas.79.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Barker D. F., Ross D. G., Botstein D. Properties of the translocatable tetracycline-resistance element Tn10 in Escherichia coli and bacteriophage lambda. Genetics. 1978 Nov;90(3):427–461. doi: 10.1093/genetics/90.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. II. Characterization of constitutive membrane-bound enzymes II of the Escherichia coli phosphotransferase system. J Biol Chem. 1971 Mar 10;246(5):1407–1418. [PubMed] [Google Scholar]
- Leifer Z., Engel R., Tropp B. E. Transport of 3,4-dihydroxybutyl-1-phosphonate, an analogue of sn-glycerol 3-phosphate. J Bacteriol. 1977 May;130(2):968–971. doi: 10.1128/jb.130.2.968-971.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Lin E. C. Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol. 1976;30:535–578. doi: 10.1146/annurev.mi.30.100176.002535. [DOI] [PubMed] [Google Scholar]
- Ohta A., Shibuya I. Membrane phospholipid synthesis and phenotypic correlation of an Escherichia coli pss mutant. J Bacteriol. 1977 Nov;132(2):434–443. doi: 10.1128/jb.132.2.434-443.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Hirota Y., Overath P. Function of phospholipids in Escherichia coli. Characterization of a mutant deficient in cardiolipin synthesis. J Biol Chem. 1978 Jul 25;253(14):5048–5055. [PubMed] [Google Scholar]
- Pluschke G., Overath P. Function of phospholipids in Escherichia coli. Influence of changes in polar head group composition on the lipid phase transition and characterization of a mutant containing only saturated phospholipid acyl chains. J Biol Chem. 1981 Apr 10;256(7):3207–3212. [PubMed] [Google Scholar]
- Raetz C. R. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978 Sep;42(3):614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Kantor G. D., Nishijima M., Newman K. F. Cardiolipin accumulation in the inner and outer membranes of Escherichia coli mutants defective in phosphatidylserine synthetase. J Bacteriol. 1979 Aug;139(2):544–551. doi: 10.1128/jb.139.2.544-551.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Newman K. F. Neutral lipid accumulation in the membranes of Escherichia coli mutants lacking diglyceride kinase. J Biol Chem. 1978 Jun 10;253(11):3882–3887. [PubMed] [Google Scholar]
- Schmidt G., Schlecht S., Westphal O. Untersuchungen zur Typisierung von Salmonella-R-Formen. 3. Typisierung von S. minnesota-Mutanten mittels chemischer Agenzien. Zentralbl Bakteriol Orig. 1969 Dec;212(1):88–96. [PubMed] [Google Scholar]
- Schulman H., Kennedy E. P. Identification of UDP-glucose as an intermediate in the biosynthesis of the membrane-derived oligosaccharides of Escherichia coli. J Biol Chem. 1977 Sep 25;252(18):6299–6303. [PubMed] [Google Scholar]
- Schulman H., Kennedy E. P. Relation of turnover of membrane phospholipids to synthesis of membrane-derived oligosaccharides of Escherichia coli. J Biol Chem. 1977 Jun 25;252(12):4250–4255. [PubMed] [Google Scholar]
- Shopsis C. S., Engel R., Tropp B. E. Effects of phosphonic acid analogues of glycerol-3-phosphate on the growth of Escherichia coli. J Bacteriol. 1972 Oct;112(1):408–412. doi: 10.1128/jb.112.1.408-412.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shopsis C. S., Engel R., Tropp B. E. The inhibition of phosphatidylglycerol synthesis in Escherichia coli by 3,4-dihydroxybutyl-1-phosphonate. J Biol Chem. 1974 Apr 25;249(8):2473–2477. [PubMed] [Google Scholar]
- Shopsis C. S., Nunn W. D., Engel R., Tropp B. E. Effects of phosphonic acid analogues of glycerol-3-phosphate on the growth of Escherichia coli: phospholipid metabolism. Antimicrob Agents Chemother. 1973 Oct;4(4):467–473. doi: 10.1128/aac.4.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Tang C. T., Engel R., Tropp B. E. Glycerol 3-phosphate analogues as metabolic inhibitors in Escherichia coli, 3-hydroxy-4-oxobutyl-1-phosphonate, a drug that interferes with normal phosphoglyceride metabolism. Biochim Biophys Acta. 1979 Mar 29;572(3):472–482. doi: 10.1016/0005-2760(79)90154-1. [DOI] [PubMed] [Google Scholar]
- Van Dijck P. W., Ververgaert P. H., Verkleij A. J., Van Deenen L. L., De Gier J. Influence of Ca2+ and Mg2+ on the thermotropic behaviour and permeability properties of liposomes prepared from dimyristoyl phosphatidylglycerol and mixtures of dimyristoyl phosphatidylglycerol and dimyristoyl phosphatidylcholine. Biochim Biophys Acta. 1975 Nov 3;406(4):465–478. doi: 10.1016/0005-2736(75)90025-5. [DOI] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Hou C., Lin J. J., Yem D. W. Biochemical characterization of a mutant lipoprotein of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1388–1392. doi: 10.1073/pnas.74.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Golde L. M. Metabolism of membrane phospholipids and its relation to a novel class of oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1368–1372. doi: 10.1073/pnas.70.5.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]


