Summary
Arf GTPases regulate membrane trafficking and actin dynamics. Grp1, ARNO, and Cytohesin-1 comprise a family of phosphoinositide-dependent Arf GTPase exchange factors with a Sec7-pleckstrin homology (PH) domain tandem. Here, we report that the exchange activity of the Sec7 domain is potently autoinhibited by conserved elements proximal to the PH domain. The crystal structure of the Grp1 Sec7-PH tandem reveals a pseudo-substrate mechanism of autoinhibition in which the linker region between domains and a C-terminal amphipathic helix physically block the docking sites for the switch regions of Arf GTPases. Mutations within either element result in partial or complete activation. Critical determinants of autoinhibition also contribute to insulin stimulated plasma membrane recruitment. Autoinhibition can be largely reversed by binding of active Arf6 to Grp1 and by phosphorylation of tandem PKC sites in Cytohesin-1. These observations suggest that Grp1 family GEFs are autoregulated by mechanisms that depend on plasma membrane recruitment for activation.
Introduction
Arf GTPases function in vesicular trafficking and cytoskeletal dynamics by cycling between active (GTP-bound) and inactive (GDP-bound) conformations (Collins, 2003; Donaldson and Jackson, 2000; Nie et al., 2003). Activation is tightly regulated by guanine nucleotide exchange factors (GEFs). Arf GTPases are N-terminally myristoylated, a modification required for membrane localization (D’Souza-Schorey and Stahl, 1995; Haun et al., 1993; Sewell and Kahn, 1988; Taylor et al., 1992). Arf1 is recruited to the Golgi by a switching mechanism that exposes the myristoylated N-terminus (Goldberg, 1998; Randazzo et al., 1995). Subsequent interactions with COPI coat proteins or clathrin adaptors initiate vesicle budding (Bonifacino and Lippincott-Schwartz, 2003; Serafini et al., 1991). Arf6 localizes to the plasma membrane in both GDP- and GTP-bound conformations (Macia et al., 2004; Song et al., 1998). Arf1 and Arf6 activate phospholipase D (Brown et al., 1993; Singer et al., 1996) and phosphatidylinositol (4) phosphate 5-kinase (Honda et al., 1999). Arf6 also regulates actin remodeling (Radhakrishna and Donaldson, 1997; Radhakrishna et al., 1996), endocytosis (Altschuler et al., 1999) and cytokinesis (Fielding et al., 2005; Schweitzer and D’Souza-Schorey, 2005).
Arf GEFs possess a catalytic Sec7 domain homologous to the yeast Sec7 protein, which is essential for intra-Golgi transport (Achstetter et al., 1988). A subset of Sec7 domain GEFs are inhibited by Brefeldin A (BFA), which binds at the interface of the Sec7 domain complex with Arf1-GDP (Mansour et al., 1999; Peyroche et al., 1999). The BFA sensitive yeast GEFs Sec7p and Gea1p/Gea2p, and the mammalian homologous BIG1/BIG2 and GBF1, activate Arf1 at the Golgi and TGN, respectively (Shin and Nakayama, 2004; Zhao et al., 2002). The mammalian proteins Grp1, ARNO and Cytohesin-1 represent a subfamily of BFA insensitive GEFs consisting of heptad repeats, a Sec7 domain, a PH domain and a polybasic motif (Chardin et al., 1996; Langille et al., 1999; Meacci et al., 1997). All three proteins are recruited to the plasma membrane in response to receptor stimulation (Frank et al., 1998a; Langille et al., 1999; Nagel et al., 1998b; Venkateswarlu et al., 1998). Plasma membrane recruitment requires phosphoinositide 3-kinase (PI3K) activity and depends on the PH domain, which binds phosphatidyl inositol (PtdIns) 3,4,5-trisphosphate (Kavran et al., 1998; Klarlund et al., 1997; Venkateswarlu et al., 1998). Grp1 family GEFs have been implicated in actin dynamics (Clodi et al., 1998; Frank et al., 1998b), receptor endocytosis (Claing et al., 2001) and insulin signaling (Fuss et al., 2006; Hafner et al., 2006).
Within the Grp1 family, diglycine (2G) and triglycine (3G) splice variants, differing only in the number of glycine residues in the β1/β2 loop of the PH domain, strongly influence the affinity and specificity for phosphoinositides (Cronin et al., 2004; Klarlund et al., 2000). Whereas the 2G variants selectively bind PtdIns(3,4,5)P3 with high affinity, the 3G variants bind PtdIns(3,4,5)P3 with ~30 fold lower affinity and require the polybasic region for plasma membrane targeting (Macia et al., 2000; Nagel et al., 1998a; Santy et al., 1999).
Crystallographic studies of Sec7 and PH domains alone and in complex with Arf GTPases, Brefeldin A and inositol polyphosphates have provided detailed insights into the exchange reaction (Cherfils et al., 1998; Goldberg, 1998; Mossessova et al., 2003; Mossessova et al., 1998; Renault et al., 2003) and determinants of phosphoinositide recognition (Cronin et al., 2004; DiNitto et al., 2003; Ferguson et al., 2000; Lietzke et al., 2000). Although little is known regarding the structural organization or functional coordination of the modular domains in Arf GEFs, the exchange activity of full length Cytohesin-1 is weak compared to the isolated Sec7 domain (Pacheco-Rodriguez et al., 1998), suggesting that Arf GEFs may be autoregulated as observed for Ras and Rho family GEFs (Aghazadeh et al., 2000; Corbalan-Garcia et al., 1998; Hall et al., 2002; Margarit et al., 2003; Sondermann et al., 2004). Here, we show that Grp1 family GEFs are autoinhibited by a pseudosubstrate mechanism, which can be partially reversed by binding to the active form of Arf6 or by phosphorylation of protein kinase C (PKC) sites in Cytohesin-1.
Results
The polybasic motif suppresses exchange activity
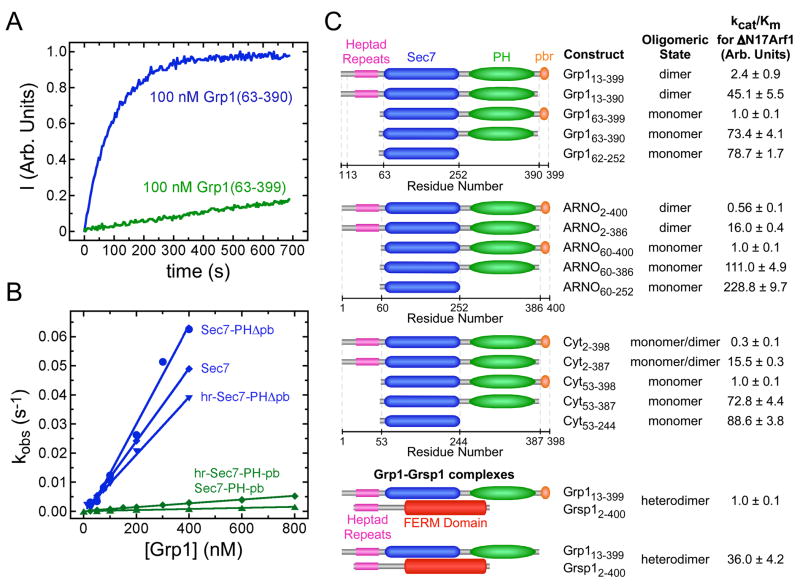
The catalytic efficiency of a Grp1 construct extending from the N-terminal heptad repeats through the C-terminus is ~30 fold lower than that of the isolated Sec7 domain (Figure 1 and Supplementary Table 1). Given that this construct purifies as a homodimer whereas the Sec7 domain is monomeric, it is possible that the weak exchange activity is a consequence of dimerization. To address this issue and identify potential autoinhibitory elements, catalytic efficiency and oligomeric state were determined for truncation constructs of Grp1 family GEFs. Although truncation of the heptad repeats eliminates dimerization, catalytic efficiency is not substantially effected. Conversely, truncation of the polybasic motif increases catalytic efficiency by 20–100 fold but has no effect on oligomeric state.
Figure 1. Grp1 family Arf GEFs are autoinhibited by the polybasic motif.
(A) Representative time courses for NΔ17Arf1 nucleotide exchange catalyzed by Grp1 constructs including (green) or lacking (blue) the polybasic motif.
(B) Concentration dependence of the observed rate constant for NΔ17Arf1 nucleotide exchange catalyzed by Grp1 constructs including (green) or lacking (blue) the polybasic motif.
(C) Summary of catalytic efficiencies (kcat/Km) and oligomeric state for Grp1, ARNO, and Cytohesin-1 constructs and Grp1-Grsp1 complexes. Oligomeric state was determined by sedimentation equilibrium at concentrations of 4.5–37 μM. Values are mean ± s.d. for n = 2.
Grp1 forms an endogenous complex with Grsp1, which contains a FERM domain, heptad repeats and a serine/threonine rich region (Klarlund et al., 2001). The Grsp1 FERM domain conserves acidic residues that, in some FERM domains, interact with polybasic motifs (Denker et al., 2000; Yonemura et al., 1998). We therefore examined the catalytic efficiency of Grp1 in complex with a Grsp1 construct that includes the FERM domain and heptad repeats required for binding to the heptad repeats in Grp1. This construct equilibrates with Grp1 homodimers to yield a uniform heterodimeric complex (manuscript in preparation) but has little effect on the catalytic efficiency (Figure 1 and Supplementary Table 1). Furthermore, the Grsp1 FERM domain does not appear to associate with a fluorescein-labeled peptide corresponding to the polybasic motif as detected by fluorescence anisotropy nor does it co-precipitate with a GST-fusion spanning the PH domain and polybasic motif (data not shown).
Given that plasma membrane recruitment depends on the interaction of the PH domain with PtdIns(3,4,5)P3, we also tested whether head group binding influences catalytic efficiency, possibly through a conformational change analogous to that of the Akt PH domain (Calleja et al., 2003; Milburn et al., 2003). Although the head group binds with high affinity (Supplementary Figure 1), it has no effect on exchange activity (Supplementary Table 1). Considered together, these observations indicate that Grp1, ARNO and Cytohesin-1 are strongly autoinhibited through an intramolecular mechanism that requires the polybasic motif.
Structure of the autoinhibited Sec7-PH-polybasic region of Grp1
To gain insight into the structural basis of autoinhibition, we conducted crystallization screens with full length and N-terminal truncation constructs of Grp1 family GEFs. ARNO and Cytohesin-1 constructs lacking the heptad repeats crystallized in complex with Ins(1,3,4,5)P4 but diffracted poorly. An analogous Grp1 construct in complex with Ins(1,3,4,5)P4 yielded crystals that diffracted anisotropically to ~3 Å. Although without effect on exchange activity, mutation of a disordered surface lysine to alanine (K68A) resulted in crystals that diffracted uniformly with two molecules in the asymmetric unit. The structure was solved by multiwavelength anomalous diffraction (MAD) and refined to 2.0 Å (Figure 2; Table 1; Supplementary Figures 2 and 3).
Figure 2. Structural organization of an autoinhibited Grp1 construct.
(A) Ribbon rendering showing the Sec7 domain (blue), linker (red), PH domain (green), and C-terminal helix (orange). Ins(1,3,4,5)P4 is represented by red spheres (oxygen atoms) and yellow spheres (carbon and phosphate atoms).
(B) Annotated sequence alignment of Grp1 family paralogs/homologs.
Table 1.
Structure Determination and Refinement
| Data Collection | |||||
|---|---|---|---|---|---|
| Crystal | Se1 | Se1 | Se1 | Native | |
| Wavelength | λ1 (0.979104 Å) | λ2 (0.97872 Å) | λ3 (1.1627 Å) | 0.9791 Å | |
| Source | NSLS x25 | NSLS x25 | NSLS x25 | NSLS x29 | |
| Resolution | 20–1.95 | 20–1.95 | 20–1.95 | 20-2.04 | |
| Rsym (%) | 5.1 (37.2) | 5.0 (30.6) | 4.8 (41.1) | 5.7 (38.1) | |
| <I/σ> | 29.9 (3.3) | 31.1 (4.1) | 33.0 (2.9) | 35.8 (3.6) | |
| Completeness(%) | 99.7 (100.0) | 99.0 (100.0) | 99.5 (100.0) | 99.8 (99.7) | |
| Redundancy | 6 | 6 | 6 | 7 | |
|
| |||||
| Phasing Power (Bijvoet Differences)a | |||||
|
| |||||
| Wavelength | Se 1 λ1 | Se 1 λ2 | Se 1 λ3 | ||
| Acentric PP | 1.29 (0.31) | 2.44 (0.67) | 0.302 (0.74) | ||
|
| |||||
| Phasing Power and FOM (Dispersive Differences)a | |||||
|
| |||||
| Wavelength | λ1 vs. λ3 | λ2 vs. λ3 | |||
| Acentric PP | 1.25 (0.547) | 1.22 (0.730) | |||
| Centric PP | 0.919 (0.346) | 0.886 (0.458) | |||
|
| |||||
| Figure of Merit (FOM) | |||||
|
| |||||
| Acentric FOM | 0.585 (0.229) | ||||
| Centric FOM | 0.457 (0.143) | ||||
|
| |||||
| Refinement | |||||
|
| |||||
| RMS deviations | |||||
| Crystal | Resolution (Å) | R Factor (%) | R free (%) | Bond Length (Å) | Bond Angle (o) |
| Se1a | 20.0-1.9 | 20.4 | 24.1 | 0.008 | 1.1 |
| Nativeb | 20.0-2.04 | 20.8 | 24.2 | 0.008 | 1.1 |
|
| |||||
| B-factors | |||||
|
| |||||
| Crystal | Chain A | Chain B | |||
| main chain | overall | main chain | overall | ||
|
|
|||||
| Se1a | 26.2 | 27.1 | 36.7 | 37.2 | |
| Nativeb | 33.1 | 33.8 | 47.5 | 48.1 | |
Grp163-399 K68A/H260Y
Grp163-399 K68A
Values in parentheses represent the highest resolution shell.
Rsym = ΣhΣj|Ij(h) − <I(h)>/ΣhΣjIj(h).
R value for a 5% subset of reflections selected at random and omitted from refinement.
The individual domains in the Grp1 Sec7-PH tandem are structurally similar to the isolated ARNO Sec7 domain (Cherfils et al., 1998; Mossessova et al., 1998) and Grp1 PH domain (Cronin et al., 2004; Ferguson et al., 2000; Lietzke et al., 2000). The Sec7 domain consists of an N-terminal subdomain with 7 helices (αA-αG) and a C-terminal three helix bundle (αH-αJ). The PH domain consists of a 9 stranded partly open β-barrel (β1-β7 and βi1-βi2) capped by a C-terminal helix (α1). Ins(1,3,4,5)P4 is bound at the open end of the β-barrel as described (Ferguson et al., 2000; Lietzke et al., 2000). Residues 251-265 in the Sec7-PH domain linker adopt an ordered though non-uniform secondary structure. After the PH domain, residues 383-395 form an amphipathic helix, hereafter referred to as the C-terminal helix. The C-terminus of this helix coincides with the first 5 residues of the polybasic motif (391RKRRI395).
Both molecules in the asymmetric unit exhibit a similar organization in which the Sec7 domain interacts extensively with the linker and C-terminal helix but does not directly contact the PH domain (Figure 2). Despite differences in the orientation of the Sec7 and PH domains, the linker and C-terminal helix adopt similar conformations and mediate equivalent interactions with the Sec7 domain (Supplementary Figure 4). The electron density is weaker for chain B, reflecting higher overall B-factors (Supplementary Figure 5 and Table 1). The B-factors are also higher for the linker and C-terminal helix in both molecules, consistent with the role of these regions as surface regulatory elements.
As shown in Figure 3, the non-polar linker residues Phe 262 and Leu 258 occupy a hydrophobic pocket bounded by Ile 165 in αG and Phe 195, Ile 198 and Met 199 in αH while a pair of acidic residues (Asp 254 and Asp 257) engage Arg 157 in the αF-αG loop. Likewise, Pro 383, Phe 384, Met 387, Leu 388 and Lys 392 from the non-polar face of the C-terminal helix pack with non-polar residues in αG (Ala 162 and Ile 165) and αH (Tyr 191, Val 192, and Phe 195). In addition, Arg 391 and Arg 394 from the polybasic motif contact acidic residues in αG (Glu 170) and αH (Asp 188).
Figure 3. Pseudosubstrate autoinhibition by the Sec7-PH linker and C-terminal helix.
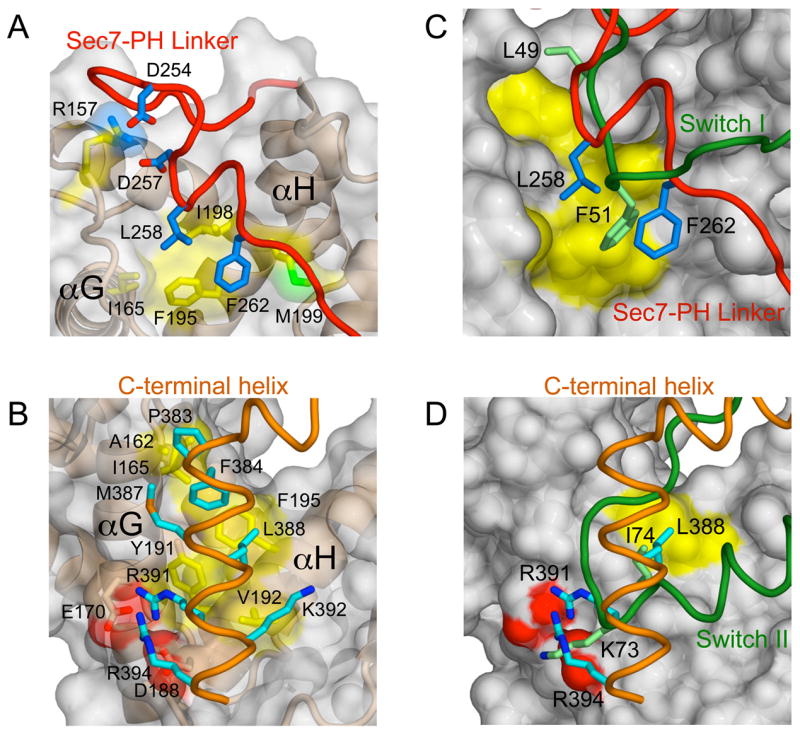
(A) Intramolecular interactions at the interface between the linker and Sec7 domain.
(B) Intramolecular interactions at the interface between the C-terminal helix and Sec7 domain. (C and D) Comparison of the linker and C-terminal helix of Grp1 with the switch I and II regions of Arf1-GDP from the complex with the E156K mutant of the ARNO Sec7 domain (PDB ID code 1R8S) after superposition of Cα atoms.
Pseudo-substrate mechanism of autoinhibition
In Sec7 domain complexes with Arf1, the switch I region docks in a hydrophobic groove formed by the termini of αF, αG and αH whereas the switch II region engages residues in αG and αH (Goldberg, 1998; Mossessova et al., 2003; Renault et al., 2003). A glutamate in the αF-αG loop, termed the glutamic acid finger, promotes GDP release through electrostatic repulsion with the β phosphate (Beraud-Dufour et al., 1998; Renault et al., 2003) and contacts the invariant P-loop lysine in the nucleotide free complex (Goldberg, 1998). As shown in Figure 3, the linker and C-terminal helix in the autoinhibited Grp1 structure occlude the docking sites for the switch I and II regions, respectively. Moreover, the mode of interaction strongly resembles that of the switch regions, despite differences in detail. Thus, Grp1 family GEFs are autoinhibited by a pseudo-substrate mechanism in which elements proximal to the PH domain occlude the Arf binding site but do not alter the disposition of the N- and C-terminal subdomains of the Sec7 domain.
Structure-based mutational analysis of the determinants of autoinhibition
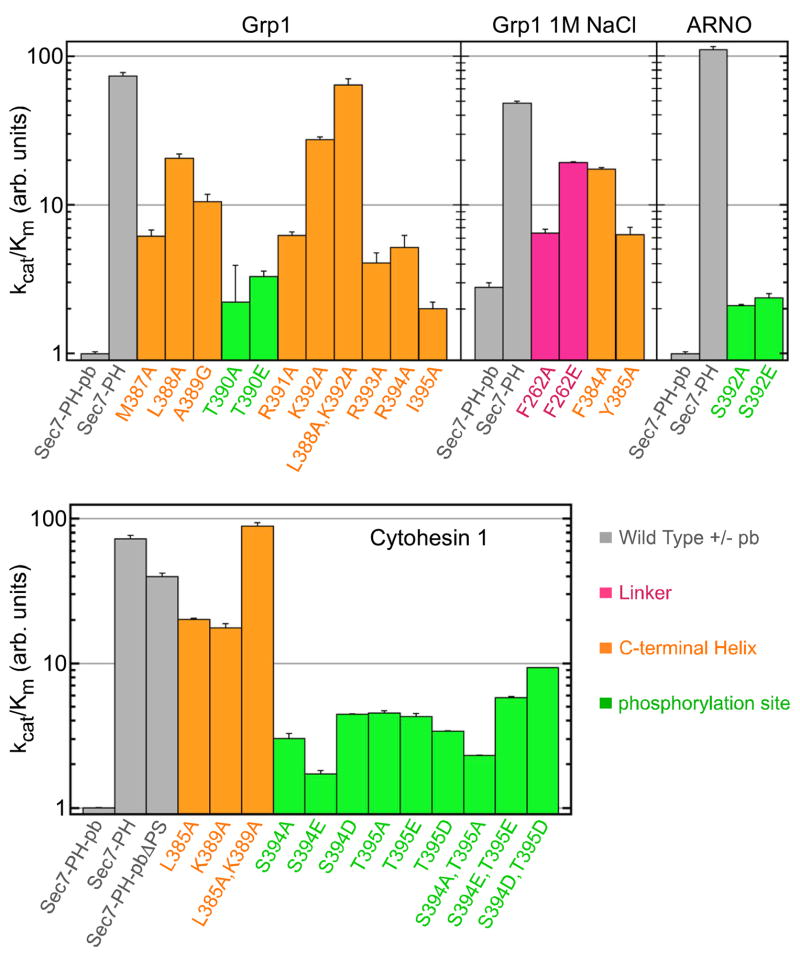
To identify determinants of autoinhibition, we analyzed the effects of amino acid substitutions on the catalytic efficiency of the Grp1(63-399) construct. All mutants expressed in a soluble form at wild type levels. As shown in Figure 4, substitutions in the C-terminal helix resulted in partial activation, with the largest increases in kcat/Km observed for L388A and K392A (20 and 27 fold, respectively). Both residues are located on the non-polar face of the C-terminal helix. Leu 388 is buried in the hydrophobic groove between αG and αH. Although the amino group of Lys 392 is exposed and does not participate in intramolecular interactions, the aliphatic portion of the side chain buries otherwise exposed hydrophobic residues on αH. The double substitution L388A/K392A is as strongly activating as deletion of the polybasic motif. A large (10 fold) effect was also observed for the helix destabilizing substitution A389G. Substitution of other interfacial residues resulted in weaker though still substantial (3–6 fold) effects. Although less extensively characterized, similar results were obtained for Cytohesin-1 and ARNO, consistent with a conserved autoinhibitory mechanism.
Figure 4. Structure-based mutational analysis of the determinants of autoinhibition.
Catalytic efficiencies are plotted on a log scale in units of the catalytic efficiency for the corresponding wild type Sec7-PH-polybasic construct. Mean values and standard deviations were calculated for two independent measurements.
Substitutions involving linker residues in the interface with the switch I docking site are prone to aggregation, preventing characterization of the L258A and L258E mutants. The F262A and F262E mutants, however, are soluble in 1M NaCl. Under these conditions, the catalytic efficiency of the construct lacking the polybasic motif is diminished by less than 40% while the catalytic activity of the corresponding construct with an intact polybasic motif increases 3 fold. The F262A and F262E substitutions further increase kcat/Km by 2 fold and 7 fold, respectively, suggesting that the linker contributes to the stability of the autoinhibited conformation, in addition to blocking the switch I docking site.
Determinants of autoinhibition are important for plasma membrane targeting
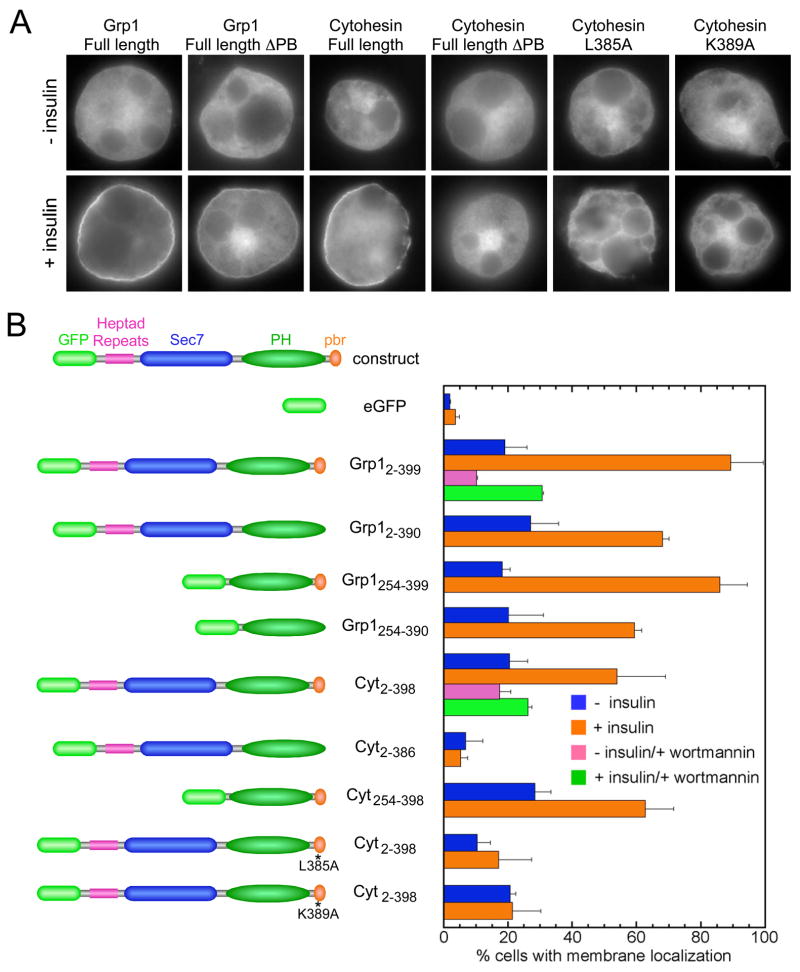
The polybasic motif enhances partitioning of ARNO and Cytohesin-1 with liposomes containing PtdIns(4,5)P2 and/or phosphatidyl serine (PtdSer) (Macia et al., 2000; Nagel et al., 1998a) and is required for plasma membrane targeting of Cytohesin-1 in Jurkat cells (Nagel et al., 1998a). To gain further insight into the relationship between autoregulation of exchange activity and plasma membrane targeting, we examined the effects of truncation and site specific mutations on insulin stimulated recruitment of 2G Grp1 and 3G Cytohesin-1 constructs in 3T3 L1 adipocytes. The rounded morphology of 3T3 L1 adipocytes and limited extent of membrane ruffling simplifies the analysis of plasma membrane targeting (Huang et al., 2004).
Full length 2G Grp1 exhibits robust insulin stimulated accumulation at the plasma membrane compared with the predominately cytoplasmic distribution in serum starved cells (Figure 5). A construct spanning the PH domain and polybasic motif also targets the plasma membrane in >80% of cells; however, the degree of accumulation appears to be reduced, suggesting that elements N-terminal to the PH domain contribute weakly to plasma membrane targeting, possibly through homo/hetero-dimerization mediated by the heptad repeats or through interaction of the Sec7 domain with Arf6. Deletion of the polybasic motif diminishes but does not eliminate plasma membrane targeting.
Figure 5. Determinants of plasma membrane targeting in 3T3 L1 adipocytes.
(A) Representative examples of the localization of GFP-fusion constructs in serum starved (−) and insulin stimulated (+) cells.
(B) Percentage of cells for which plasma membrane targeting was observed. Mean values and standard deviations are plotted for 2 independent experiments. Approximately 100 cells were analyzed for each experiment.
For 3G Cytohesin-1, plasma membrane accumulation is evident in 50–60% of cells expressing the full length protein or a construct spanning the PH domain and polybasic motif. Consistent with earlier studies in Jurkat cells (Nagel et al., 1998a), little insulin stimulated plasma membrane localization is observed for a construct lacking only the polybasic motif, even though PtdIns(3,4,5)P3 production is required as indicated by sensitivity to the PI 3-kinase inhibitor wortmannin. The requirement of the polybasic motif for 3G Cytohesin-1 but not 2G Grp1 is consistent with the ~30 fold lower affinity of the 3G variants for PtdIns(3,4,5)P3 (Cronin et al., 2004). Given that the plasma membrane localization of 2G Grp1 is less sensitive to deletion of the polybasic motif, the effects of individual amino acid substitutions were examined in the context of full length 3G Cytohesin-1. Remarkably, both the L385A and K389A mutants remain cytoplasmic upon insulin stimulation, indicating that critical determinants of autoinhibition also contribute to insulin stimulated plasma membrane recruitment. Finally, the basal localization of Grp1 and Cytohesin-1 constructs observed in 5–30% of serum starved cells probably reflects a combination of factors including binding to PtdIns(4,5)P2, non-specific interactions between the electropositive surface of the PH domain and the negatively charged inner leaflet of the plasma membrane, and residual levels of PtdIns(3,4,5)P3.
Mechanisms for reversal of autoinhibition
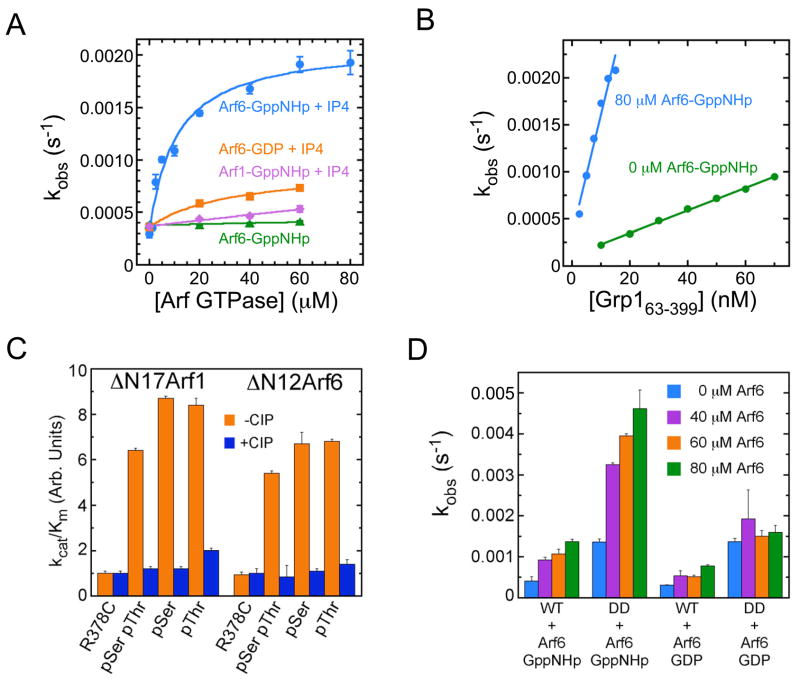
Active Arf6, but not Arf1, has recently been shown to co-precipitate Grp1 as well as ARNO and enhance PtdIns(3,4,5)P3-dependent plasma membrane recruitment of both proteins (Cohen et al., 2007). Active Arf6 binds directly to the PH domain-polybasic region of Grp1, but only in the presence of Ins(1,3,4,5)P4 (Cohen et al., 2007). To explore the hypothesis that autoinhibition can be reversed by Ins(1,3,4,5)P4-dependent Arf6 binding, the Arf1 exchange activity of Grp163-399 and Grp163-390 was characterized as a function of the concentration of GppNHp-loaded Arf6 in the presence and absence of Ins(1,3,4,5)P4. As shown in Figure 6A, Arf6-GppNHp strongly stimulates the exchange activity of the autoinhibited Grp163-399 construct. Stimulation requires Ins(1,3,4,5)P4 and exhibits a hyperbolic dependence on the concentration of Arf6-GppNHp. The observed rate constant is well described by a 1:1 binding isotherm, yielding a maximum activation of 8 fold at saturation and a half maximal activation constant of 14 μM. A more rigorous kinetic analysis at a saturating Arf6-GppNHp concentration of 80 μM indicates a 10 fold increase in kcat/Km (Figure 6B). Ins(1,3,4,5)P4 alone or in combination with GppNHp-loaded Arf1 has no effect (Figure 6A). Likewise, the combination of GDP-loaded Arf6 and Ins(1,3,4,5)P4 only weakly stimulates exchange activity. In contrast, Arf6-GppNHp in combination with Ins(1,3,4,5)P4 does not stimulate that exchange activity of the nearly fully active Grp163-390 construct lacking the polybasic motif.
Figure 6. Mechanisms for partial activation of Grp1 family GEFs.
(A) Dependence of the observed rate constant for NΔ17Arf1 exchange catalyzed by Grp163-399 on the concentration of GppNHp-loaded or GDP-loaded Arf GTPases. Solid lines represent fitted model functions for a 1:1 binding/activation isotherm.
(B) Dependence of the observed rate constant for NΔ17Arf1 exchange on the concentration of Grp163-399 in the presence and absence of 80 μM Arf6-GppNHp and 1 μM Ins(1,3,4,5)P4.
(C) Catalytic efficiencies for Cyothesin-1 phosphoproteins compared with the R378C reference protein.
(D) Dependence of the observed rate constant for NΔ17Arf1 exchange catalyzed by wild type (WT) Cytohesin-153-398 or the 394DD395 double mutant on the concentration Arf6 loaded with GppNHp or GDP in the presence of 10 μM Ins(1,3,4,5)P4. Values and error bars for panels A, C, and D are mean ± s.d. for n = 2.
Cytohesin-1 and ARNO are specifically phosphorylated by PKC at sites (Ser 392 in ARNO; Ser 394 and Thr 395 in Cytohesin-1) within the polybasic region in response to phorbol ester stimulation (Dierks et al., 2001; Santy et al., 1999). Pseudophosphorylation mutations (S392E in ARNO; S394D/E or T395D/E in Cytohesin-1; and T390E in Grp1) result in 2–9 fold increases in kcat/Km (Figure 4). The corresponding alanine substitutions also increase kcat/Km. To assess the effect of phosphorylation, intein mediated ligation was used to prepare Cytohesin-1 constructs that are fully and specifically phosphorylated on Ser 394 and/or Thr 395. As a consequence of the ligation reaction, the resulting phosphoproteins contain a substitution (R378C) in the loop connecting the PH domain to the C-terminal helix. The R378C substitution has a negligible (<2 fold) effect on the exchange activity and was used as a reference for the ligated phosphoproteins, which were separated from unligated reactants by purification over a phosphoprotein binding column followed by gel filtration on Superdex-75 (Supplementary Figure 6). As shown in Figure 6C, the catalytic efficiencies of the ligated phosphoproteins are 5–9 fold higher than that of the reference protein. Dephosphorylation with alkaline phosphatase eliminates the increased catalytic efficiency of the ligated phosphoproteins but has no effect on the reference protein. Thus, phosphorylation of either Ser 394 or Thr 395 in the polybasic region of Cytohesin-1 results in a large increase in exchange activity. Conversely, partitioning with liposomes containing 20% PtdSer and 3% PtdIns(3,4,5)P3 with or without 10% PtdIns(4,5)P2 has no detectable effect on the catalytic efficiency of either Grp1 or Cytohesin-1 (Supplementary Figures 7 and 8). Finally, we note that Arf6-GppNHp in combination with Ins(1,3,4,5)P4 stimulates the exchange activity of Cytohesin-153-398 as well as the double pseudophosphorylation mutant (Figure 6D). In this case, the half maximal activation constant appears to be greater than 100 μM (data not shown), possibly reflecting the lower affinity of the 3G splice variants for Ins(1,3,4,5)P4.
Discussion
Autoinhibition has been observed in GEFs for several GTPase families and may represent a widespread regulatory mechanism (Bos et al., 2007; Delprato and Lambright, 2007). The underlying structural bases have been characterized for Vav (Aghazadeh et al., 2000) and Sos (Margarit et al., 2003; Sondermann et al., 2004). Both GEFs contain a DH-PH tandem that activates Rho GTPases (Abe et al., 2000; Mosteller et al., 2000; Nimnual et al., 1998). Sos also contains a CDC25 domain that activates Ras (Chardin et al., 1993). The Vav DH domain is autoinhibited by an N-terminal extension, which blocks the exchange site through an interface centered on a tyrosine residue in the N-terminal extension (Aghazadeh et al., 2000). Tyrosine phosphorylation releases the N-terminal extension, exposing the catalytic site. The Ras exchange activity of Sos is regulated by GTP-bound Ras, which stabilizes a high activity conformation of Sos by binding to a distal allosteric site (Margarit et al., 2003). The Ras exchange activity is further autoinhibited by the DH-PH tandem, which blocks the allosteric site (Sondermann et al., 2004).
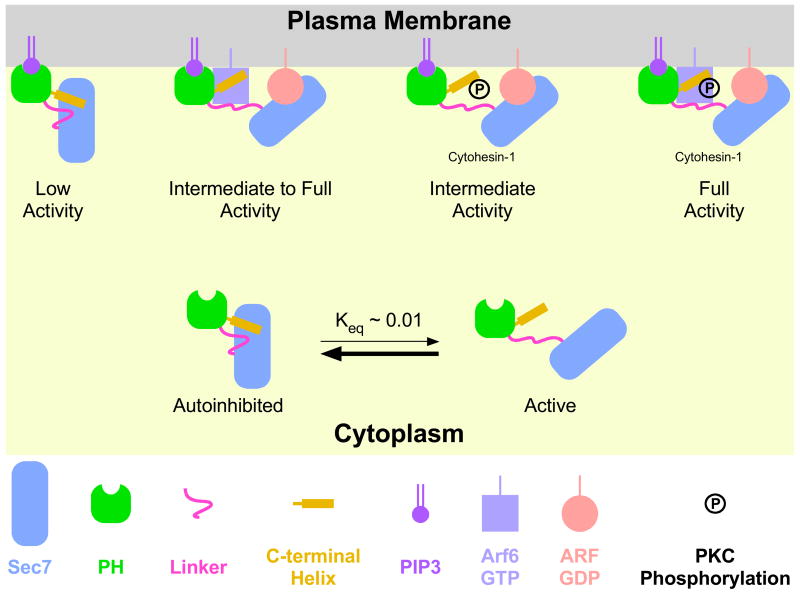
In Grp1 family GEFs, the linker and C-terminal helix block the docking sites for the switch regions of Arf GTPases. The simplest activation mechanism would require two states, a ‘closed’ state corresponding to the autoinhibited conformation and an ‘open’ state in which the Arf binding site is accessible. Like Vav, the autoinhibited state is catalytically incompetent. Consequently, the residual exchange activity can be interpreted as evidence that a small fraction of the total population exists in the open conformation at equilibrium. Assuming the open conformation has a catalytic activity equivalent to the isolated Sec7 domain, the fractional concentration can be estimated as 1–2%, implying an equilibrium constant of 1–2 × 10−2. Active Arf6 might in principle shift the equilibrium to the open conformation by binding simultaneously to the PH domain, C-terminal helix and/or linker region. Such a binding modality involving a PH domain and a C-terminal helix has recently been observed in the crystal structure of active Arf1 in complex with the Arf binding region of ARHGAP21 (Menetrey et al., 2007). Alternatively, the binding of active Arf6 to the PH domain may selectively destabilize the closed conformation through steric overlap with the Sec7 domain. The requirement for Ins(1,3,4,5)P4 may be due to a small conformational change in the PH domain and/or the reduction of positive electrostatic potential (Lietzke et al., 2000). Phosphorylation of PKC sites in the polybasic motif of Cytohesin-1 might shift the equilibrium towards the open conformation through an intramolecular rearrangement stabilized by attractive electrostatic interactions between the doubly negatively charged phosphate groups and positively charged residues in the polybasic motif or through destabilization of the C-terminal helix resulting from repulsive interactions between the phosphate groups and the negative end of the macroscopic helix dipole. A detailed understanding of the structural basis for activation awaits future studies.
Grp1 family GEFs exhibit a clear preference for Arf1 in vitro (Frank et al., 1998a; Macia et al., 2001), with a 5–20 fold higher kcat/Km compared with Arf6 (Supplementary Table 1). In transfected cells, Grp1 family GEFs activate Arf6 (Frank et al., 1998a; Langille et al., 1999) as well as Arf1 (Cohen et al., 2007). Although autoinhibition has little effect on substrate specificity in vitro, it may contribute indirectly to the specificity in vivo by reducing the basal level of Arf GTPase activation such that efficient exchange would require co-localization and/or relief of autoinhibition. Provided that the effect of autoinhibition is primarily on Km rather than kcat, as expected for a pseudosubstrate mechanism involving occlusion of the active site, the high effective concentrations achieved by PtdIns(3,4,5)P3-dependent plasma membrane co-localization of Arf6 with Grp1 family GEFs would be expected to selectively enhance activation of Arf6.
Polybasic regions have been implicated in the targeting and activation of signaling and trafficking complexes. In MARCKS, for example, a polybasic region consisting of alternating basic and hydrophobic residues exhibits multivalent binding to anionic membranes with a cooperative dependence on the mole fraction of PtdIns(4,5)P2 (Wang et al., 2001). Although natively unfolded, the MARCKS polybasic region interacts with membranes in an extended conformation, allowing the hydrophobic side chains to partition into the non-polar region of the bilayer while the basic side chains sequester multiple molecules of PtdIns(4,5)P2 through long range electrostatic interactions (Gambhir et al., 2004; Zhang et al., 2003). Polybasic motifs further contribute to the plasma membrane targeting of many small GTPases through a mechanism that requires PtdIns(3,4,5)P3 as well as PtdIns(4,5)P2 (Heo et al., 2006). In another well characterized example, the actin regulatory protein N-WASP is autoinhibited through intramolecular interactions involving a binding site for the Cdc42 GTPase (Kim et al., 2000) and an adjacent polybasic region (Prehoda et al., 2000). Autoinhibition can be relieved in a combinatorial manner by Cdc42 binding (Kim et al., 2000) and/or though cooperative, multivalent binding of the polybasic region to membranes containing PtdIns(4,5)P2 (Papayannopoulos et al., 2005). Given the distal location of the C-terminal helix relative to the head group binding site in the PH domain, simultaneous head group binding and non-specific membrane partitioning seems unlikely unless the C-terminal helix adopts an extended structure in the open conformation, in which case the terminal residues of the polybasic motif might be appropriately disposed for non-specific membrane interactions. Even in the absence of direct contact with membranes, the high net positive charge (+6 to +8) of the polybasic motif would be expected to enhance the positive electrostatic potential of the PH domain and thereby contribute indirectly to the non-specific component of membrane partitioning. Phosphorylation of PKC sites in Cytohesin-1 may reduce the non-specific contribution to membrane partitioning as observed for ARNO (Santy et al., 1999). However, the double pseudo-phosphorylation mutant evidently does not alter the PtdIns(3,4,5)P3-dependent localization of Cytohesin-1 (Dierks et al., 2001).
Under the conditions of our experiments, membrane partitioning does not appear to directly influence the stability of the autoinhibited conformation. It remains possible that a specific lipid component and/or membrane composition would be capable of shifting the equilibrium towards the open conformation. Our experiments also do not exclude the possibility of a more elaborate activation mechanism that would require the full length myristoylated form of Arf substrates. Nevertheless, the observation that insulin stimulated membrane targeting of Cytohesin-1 is abrogated by site specific substitutions in the C-terminal helix that also relieve autoinhibition suggests that activation is mechanistically coupled to membrane targeting. An intriguing possibility is that the binding site for active Arf6, which contributes to both plasma membrane targeting (Cohen et al., 2007) and relief of autoinhibition, would include the C-terminal helix.
Taking into account the observations discussed above, we propose the model shown in Figure 7, in which the initial plasma membrane recruitment of Grp1 family GEFs in response to PtdIns(3,4,5)P3 production is followed by lateral association with GTP-bound Arf6. In addition to enhancing PtdIns(3,4,5)P3-dependent membrane recruitment (Cohen et al., 2007), lateral association with GTP-bound Arf6 would simultaneously shift the equilibrium towards the active conformation. Other plasma membrane proximal events, including phosphorylation of PKC sites in the polybasic motif of Cyothesin-1, may further shift the equilibrium to achieve full activation or otherwise represent an independent pathway for activation. Finally, it is interesting to note that the constitutively active form of the Arf-like GTPase Arl4, which depends on a C-terminal polybasic motif for localization to the plasma membrane, has recently been shown to bind to the PH-polybasic region of ARNO and facilitate plasma membrane recruitment of ARNO as well as the other members of the Grp1 family (Hofmann et al., 2007). Additional experiments will be necessary to test the proposed model and investigate other potential mechanisms for activation.
Figure 7. Model for autoregulation of Grp1 family GEFs.
After PtdIns(3,4,5)P3-dependent plasma membrane recruitment of Grp1 family GEFs, lateral association with Arf6-GTP simultaneously enhances membrane partitioning and shifts the equilibrium towards the catalytically competent conformation. Other mechanisms, including phosphorylation of PKC sites in the polybasic motif of Cytohesin-1, may be required for full activation.
Experimental Procedures
Constructs, Expression and Purification
Constructs were amplified with Vent polymerase (NEB), digested with SalI and BamHI or BglII, and ligated into a modified pET15b vector incorporating an N-terminal 6xHis tag (MGHHHHHHGS). Mutants were generated with the Quick-Change II XL kit (Stratagene). All constructs were sequenced and expressed in BL21(DE3)RIL cells (Stratagene) cultured in 2X YT-amp (16 g tryptone, 10 g yeast extract, 5 g NaCl, 100 mg ampicillin per L). Cultures were grown at 20°C to an OD600 of 0.4 and induced with 0.05 mM IPTG for 16 hrs. Cells were disrupted by sonication in 50 mM Tris, pH 8.0, 0.1% 2-mercaptoethanol, 0.1 mM PMSF, 1 mg/ml lysozyme, 2 mM MgCl2, 0.01 mg/ml DNase I. Lysates were supplemented with 0.5% Triton X-100 and centrifuged at 35000 g for 45 min. Supernatants were loaded onto NiNTA-agarose columns (Qiagen) equilibrated with 50 mM Tris, pH 8.0, 0.1% 2-mercaptoethanol. The columns were washed with buffer containing 15 mM imidazole and eluted with a gradient of 10–250 mM imidazole. For crystallization, proteins were further purified by chromatography on Source Q and Superdex-200 (GE Health Care).
Nucleotide Loading
Arf GTPases were incubated with a 20 fold excess of nucleotide (GppNHp, GDP or mant-GDP) for 1–5 hrs at 20°C in 50 mM Tris, pH 8.0, 150 mM NaCl, 1 mM EDTA. MgCl2 was added to 10 mM and unbound nucleotide removed by gel filtration over a D-salt column (Pierce).
Exchange Assays
Exchange kinetics were monitored using the increase in intrinsic tryptophan emission intensity accompanying Arf GTPase activation or the decrease in emission intensity accompanying release of mant-GDP. Exchange reactions were initiated by mixing GDP-loaded or mant-GDP-loaded ΔN17Arf1 or ΔN12Arf6 at a final concentration of 1 μM with varying concentrations of the GEF in the presence of 200 or 250 μM GppNHp. Data were collected with a Saphire microplate spectrophotometer (Tecan) or a PC1 spectrofluorimeter (ISS) using excitation and emission wavelengths of 300 nm and 335 nm (tryptophan fluorescence) or 360 nm and 440 nm (mant fluorescence). Observed pseudo first order rate constants (kobs) were extracted from a non-linear least squares fit to
where I(t) is the emission intensity as a function of time and I0 and I∞ are the initial and final emission intensities. Catalytic efficiency (kcat/Km) was obtained from the slope of a linear least squares fit to
where kintr is the intrinsic rate constant for GDP or mant-GDP release.
Crystallization and Data Collection
Selenomethionine substituted Grp163-399 with two substitutions (K68A and H260Y) was mixed in a 1:1.2 molar ratio with Ins(1,3,4,5)P4 and crystallized at 4°C in hanging drops containing 10–15% PEG 6000, 50 mM Tris, pH 8.0, 0.2 M Li2SO4. H260Y is a spurious mutation that has no effect on the catalytic efficiency. Crystals were transferred to a cryoprotectant solution (15% PEG 6000, 25% PEG 400, 50 mM Tris, pH 8.0, 0.2 M Li2SO4) and frozen in liquid propane. The crystals are in the space group P212121 with a = 83.5 Å, b = 94.6 Å, c = 115.3 Å. There are 2 molecules in the asymmetric unit with a solvent content of 58%. Using the NSLS X25 beamline, complete data sets were collected on a single crystal at the f′ maximum and f″ minimum of the selenium edge and at a high energy remote wavelength. The crystal was maintained at 100°K in a nitrogen cryostream (Oxford cryosystems). Crystals of the single K68A mutant in complex with Ins(1,3,4,5)P4 were subsequently obtained at 4°C in hanging drops containing 10% PEG 4000, 50 mM Tris, pH 8.2, 50 mM Li2SO4. The crystals are in the space group P212121 with cell constants a = 83.0 Å, b = 94.7 Å, c = 115.3 Å. Crystals were soaked in a cryoprotectant solution (15% PEG 4000, 25% PEG 400, 50 mM Tris, pH 8.2, 0.05 M Li2SO4) and frozen in liquid nitrogen. A data set complete to 2.0 Å was collected at the NSLS X29 beamline. Data were processed with Denzo and scaled with Scalepack (Otwinowski and Minor, 1997).
Structure Determination and Refinement
The structure of K68A/H260Y Grp163-399 in complex with Ins(1,3,4,5)P4 was solved by MAD (Table 1 and Supplementary Figure 3). Selenium sites were identified from a 3.0 Å molecular replacement solution and refined with SHARP (La Fortelle and Bricogne, 1997). After solvent flipping, a σA-weighted Fourier summation yielded an interpretable map. An initial model constructed with ARP/wARP was completed by manual building with O (CCP4, 1994; Jones et al., 1991) and refined by simulated annealing with CNS (Brunger et al., 1998), atom updating with ARP/wARP (Perrakis et al., 2001), positional refinement with Refmac5 (CCP4, 1994), and manual rebuilding in O. The final model includes residues 63-396 (molecule A), residues 63-394 (molecule B), 809 water molecules, 7 sulfate ions and 2 molecules of PEG. The structure of K68A Grp163-399 was solved by molecular replacement with Phaser (McCoy et al., 2005) and refined as described for the double mutant (Table 1 and Supplementary Figure 4). The final refined model includes residues 63-396 (chain A), residues 63-394 (chain B), 535 water molecules, 4 sulfate ions and 3 molecules of PEG.
Adipocyte Localization Experiments
Constructs were cloned into the expression vector pEGFP-c1 (GE Health Care). 3T3 L1 fibroblasts were cultured in DMEM supplemented with 10% FBS, 50 units/ml penicillin, 50 μg/ml streptomycin and differentiated to adipocytes (Harrison et al., 1990). Day 6–8 3T3 L1 adipocytes were electroporated with 40 μg of plasmid DNA, seeded on sterile glass cover slips, cultured for 12 hrs, serum starved for 4 hrs, stimulated with 100 nM human insulin (Eli Lilly) or treated with an equivalent volume of serum free medium, and incubated for 30 min at 37°C. Where indicated, wortmannin was added to a final concentration of 100 nM followed by incubation at 37°C for 15 min prior to stimulation with insulin. Cover slips containing adherent cell cultures were washed 3 times with PBS, incubated at 25°C for 20 min in 4% paraformaldehyde/PBS. After fixation, cover slips were washed with PBS and mounted on glass slides with ProLong Gold antifade reagent (Invitrogen). Cells were visualized with a Zeiss Axiovert 200 microscope using 63x or 100x oil immersion objectives and filters for FITC. Digital images were acquired with a cooled CCD camera using the Axiovision software supplied by the manufacturer. A minimum of 2 images were acquired for each cell at different z-planes.
Formation of Arf6-GppNHp complexes with Grp1 family GEFs
Grp1, Cytohesin-1 and ARNO at 100 nM were incubated with 1–10 μM Ins(1,4,5)P3 or Ins(1,3,4,5)P4 for 30 min at 25°C in 50 mM Tris, pH 8.0, 150 mM NaCl, 2 mM MgCl2. Varying concentrations of Arf6-GppNHp were added and the incubation continued for 3 hrs.
Generation of phosphorylated Cytohesin-1
A Cytohesin-1 construct (residues 53-377) was sub-cloned into pTXB1 (NEB) upstream of the GyrA intein and chitin-binding domain (CBD). After expression in BL21(DE3)RILP cells at 20°C for 16 hrs, cells were lysed by sonication. The lysate was loaded onto a chitin column equilibrated with 20 mM Tris, pH 8.0, 150 mM NaCl. After extensive washing with 20 mM Tris, pH 8.0, 1.0 M NaCl, 1 mM EDTA, 0.1% Triton X-100, the column was incubated with 50 mM Tris, pH 8.8, 150 mM NaCl, 10% glycerol, 100 mM PMSF, 100 mM MESNA for 48 hrs at 25°C. The resulting Cyt53-377-MESNA derivative was concentrated and purified on Superdex-75 in buffer containing 50 mM MESNA. Native chemical ligation was performed by incubation of a 5–10 fold excess of the mono- or di-phosphorylated synthetic peptide CDPFYEMLAARKKKVS-(S/pS)-(T/pT)-KRH with 0.2 mM Cyt53-377-MESNA in a buffer containing 320 mM MESNA for 18 hrs at 25°C. The ligated product was isolated over a phosphoprotein purification column (Qiagen) followed by gel filtration chromatography on Superdex-75 equilibrated with 10 mM Tris, pH 8.0, 150 mM NaCl, 50 mM MESNA.
Supplementary Material
Acknowledgments
We thank Drs. Michael Becker and Howard Robinson at NSLS beamlines X25 and X29 for assistance with data collection and Dr. Sean Munro (MRC Laboratory of Molecular Biology, Cambridge, UK) for comments on the manuscript. This work was supported by NIH grant DK60564.
Footnotes
Accession Numbers
Coordinates and structure factors have been deposited with the Protein Data Bank under the ID codes 2R09 (K68A/H260Y) and 2R0D (K68A).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe K, Rossman KL, Liu B, Ritola KD, Chiang D, Campbell SL, Burridge K, Der CJ. Vav2 is an activator of Cdc42, Rac1, and RhoA. J Biol Chem. 2000;275:10141–10149. doi: 10.1074/jbc.275.14.10141. [DOI] [PubMed] [Google Scholar]
- Achstetter T, Franzusoff A, Field C, Schekman R. SEC7 encodes an unusual, high molecular weight protein required for membrane traffic from the yeast Golgi apparatus. J Biol Chem. 1988;263:11711–11717. [PubMed] [Google Scholar]
- Aghazadeh B, Lowry WE, Huang XY, Rosen MK. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 2000;102:625–633. doi: 10.1016/s0092-8674(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Altschuler Y, Liu S, Katz L, Tang K, Hardy S, Brodsky F, Apodaca G, Mostov K. ADP-ribosylation factor 6 and endocytosis at the apical surface of Madin-Darby canine kidney cells. J Cell Biol. 1999;147:7–12. doi: 10.1083/jcb.147.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraud-Dufour S, Robineau S, Chardin P, Paris S, Chabre M, Cherfils J, Antonny B. A glutamic finger in the guanine nucleotide exchange factor ARNO displaces Mg2+ and the beta-phosphate to destabilize GDP on ARF1. EMBO J. 1998;17:3651–3659. doi: 10.1093/emboj/17.13.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Lippincott-Schwartz J. Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol. 2003;4:409–414. doi: 10.1038/nrm1099. [DOI] [PubMed] [Google Scholar]
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Brown HA, Gutowski S, Moomaw CR, Slaughter C, Sternweis PC. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993;75:1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr, D Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- Calleja V, Ameer-Beg SM, Vojnovic B, Woscholski R, Downward J, Larijani B. Monitoring conformational changes of proteins in cells by fluorescence lifetime imaging microscopy. Biochem J. 2003;372:33–40. doi: 10.1042/BJ20030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CCP4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- Chardin P, Camonis JH, Gale NW, van Aelst L, Schlessinger J, Wigler MH, Bar-Sagi D. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science. 1993;260:1338–1343. doi: 10.1126/science.8493579. [DOI] [PubMed] [Google Scholar]
- Chardin P, Paris S, Antonny B, Robineau S, Beraud-Dufour S, Jackson CL, Chabre M. A human exchange factor for ARF contains Sec7- and pleckstrin-homology domains. Nature. 1996;384:481–484. doi: 10.1038/384481a0. [DOI] [PubMed] [Google Scholar]
- Cherfils J, Menetrey J, Mathieu M, Le Bras G, Robineau S, Beraud-Dufour S, Antonny B, Chardin P. Structure of the Sec7 domain of the Arf exchange factor ARNO. Nature. 1998;392:101–105. doi: 10.1038/32210. [DOI] [PubMed] [Google Scholar]
- Claing A, Chen W, Miller WE, Vitale N, Moss J, Premont RT, Lefkowitz RJ. beta-Arrestin-mediated ADP-ribosylation factor 6 activation and beta 2-adrenergic receptor endocytosis. J Biol Chem. 2001;276:42509–42513. doi: 10.1074/jbc.M108399200. [DOI] [PubMed] [Google Scholar]
- Clodi M, Vollenweider P, Klarlund J, Nakashima N, Martin S, Czech MP, Olefsky JM. Effects of general receptor for phosphoinositides 1 on insulin and insulin-like growth factor I-induced cytoskeletal rearrangement, glucose transporter-4 translocation, and deoxyribonucleic acid synthesis. Endocrinology. 1998;139:4984–4990. doi: 10.1210/endo.139.12.6351. [DOI] [PubMed] [Google Scholar]
- Cohen LA, Honda A, Varnai P, Brown FD, Balla T, Donaldson JG. Active Arf6 recruits ARNO/cytohesin GEFs to the PM by binding their PH domains. Mol Biol Cell. 2007;18:2244–2253. doi: 10.1091/mbc.E06-11-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RN. Rab and ARF GTPase regulation of exocytosis. Mol Membr Biol. 2003;20:105–115. doi: 10.1080/0968768031000085892. [DOI] [PubMed] [Google Scholar]
- Corbalan-Garcia S, Margarit SM, Galron D, Yang SS, Bar-Sagi D. Regulation of Sos activity by intramolecular interactions. Mol Cell Biol. 1998;18:880–886. doi: 10.1128/mcb.18.2.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin TC, DiNitto JP, Czech MP, Lambright DG. Structural determinants of phosphoinositide selectivity in splice variants of Grp1 family PH domains. EMBO J. 2004;23:3711–3720. doi: 10.1038/sj.emboj.7600388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delprato A, Lambright DG. Structural basis for Rab GTPase activation by VPS9 domain exchange factors. Nat Struct Mol Biol. 2007;14:406–412. doi: 10.1038/nsmb1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza-Schorey C, Stahl PD. Myristoylation is required for the intracellular localization and endocytic function of ARF6. Exp Cell Res. 1995;221:153–159. doi: 10.1006/excr.1995.1362. [DOI] [PubMed] [Google Scholar]
- Denker SP, Huang DC, Orlowski J, Furthmayr H, Barber DL. Direct binding of the Na--H exchanger NHE1 to ERM proteins regulates the cortical cytoskeleton and cell shape independently of H(+) translocation. Mol Cell. 2000;6:1425–1436. doi: 10.1016/s1097-2765(00)00139-8. [DOI] [PubMed] [Google Scholar]
- Dierks H, Kolanus J, Kolanus W. Actin cytoskeletal association of cytohesin-1 is regulated by specific phosphorylation of its carboxyl-terminal polybasic domain. J Biol Chem. 2001;276:37472–37481. doi: 10.1074/jbc.M101502200. [DOI] [PubMed] [Google Scholar]
- DiNitto JP, Cronin TC, Lambright DG. Membrane recognition and targeting by lipid-binding domains. Sci STKE 2003. 2003:re16. doi: 10.1126/stke.2132003re16. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Jackson CL. Regulators and effectors of the ARF GTPases. Curr Opin Cell Biol. 2000;12:475–482. doi: 10.1016/s0955-0674(00)00119-8. [DOI] [PubMed] [Google Scholar]
- Ferguson KM, Kavran JM, Sankaran VG, Fournier E, Isakoff SJ, Skolnik EY, Lemmon MA. Structural basis for discrimination of 3-phosphoinositides by pleckstrin homology domains. Mol Cell. 2000;6:373–384. doi: 10.1016/s1097-2765(00)00037-x. [DOI] [PubMed] [Google Scholar]
- Fielding AB, Schonteich E, Matheson J, Wilson G, Yu X, Hickson GR, Srivastava S, Baldwin SA, Prekeris R, Gould GW. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 2005;24:3389–3399. doi: 10.1038/sj.emboj.7600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Upender S, Hansen SH, Casanova JE. ARNO is a guanine nucleotide exchange factor for ADP-ribosylation factor 6. J Biol Chem. 1998a;273:23–27. doi: 10.1074/jbc.273.1.23. [DOI] [PubMed] [Google Scholar]
- Frank SR, Hatfield JC, Casanova JE. Remodeling of the actin cytoskeleton is coordinately regulated by protein kinase C and the ADP-ribosylation factor nucleotide exchange factor ARNO. Mol Biol Cell. 1998b;9:3133–3146. doi: 10.1091/mbc.9.11.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss B, Becker T, Zinke I, Hoch M. The cytohesin Steppke is essential for insulin signalling in Drosophila. Nature. 2006;444:945–948. doi: 10.1038/nature05412. [DOI] [PubMed] [Google Scholar]
- Gambhir A, Hangyas-Mihalyne G, Zaitseva I, Cafiso DS, Wang J, Murray D, Pentyala SN, Smith SO, McLaughlin S. Electrostatic sequestration of PIP2 on phospholipid membranes by basic/aromatic regions of proteins. Biophys J. 2004;86:2188–2207. doi: 10.1016/S0006-3495(04)74278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. Structural basis for activation of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell. 1998;95:237–248. doi: 10.1016/s0092-8674(00)81754-7. [DOI] [PubMed] [Google Scholar]
- Hafner M, Schmitz A, Grune I, Srivatsan SG, Paul B, Kolanus W, Quast T, Kremmer E, Bauer I, Famulok M. Inhibition of cytohesins by SecinH3 leads to hepatic insulin resistance. Nature. 2006;444:941–944. doi: 10.1038/nature05415. [DOI] [PubMed] [Google Scholar]
- Hall BE, Yang SS, Bar-Sagi D. Autoinhibition of Sos by intramolecular interactions. Front Biosci. 2002;7:d288–294. doi: 10.2741/hall. [DOI] [PubMed] [Google Scholar]
- Harrison SA, Buxton JM, Clancy BM, Czech MP. Insulin regulation of hexose transport in mouse 3T3-L1 cells expressing the human HepG2 glucose transporter. J Biol Chem. 1990;265:20106–20116. [PubMed] [Google Scholar]
- Haun RS, Tsai SC, Adamik R, Moss J, Vaughan M. Effect of myristoylation on GTP-dependent binding of ADP-ribosylation factor to Golgi. J Biol Chem. 1993;268:7064–7068. [PubMed] [Google Scholar]
- Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann I, Thompson A, Sanderson CM, Munro S. The Arl4 family of small G proteins can recruit the cytohesin Arf6 exchange factors to the plasma membrane. Curr Biol. 2007;17:711–716. doi: 10.1016/j.cub.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, Watanabe H, Kawamoto K, Nakayama K, Morris AJ, Frohman MA, Kanaho Y. Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99:521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- Huang S, Lifshitz L, Patki-Kamath V, Tuft R, Fogarty K, Czech MP. Phosphatidylinositol-4,5-bisphosphate-rich plasma membrane patches organize active zones of endocytosis and ruffling in cultured adipocytes. Mol Cell Biol. 2004;24:9102–9123. doi: 10.1128/MCB.24.20.9102-9123.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Zou JY, Cowan SW, Kjeldgaard Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. 1991;A47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kavran JM, Klein DE, Lee A, Falasca M, Isakoff SJ, Skolnik EY, Lemmon MA. Specificity and promiscuity in phosphoinositide binding by pleckstrin homology domains. J Biol Chem. 1998;273:30497–30508. doi: 10.1074/jbc.273.46.30497. [DOI] [PubMed] [Google Scholar]
- Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature. 2000;404:151–158. doi: 10.1038/35004513. [DOI] [PubMed] [Google Scholar]
- Klarlund JK, Guilherme A, Holik JJ, Virbasius JV, Chawla A, Czech MP. Signaling by phosphoinositide-3,4,5-trisphosphate through proteins containing pleckstrin and Sec7 homology domains. Science. 1997;275:1927–1930. doi: 10.1126/science.275.5308.1927. [DOI] [PubMed] [Google Scholar]
- Klarlund JK, Holik J, Chawla A, Park JG, Buxton J, Czech MP. Signaling complexes of the FERM domain-containing protein GRSP1 bound to ARF exchange factor GRP1. J Biol Chem. 2001;276:40065–40070. doi: 10.1074/jbc.M105260200. [DOI] [PubMed] [Google Scholar]
- Klarlund JK, Tsiaras W, Holik JJ, Chawla A, Czech MP. Distinct polyphosphoinositide binding selectivities for pleckstrin homology domains of GRP1-like proteins based on diglycine versus triglycine motifs. J Biol Chem. 2000;275:32816–32821. doi: 10.1074/jbc.M002435200. [DOI] [PubMed] [Google Scholar]
- La Fortelle E, Bricogne G. Maximum-likelihood heavy-atom parameter refinement for the multiple isomorphous replacement and multiwavelength anomalous diffraction methods. Methods Enzymol. 1997;276:472–494. doi: 10.1016/S0076-6879(97)76073-7. [DOI] [PubMed] [Google Scholar]
- Langille SE, Patki V, Klarlund JK, Buxton JM, Holik JJ, Chawla A, Corvera S, Czech MP. ADP-ribosylation factor 6 as a target of guanine nucleotide exchange factor GRP1. J Biol Chem. 1999;274:27099–27104. doi: 10.1074/jbc.274.38.27099. [DOI] [PubMed] [Google Scholar]
- Lietzke SE, Bose S, Cronin T, Klarlund J, Chawla A, Czech MP, Lambright DG. Structural basis of 3-phosphoinositide recognition by pleckstrin homology domains. Mol Cell. 2000;6:385–394. doi: 10.1016/s1097-2765(00)00038-1. [DOI] [PubMed] [Google Scholar]
- Macia E, Chabre M, Franco M. Specificities for the small G proteins ARF1 and ARF6 of the guanine nucleotide exchange factors ARNO and EFA6. J Biol Chem. 2001;276:24925–24930. doi: 10.1074/jbc.M103284200. [DOI] [PubMed] [Google Scholar]
- Macia E, Luton F, Partisani M, Cherfils J, Chardin P, Franco M. The GDP-bound form of Arf6 is located at the plasma membrane. J Cell Sci. 2004;117:2389–2398. doi: 10.1242/jcs.01090. [DOI] [PubMed] [Google Scholar]
- Macia E, Paris S, Chabre M. Binding of the PH and polybasic C-terminal domains of ARNO to phosphoinositides and to acidic lipids. Biochemistry. 2000;39:5893–5901. doi: 10.1021/bi992795w. [DOI] [PubMed] [Google Scholar]
- Mansour SJ, Skaug J, Zhao XH, Giordano J, Scherer SW, Melancon P. p200 ARF-GEP1: a Golgi-localized guanine nucleotide exchange protein whose Sec7 domain is targeted by the drug brefeldin A. Proc Natl Acad Sci USA. 1999;96:7968–7973. doi: 10.1073/pnas.96.14.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margarit SM, Sondermann H, Hall BE, Nagar B, Hoelz A, Pirruccello M, Bar-Sagi D, Kuriyan J. Structural evidence for feedback activation by Ras. GTP of the Ras-specific nucleotide exchange factor SOS. Cell. 2003;112:685–695. doi: 10.1016/s0092-8674(03)00149-1. [DOI] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Storoni LC, Read RJ. Likelihood-enhanced fast translation functions. Acta Crystallogr D Biol Crystallogr. 2005;61:458–464. doi: 10.1107/S0907444905001617. [DOI] [PubMed] [Google Scholar]
- Meacci E, Tsai SC, Adamik R, Moss J, Vaughan M. Cytohesin-1, a cytosolic guanine nucleotide-exchange protein for ADP-ribosylation factor. Proc Natl Acad Sci USA. 1997;94:1745–1748. doi: 10.1073/pnas.94.5.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menetrey J, Perderiset M, Cicolari J, Dubois T, Elkhatib N, El Khadali F, Franco M, Chavrier P, Houdusse A. Structural basis for ARF1-mediated recruitment of ARHGAP21 to Golgi membranes. EMBO J. 2007;26:1953–1962. doi: 10.1038/sj.emboj.7601634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn CC, Deak M, Kelly SM, Price NC, Alessi DR, Van Aalten DM. Binding of phosphatidylinositol 3,4,5-trisphosphate to the pleckstrin homology domain of protein kinase B induces a conformational change. Biochem J. 2003;375:531–538. doi: 10.1042/BJ20031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossessova E, Corpina RA, Goldberg J. Crystal structure of ARF1*Sec7 complexed with Brefeldin A and its implications for the guanine nucleotide exchange mechanism. Mol Cell. 2003;12:1403–1411. doi: 10.1016/s1097-2765(03)00475-1. [DOI] [PubMed] [Google Scholar]
- Mossessova E, Gulbis JM, Goldberg J. Structure of the guanine nucleotide exchange factor Sec7 domain of human arno and analysis of the interaction with ARF GTPase. Cell. 1998;92:415–423. doi: 10.1016/s0092-8674(00)80933-2. [DOI] [PubMed] [Google Scholar]
- Mosteller R, Han J, Das B, Broek D. Biochemical analysis of regulation of Vav, a guanine-nucleotide exchange factor for Rho family of GTPases. Methods Enzymol. 2000;325:38–51. doi: 10.1016/s0076-6879(00)25429-3. [DOI] [PubMed] [Google Scholar]
- Nagel W, Schilcher P, Zeitlmann L, Kolanus W. The PH domain and the polybasic c domain of cytohesin-1 cooperate specifically in plasma membrane association and cellular function. Mol Biol Cell. 1998a;9:1981–1994. doi: 10.1091/mbc.9.8.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel W, Zeitlmann L, Schilcher P, Geiger C, Kolanus J, Kolanus W. Phosphoinositide 3-OH kinase activates the beta2 integrin adhesion pathway and induces membrane recruitment of cytohesin-1. J Biol Chem. 1998b;273:14853–14861. doi: 10.1074/jbc.273.24.14853. [DOI] [PubMed] [Google Scholar]
- Nie Z, Hirsch DS, Randazzo PA. Arf and its many interactors. Curr Opin Cell Biol. 2003;15:396–404. doi: 10.1016/s0955-0674(03)00071-1. [DOI] [PubMed] [Google Scholar]
- Nimnual AS, Yatsula BA, Bar-Sagi D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science. 1998;279:560–563. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Pacheco-Rodriguez G, Meacci E, Vitale N, Moss J, Vaughan M. Guanine nucleotide exchange on ADP-ribosylation factors catalyzed by cytohesin-1 and its Sec7 domain. J Biol Chem. 1998;273:26543–26548. doi: 10.1074/jbc.273.41.26543. [DOI] [PubMed] [Google Scholar]
- Papayannopoulos V, Co C, Prehoda KE, Snapper S, Taunton J, Lim WA. A polybasic motif allows N-WASP to act as a sensor of PIP(2) density. Mol Cell. 2005;17:181–191. doi: 10.1016/j.molcel.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Perrakis A, Harkiolaki M, Wilson KS, Lamzin VS. ARP/wARP and molecular replacement. Acta Crystallogr D Biol Crystallogr. 2001;57:1445–1450. doi: 10.1107/s0907444901014007. [DOI] [PubMed] [Google Scholar]
- Peyroche A, Antonny B, Robineau S, Acker J, Cherfils J, Jackson CL. Brefeldin A acts to stabilize an abortive ARF-GDP-Sec7 domain protein complex: involvement of specific residues of the Sec7 domain. Mol Cell. 1999;3:275–285. doi: 10.1016/s1097-2765(00)80455-4. [DOI] [PubMed] [Google Scholar]
- Prehoda KE, Scott JA, Mullins RD, Lim WA. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science. 2000;290:801–806. doi: 10.1126/science.290.5492.801. [DOI] [PubMed] [Google Scholar]
- Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol. 1997;139:49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna H, Klausner RD, Donaldson JG. Aluminum fluoride stimulates surface protrusions in cells overexpressing the ARF6 GTPase. J Cell Biol. 1996;134:935–947. doi: 10.1083/jcb.134.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo PA, Terui T, Sturch S, Fales HM, Ferrige AG, Kahn RA. The myristoylated amino terminus of ADP-ribosylation factor 1 is a phospholipid- and GTP-sensitive switch. J Biol Chem. 1995;270:14809–14815. doi: 10.1074/jbc.270.24.14809. [DOI] [PubMed] [Google Scholar]
- Renault L, Guibert B, Cherfils J. Structural snapshots of the mechanism and inhibition of a guanine nucleotide exchange factor. Nature. 2003;426:525–530. doi: 10.1038/nature02197. [DOI] [PubMed] [Google Scholar]
- Santy LC, Frank SR, Hatfield JC, Casanova JE. Regulation of ARNO nucleotide exchange by a PH domain electrostatic switch. Curr Biol. 1999;9:1173–1176. doi: 10.1016/S0960-9822(00)80019-6. [DOI] [PubMed] [Google Scholar]
- Schweitzer JK, D’Souza-Schorey C. A requirement for ARF6 during the completion of cytokinesis. Exp Cell Res. 2005;311:74–83. doi: 10.1016/j.yexcr.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Serafini T, Orci L, Amherdt M, Brunner M, Kahn RA, Rothman JE. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell. 1991;67:239–253. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- Sewell JL, Kahn RA. Sequences of the bovine and yeast ADP-ribosylation factor and comparison to other GTP-binding proteins. Proc Natl Acad Sci USA. 1988;85:4620–4624. doi: 10.1073/pnas.85.13.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HW, Nakayama K. Guanine nucleotide-exchange factors for arf GTPases: their diverse functions in membrane traffic. J Biochem (Tokyo) 2004;136:761–767. doi: 10.1093/jb/mvh185. [DOI] [PubMed] [Google Scholar]
- Singer WD, Brown HA, Jiang X, Sternweis PC. Regulation of phospholipase D by protein kinase C is synergistic with ADP-ribosylation factor and independent of protein kinase activity. J Biol Chem. 1996;271:4504–4510. doi: 10.1074/jbc.271.8.4504. [DOI] [PubMed] [Google Scholar]
- Sondermann H, Soisson SM, Boykevisch S, Yang SS, Bar-Sagi D, Kuriyan J. Structural analysis of autoinhibition in the Ras activator Son of sevenless. Cell. 2004;119:393–405. doi: 10.1016/j.cell.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Song J, Khachikian Z, Radhakrishna H, Donaldson JG. Localization of endogenous ARF6 to sites of cortical actin rearrangement and involvement of ARF6 in cell spreading. J Cell Sci. 1998;111:2257–2267. doi: 10.1242/jcs.111.15.2257. [DOI] [PubMed] [Google Scholar]
- Taylor TC, Kahn RA, Melancon P. Two distinct members of the ADP-ribosylation factor family of GTP-binding proteins regulate cell-free intra-Golgi transport. Cell. 1992;70:69–79. doi: 10.1016/0092-8674(92)90534-j. [DOI] [PubMed] [Google Scholar]
- Venkateswarlu K, Oatey PB, Tavare JM, Cullen PJ. Insulin-dependent translocation of ARNO to the plasma membrane of adipocytes requires phosphatidylinositol 3-kinase. Curr Biol. 1998;8:463–466. doi: 10.1016/s0960-9822(98)70181-2. [DOI] [PubMed] [Google Scholar]
- Wang J, Arbuzova A, Hangyas-Mihalyne G, McLaughlin S. The effector domain of myristoylated alanine-rich C kinase substrate binds strongly to phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2001;276:5012–5019. doi: 10.1074/jbc.M008355200. [DOI] [PubMed] [Google Scholar]
- Yonemura S, Hirao M, Doi Y, Takahashi N, Kondo T, Tsukita S, Tsukita S. Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J Cell Biol. 1998;140:885–895. doi: 10.1083/jcb.140.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Crocker E, McLaughlin S, Smith SO. Binding of peptides with basic and aromatic residues to bilayer membranes: phenylalanine in the myristoylated alanine-rich C kinase substrate effector domain penetrates into the hydrophobic core of the bilayer. J Biol Chem. 2003;278:21459–21466. doi: 10.1074/jbc.M301652200. [DOI] [PubMed] [Google Scholar]
- Zhao X, Lasell TK, Melancon P. Localization of large ADP-ribosylation factor-guanine nucleotide exchange factors to different Golgi compartments: evidence for distinct functions in protein traffic. Mol Biol Cell. 2002;13:119–133. doi: 10.1091/mbc.01-08-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.