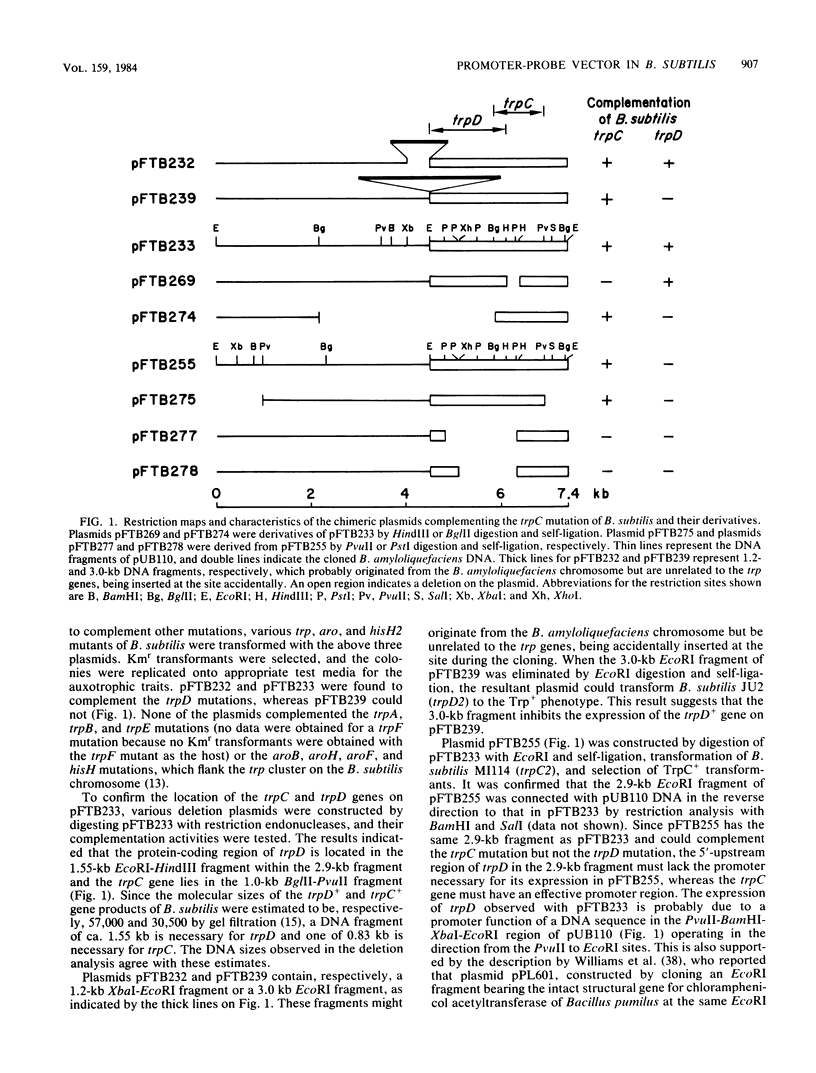
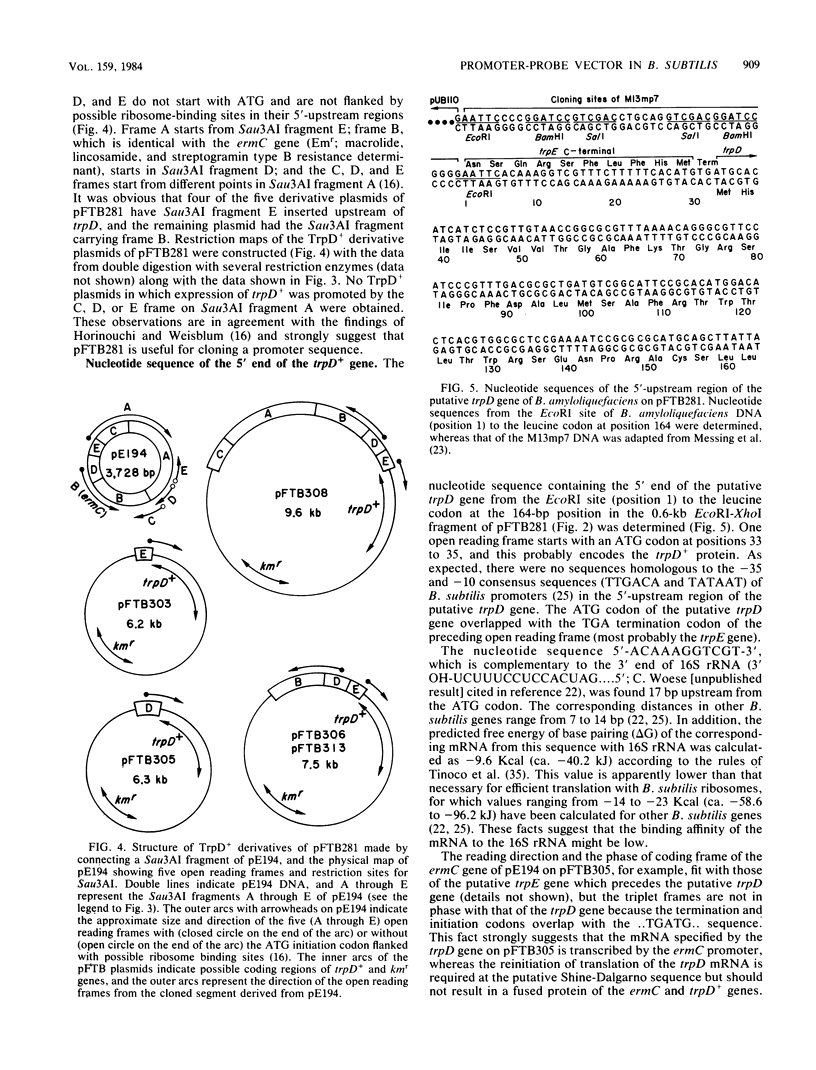
Abstract
The trp gene cluster of Bacillus amyloliquefaciens was found to be structurally similar to that of the Enterobacteriaceae. The translation termination codon of the putative trpE gene and the initiation codon for the putative trpD gene overlap at the trpE-trpD junction, and a promoter for the putative trpC gene is suggested to exist. A promoter-probe vector of Bacillus subtilis, pFTB281, was constructed with a DNA fragment of B. amyloliquefaciens, complementing the trpC and trpD mutations of B. subtilis, a 42-base-pair DNA fragment of M13mp7, and the larger EcoRI-PvuII fragment of pUB110, which confers an autonomous replication function and the kanamycin-resistance phenotype to the chimeric plasmid. pFTB281 has BamHI, EcoRI, and SalI cloning sites in the 5'-upstream portion of the protein-coding region of the putative trpD gene, and the insertion of a certain DNA fragment at any of these sites allowed the plasmid to transform a trpD mutant of B. subtilis to the TrpD+ phenotype. DNA fragments showing the promoter function for the trpD gene were obtained from B. amyloliquefaciens and Saccharomyces cerevisiae chromosomes and rho 11 and lambda phage DNAs, but rarely from the DNAs of Escherichia coli and pBR322.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band L., Shimotsu H., Henner D. J. Nucleotide sequence of the Bacillus subtilis trpE and trpD genes. Gene. 1984 Jan;27(1):55–65. doi: 10.1016/0378-1119(84)90238-5. [DOI] [PubMed] [Google Scholar]
- Band L., Yansura D. G., Henner D. J. Construction of a vector for cloning promoters in Bacillus subtilis. Gene. 1983 Dec;26(2-3):313–315. doi: 10.1016/0378-1119(83)90204-4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton B. C., Whitt D. D. The isolation and genetic characterization of mutants of the tryptophan system of Bacillus subtilis. Genetics. 1969 Jul;62(3):445–460. doi: 10.1093/genetics/62.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli: specific complementation of argininosuccinate lyase (argH) mutations. J Mol Biol. 1978 Apr 25;120(4):517–532. doi: 10.1016/0022-2836(78)90351-0. [DOI] [PubMed] [Google Scholar]
- Donnelly C. E., Sonenshein A. L. Promoter-probe plasmid for Bacillus subtilis. J Bacteriol. 1984 Mar;157(3):965–967. doi: 10.1128/jb.157.3.965-967.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARMER J. L., ROTHMAN F. TRANSFORMABLE THYMINE-REQUIRING MUTANT OF BACILLUS SUBTILS. J Bacteriol. 1965 Jan;89:262–263. doi: 10.1128/jb.89.1.262-263.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb D. S., Doi R. H., Rodriguez R. L. Expression of Tn9-derived chloramphenicol resistance in Bacillus subtilis. Nature. 1981 Sep 24;293(5830):309–311. doi: 10.1038/293309a0. [DOI] [PubMed] [Google Scholar]
- Gryczan T., Contente S., Dubnau D. Molecular cloning of heterologous chromosomal DNA by recombination between a plasmid vector and a homologous resident plasmid in Bacillus subtilis. Mol Gen Genet. 1980 Feb;177(3):459–467. doi: 10.1007/BF00271485. [DOI] [PubMed] [Google Scholar]
- Henner D. J., Hoch J. A. The Bacillus subtilis chromosome. Microbiol Rev. 1980 Mar;44(1):57–82. doi: 10.1128/mr.44.1.57-82.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereford L., Fahrner K., Woolford J., Jr, Rosbash M., Kaback D. B. Isolation of yeast histone genes H2A and H2B. Cell. 1979 Dec;18(4):1261–1271. doi: 10.1016/0092-8674(79)90237-x. [DOI] [PubMed] [Google Scholar]
- Hoch S. O., Anagnostopoulos C., Crawford I. P. Enzymes of the tryptophan operon of Bacillus subtilis. Biochem Biophys Res Commun. 1969 Jun 27;35(6):838–844. doi: 10.1016/0006-291x(69)90700-1. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz H., Platt T. Identification of trp-p2, an internal promoter in the tryptophan operon of Escherichia coli. J Mol Biol. 1982 Apr 5;156(2):257–267. doi: 10.1016/0022-2836(82)90327-8. [DOI] [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Internal promoter of the tryptophan operon of Escherichia coli is located in a structural gene. J Mol Biol. 1972 Aug 21;69(2):307–313. doi: 10.1016/0022-2836(72)90232-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. R., Murray C. L., Rabinowitz J. C. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981 Nov 10;256(21):11283–11291. [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. The internal low-efficiency promoter of the tryptophan operon of Escherichia coli. J Mol Biol. 1968 Dec;38(3):447–451. doi: 10.1016/0022-2836(68)90401-4. [DOI] [PubMed] [Google Scholar]
- Nichols B. P., Miozzari G. F., van Cleemput M., Bennett G. N., Yanofsky C. Nucleotide sequences of the trpG regions of Escherichia coli, Shigella dysenteriae, Salmonella typhimurium and Serratia marcescens. J Mol Biol. 1980 Oct 5;142(4):503–517. doi: 10.1016/0022-2836(80)90260-0. [DOI] [PubMed] [Google Scholar]
- Roth C. W., Nester E. W. Co-ordinate control of tryptophan, histidine and tyrosine enzyme synthesis in Bacillus subtilis. J Mol Biol. 1971 Dec 28;62(3):577–589. doi: 10.1016/0022-2836(71)90157-4. [DOI] [PubMed] [Google Scholar]
- Sanchez-Rivas C. Direct selection of complementing diploids from PEG-induced fusion of Bacillus subtilis protoplasts. Mol Gen Genet. 1982;185(2):329–333. doi: 10.1007/BF00330807. [DOI] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szybalski E. H., Szybalski W. A comprehensive molecular map of bacteriophage lambda. Gene. 1979 Nov;7(3-4):217–270. doi: 10.1016/0378-1119(79)90047-7. [DOI] [PubMed] [Google Scholar]
- Uozumi T., Hoshino T., Miwa K., Horinouchi S., Beppu T., Arima K. Restriction and modification in Bacillus species: genetic transformation of bacteria with DNA from different species, part I. Mol Gen Genet. 1977 Mar 28;152(1):65–69. doi: 10.1007/BF00264941. [DOI] [PubMed] [Google Scholar]
- Whitt D. D., Carlton B. C. Characterization of mutants with single and multiple defects in the tryptophan biosynthetic pathway in Bacillus subtilis. J Bacteriol. 1968 Oct;96(4):1273–1280. doi: 10.1128/jb.96.4.1273-1280.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Duvall E. J., Lovett P. S. Cloning restriction fragments that promote expression of a gene in Bacillus subtilis. J Bacteriol. 1981 Jun;146(3):1162–1165. doi: 10.1128/jb.146.3.1162-1165.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K., Ikenaka Y., Murai M., Tanabe M., Seki T., Oshima Y. Construction of a Bacillus subtilis cloning vehicle with heterologous DNA sequence. Gene. 1983 Oct;24(2-3):255–263. doi: 10.1016/0378-1119(83)90086-0. [DOI] [PubMed] [Google Scholar]
- Zukowski M. M., Gaffney D. F., Speck D., Kauffmann M., Findeli A., Wisecup A., Lecocq J. P. Chromogenic identification of genetic regulatory signals in Bacillus subtilis based on expression of a cloned Pseudomonas gene. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1101–1105. doi: 10.1073/pnas.80.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]



