Abstract
We have previously shown that the leader proteinase (Lpro) of foot-and-mouth disease virus (FMDV) interferes with the innate immune response by blocking the translation of interferon (IFN) protein and by reducing the immediate-early induction of beta IFN mRNA and IFN-stimulated genes. Here, we report that Lpro regulates the activity of nuclear factor κB (NF-κB). Analysis of NF-κB-dependent reporter gene expression in BHK-21 cells demonstrated that infection with wild-type (WT) virus has an inhibitory effect compared to infection with a genetically engineered mutant lacking the leader coding region. The expression of endogenous NF-κB-dependent genes tumor necrosis factor alpha and RANTES is also reduced in WT virus-infected primary porcine cells. This inhibitory effect is neither the result of a decrease in the level of the mRNA of p65/RelA, a subunit of NF-κB, nor a block on the nuclear translocation of p65/RelA, but instead appears to be a consequence of the degradation of accumulated p65/RelA. Viral Lpro is localized to the nucleus of infected cells, and there is a correlation between the translocation of Lpro and the decrease in the amount of nuclear p65/RelA. By using a recombinant cardiovirus expressing Lpro, we demonstrate that the disappearance of p65/RelA takes place in the absence of any other FMDV product. The observation that Lpro disrupts the integrity of NF-κB suggests a global mechanism by which FMDV antagonizes the cellular innate immune and inflammatory responses to viral infection.
Foot-and-mouth disease virus (FMDV) is the prototype member of the Aphthovirus genus within the family Picornaviridae and is the causative agent of foot-and-mouth disease, one of the most devastating viral diseases that affect wild and domestic cloven-hoofed animals including pigs and cattle (23). FMDV contains a single-stranded, positive-sense RNA genome of approximately 8,500 nucleotides surrounded by an icosahedral capsid composed of 60 copies each of four structural proteins. Upon infection, the viral RNA is translated as a single, long polyprotein that is cotranslationally processed by virally encoded proteinases into the four structural proteins VP1 (1D), VP2 (1B), VP3 (1C), and VP4 (1A) and eight nonstructural proteins that function in various aspects of the replication cycle: leader (Lpro), 2Apro, 2B, 2C, 3A, 3B, 3Cpro, and 3Dpol (52). Lpro, the first protein to be translated, is a papain-like proteinase (33, 47, 51, 57) that, in addition to self-cleavage from the polyprotein precursor (58), also cleaves the host translation initiation factor eIF-4G, resulting in the shutoff of host cap-dependent mRNA translation, a characteristic of most picornavirus infections (20, 32, 41). Since FMDV mRNA is translated by a cap-independent mechanism that utilizes an internal ribosome entry site and does not require intact eIF-4G, the virus takes over the host cell protein synthesis machinery for its own benefit to produce virus progeny (4, 36).
We have previously shown that Lpro plays a role in FMDV pathogenesis. A genetically engineered virus lacking the Lpro coding region, leaderless virus (47), was highly attenuated in both cattle and swine (12, 40), and, after aerosol infection of cattle, it did not spread systemically beyond the initial site of infection in the lungs (7). Studies in vitro, however, resulted in different phenotypes depending on the cell type used for analysis. Data for leaderless virus infection of primary/secondary porcine, bovine, or ovine cells resembled the results of animal studies with limited cytopathic effects, significantly lower virus yields, and the absence of plaque formation in comparison to wild-type (WT) virus infection (11, 12, 14). In contrast, in BHK-21 or IBRS-2 cells, typical cell lines used for the propagation of FMDV, leaderless virus grew almost as well as WT virus. Further studies demonstrated that supernatants of leaderless virus-infected primary cells contained higher levels of antiviral activity than supernatants from WT virus-infected cells, and this activity was type I interferon (IFN) (alpha/beta IFN [IFN-α/β]) specific (14). Utilizing mouse embryonic fibroblasts, we showed that two IFN-α/β-stimulated genes (ISGs), double-stranded RNA-dependent protein kinase (PKR) and RNase L, are involved in the inhibition of FMDV replication (11). These results suggested that Lpro blocks the innate immune response to virus infection in primary cells and susceptible animals by inhibition of host mRNA translation, including IFN-α/β mRNAs (5). The absence of Lpro in leaderless virus results in an attenuated phenotype in IFN-competent cells. Analyses of IBRS-2 and BHK-21 cell lines indicated that they both harbor either an impairment in type I IFN production or the IFN response, and therefore, they do not allow a clear phenotypic differentiation between WT and leaderless viruses (24). We further demonstrated that WT FMDV replication is inhibited by the pretreatment of cells with IFN-α/β, suggesting that FMDV cannot overcome the antiviral effects once ISG products are expressed (11).
More recently, we have examined the effect of WT and leaderless virus infection on the induction of host IFN-α/β mRNA in primary cells (18). We demonstrated that in addition to inhibiting IFN-α/β protein synthesis, Lpro interferes with the early induction of mRNAs including IFN-β and the ISGs 2′,5′-oligoadenylate synthetase, PKR, and Mx1. The addition of cycloheximide prevented the inhibition of IFN-β mRNA expression in WT but not in leaderless virus-infected cells, a result that was consistent with the hypothesis that a viral product, e.g., Lpro, is required for the inhibition of mRNA expression. Thus far, our data suggest that Lpro down-regulates the innate immune response to FMDV infection by blocking IFN production at both the transcriptional and translational levels.
In most cells, the early expression of IFN-β is tightly regulated by the coordinated activation of latent transcription factors at the IFN-β enhancer, including nuclear factor κB (NF-κB), IFN regulatory factor 3 (IRF3), IRF7, and the activating transcription factor 2/cellular Jun protein complex (ATF2/cJun, also named AP-1) (31). NF-κB acts as a molecular sensor that responds to a wide variety of changes in the environment, including those caused by viral infection (6, 26). For example, cytomegalovirus, human immunodeficiency virus, rhinovirus, Theiler's murine encephalomyelitis virus (TMEV), and measles virus, among others, have been reported to activate NF-κB and induce an inflammatory and/or antiviral response (19, 27, 35, 45, 46). NF-κB belongs to a conserved family of proteins that form multiple homo- and heterodimers with transcriptional activity (21). In most cell types, NF-κB dimers are retained in the cytoplasm by their interaction with specific inhibitors known as IκBs. The recognition of pathogen-associated molecular patterns such as double-stranded RNA (dsRNA), single-stranded RNA, lipopolysaccharide, or CpG oligonucleotides by membrane-associated receptors (Toll-like receptors) and cytoplasmic sensors (retinoic acid-inducible gene I, melanoma differentiation-associated gene 5, and PKR) leads to a series of events resulting in the ubiquitination and proteosomal degradation of the IκB subunit. NF-κB is then released and translocates to the nucleus, activating the transcription of genes involved in innate and adaptive immunity (37, 59).
In this study, we found that infection with leaderless virus resulted in significantly higher levels of NF-κB activity than infection with WT virus. At early stages of FMDV infection, NF-κB was activated and translocated into the nucleus, but at later times, the p65/RelA subunit of NF-κB disappeared only in WT virus-infected cells, suggesting a role of Lpro in this activity. We observed that the disappearance of p65/RelA from the nuclei of infected cells correlated with the translocation of Lpro into this compartment and that Lpro, in the absence of other FMDV proteins, was able to cause p65/RelA degradation. Our results suggest that Lpro-dependent degradation of NF-κB may constitute another global strategy by which FMDV counteracts the immune response.
MATERIALS AND METHODS
Cells and viruses.
Secondary porcine kidney (PK) cells were provided by the Animal, Plant, and Health Inspection Service, National Veterinary Service Laboratory, Ames, IA, or prepared by Juan Pacheco, Plum Island Animal Disease Center. The porcine kidney IBRS-2 cell line was obtained from the Foreign Animal Disease Diagnostic Laboratory at the Plum Island Animal Disease Center. All porcine cells were maintained in minimal essential medium (MEM; GIBCO BRL, Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum and supplemented with 1% antibiotics and nonessential amino acids. BHK-21 cells (baby hamster kidney cells strain 21, clone 13; ATCC CL10) obtained from the American Type Culture Collection (ATCC) (Rockville, MD) were used to propagate virus stocks, to measure virus titers, and for transfection experiments to determine reporter gene activity. BHK-21 cells were maintained in MEM containing 10% calf serum and 10% tryptose phosphate broth supplemented with 1% antibiotics and nonessential amino acids. Cell cultures were incubated at 37°C in 5% CO2.
FMDV A12-IC (WT virus) was generated from the full-length serotype A12 infectious clone pRMC35 (50), and A12-LLV2 (leaderless virus) was derived from an infectious clone lacking the Lb coding region, pRM-LLLV2 (47). Viruses were concentrated by polyethylene glycol precipitation, titers were determined using BHK-21 cells, and virus was stored at −70°C. TMEV and a chimeric TMEV containing the Lb coding region of FMDV A12 (TMEV-Lb) were constructed as previously described and grown in BHK-21 cells (48).
FMDV infection.
Porcine cell monolayers in six-well plates were infected, in duplicate, with A12-IC and A12-LLV2 at the indicated multiplicity of infection (MOI) for 1 h at 37°C. After adsorption, cells were rinsed and incubated with MEM at 37°C. For indirect immunofluorescence analyses, unabsorbed virus was removed by washing the cells with 150 mM NaCl-20 mM morpholineethanesulfonic acid (pH 6.0) before MEM was added. To neutralize the IFN-α/β protein expressed and secreted by infected cells, neutralizing antibodies were added to the culture medium. Mouse monoclonal K17 antibody (PBL Biomedical Laboratories, Piscataway, NJ) was used for IFN-α. For IFN-β, a polyclonal antibody was obtained by the direct inoculation of rabbits with a replication-defective human adenovirus containing the gene for porcine IFN-β (13).
Analysis of mRNA.
A quantitative real-time reverse transcription-PCR (RT-PCR) assay was used to evaluate the mRNA levels of porcine IFN-β, RelA, tumor necrosis factor alpha (TNF-α), and RANTES and viral genome RNA. RNA was extracted from monolayers of PK or BHK-21 cells (FMDV infected, mock infected, or treated with 10 μg/ml poly(I:C) in the presence of Lipofectamine 2000 [Invitrogen]) by using an RNeasy Mini kit (QIAGEN, Valencia, CA). Approximately 1 μg of RNA was treated with DNase I (Sigma, St. Louis, MO) and was used to synthesize cDNA with Moloney murine leukemia virus reverse transcriptase (Invitrogen) and random hexamers according to the manufacturer's directions. An aliquot (1/40) of the cDNA was used as a template for real-time PCR using TaqMan Universal PCR master mix (Applied Biosystems, Foster City, CA). Primers and TaqMan minor groove binding (MGB) probes were designed with Primer Express software v.1.5 (Applied Biosystems). Forward and reverse primers were purchased from Invitrogen, and the 6-carboxyfluorescein-labeled TaqMan MGB probes were purchased from Applied Biosystems. An 18S rRNA kit (Applied Biosystems) or porcine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control to normalize the values for each sample. The sequences for the primers and probes are listed in Table 1. Reactions were performed using an ABI Prism 7000 sequence detection system (Applied Biosystems).
TABLE 1.
Oligonucleotide primer and probe sequences for real-time RT-PCR
| Gene | Primer and probe setc | Sequence | GenBank accession no.a |
|---|---|---|---|
| FMDVb | FMDV F | ACTGGGTTTTACAAACCTGTGA | |
| FMDV R | GCGAGTCCTGCCACGGA | ||
| FMDV T | TCCTTTGCACGCCGTGGGAC | ||
| GAPDH | Porcine GAPDH-327F | CGTCCCTGAGACACGATGGT | AF017079 |
| Porcine GAPDH-380R | CCCGATGCGGCCAAAT | ||
| Porcine GAPDH-348T | AAGGTCGGAGTGAACG | ||
| IFN-β | Porcine IFN-β-11F | AGTGCATCCTCCAAATCGCT | M86762 |
| Porcine IFN-β-69R | GCTCATGGAAAGAGCTGTGGT | ||
| Porcine IFN-β-32T | TCCTGATGTGTTTCTC | ||
| RANTES | Porcine RANTES-54F | TGGCAGCAGTCGTCTTTATCA | F14636 |
| Porcine RANTES-125R | CCCGCACCCATTTCTTCTC | ||
| Porcine RANTES-101T | TGGCACACACCTGGCGGTTCTTTC | ||
| p65/RelA | Porcine RelA-786F | GGAACACGATGGCCACTTG | Homologene 32064/NM021975 |
| Porcine RelA-881R | AAGAGGACATCGAGGTGTATTTCAC | ||
| Porcine RelA-825T | AAAAGGAGCCTCGGGCCTCCCA | ||
| TNF-α | Porcine TNF-α-338F | TGGCCCCTTGAGCATCA | NM214022 |
| Porcine TNF-α-405R | CGGGCTTATCTGAGGTTTGAGA | ||
| Porcine TNF-α-356T | CCCTCTGGCCCAAGGACTCAGATCA |
NCBI GenBank accession number.
Primers and probe were obtained from reference 9.
F, forward primer; R, Reverse primer; T, TaqMan 6-carboxyfluorescein-MGB probe.
Reporter gene expression assays.
BHK-21 cells were cotransfected with pNF-κB-LUC (Stratagene, La Jolla, CA), pRL-TK (Promega, Fitchburg, WI), and Lipofectamine 2000 (Invitrogen) according to the manufacturers' directions. Twenty-four hours posttransfection the cells were infected or treated with poly(I:C)-Lipofectamine 2000 accordingly. Firefly and Renilla luciferase activities were determined using a dual-luciferase reporter assay system (Promega) according to the manufacturer's directions, and readings were performed using a GloMax 96 microplate luminometer (Promega).
Western blot analysis.
Whole-cell extracts were prepared by adding NuPAGE (Invitrogen) lithium dodecyl sulfate sample buffer to the monolayers. To prepare cytoplasmic and nuclear cell fractions, low-salt lysis buffer (10 mM HEPES [pH 7.9], 10 mM NaCl, 3 mM MgCl2, 0.5% NP-40) was added to the monolayers, followed by scraping and incubation on ice for 20 min. After centrifugation for 10 min at 6,000 × g, supernatants were kept as cytoplasmic extracts, and pellets were washed with low-salt lysis buffer and resuspended in high-salt lysis buffer (20 mM HEPES [pH 7.9], 25% glycerol, 0.42 M KCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol), followed by 5 s of sonication at medium power in a Microsom XL2005 apparatus (Heat System, Farmingdale, NY). Clarified supernatants were kept as nuclear fractions. Proteins were resolved in 10% NuPAGE Novex Bis-Tris gels (Invitrogen), transferred onto polyvinylidene difluoride membranes, and detected by Western blotting using an Immun-Star HRP chemiluminescent kit (Bio-Rad, Hercules, CA) according to the manufacturer's directions. NF-κB-p65/RelA was detected using antibody SC 8008 (Santa Cruz Biotechnology, Santa Cruz, CA) or Ab-1 RB-1638 (NeoMarkers; Lab Vision, Freemont, CA), viral protein VP1 was detected using mouse monoclonal antibody 6HC4 (2), Lpro was detected with rabbit polyclonal antibody raised against bacterially expressed recombinant protein (48), α-tubulin was detected with Ab-2 MS-581 (clone DM1A, NeoMarkers; Lab Vision), fibrillarin was detected with ab4566 (clone 38F3; Abcam, Cambridge, MA), and TATA binding protein (TBP) was detected with ab818 (clone 1TBP18; Abcam). Monoclonal antibody against TMEV VP1 (DAmAb2) was provided by Ray Roos, University of Chicago.
Indirect immunofluorescence.
Subconfluent monolayers of cells prepared in 12-mm glass coverslips were infected with FMDV at an MOI of 10 or treated with 25 μg/ml poly(I:C)-Lipofectamine 2000 for the indicated times. Cells were fixed in 4% paraformaldehyde, permeabilized with 0.5% Triton X-100 (Sigma) in phosphate-buffered saline (PBS), blocked with blocking buffer (PBS, 2% bovine serum albumin, 5% normal goat serum, 10 mM glycine), and then incubated overnight at 4°C with the respective primary antibodies. Alexa Fluor 488 (excitation wavelength of 494 nm and emission wavelength of 517 nm)- and Alexa Fluor 594 (excitation wavelength of 590 nm and emission wavelength of 617 nm) (Molecular Probes, Invitrogen)-conjugated secondary antibodies were used for detection. Nuclei were visualized by DAPI (4′,6′-diamidino-2-phenylindole) (excitation wavelength of 345 nm and emission wavelength of 455 nm) included in the mounting medium (Vector Laboratories, Burlingame, CA). Cells were examined using an Olympus BX40 fluorescence microscope, and pictures were taken with a DP-70 digital camera and DP-BSW v2.2 software (Olympus America, Central Valley, PA).
Detection of apoptosis.
Apoptosis was evaluated by the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) method using a POD in situ cell death detection kit (Roche Applied Sciences, Indianapolis, IN) according to the manufacturer's directions. Monolayers of mock- or FMDV-infected cells in 12-mm glass coverslips were fixed with 4% paraformaldehyde, washed with PBS, treated with 0.3% hydrogen peroxide in methanol for 15 min at room temperature for quenching the endogenous peroxidase activity, and permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate before staining. The color reaction was developed with 3,3′-diaminobenzidine, and coverslips were counterstained with Gill's hematoxylin. TNF-α-treated cells (10 ng/ml for 12 h) were included as a positive control, and kit controls, positive and negative, were always added for reference.
RESULTS
WT FMDV blocks NF-κB-dependent reporter gene expression.
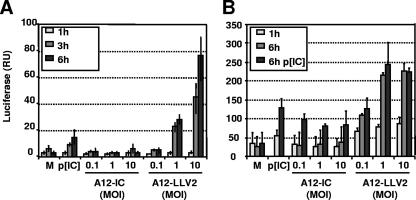
We have previously shown that infection with leaderless virus results in higher levels of expression of IFN-β mRNA than infection with WT FMDV (18). Early expression of IFN-β depends on the activation and assembly of several transcription factors at the IFN-β promoter, and among them, NF-κB plays a fundamental role (31). In order to determine if FMDV affects the function of NF-κB, we performed reporter assays in infected BHK cells to measure the levels of expression of firefly luciferase under the control of NF-κB-responsive sequences. BHK-21 cells are known to be capable of producing IFN but fail to respond to IFN (24), and therefore, no autocrine- or paracrine-activating effects may contribute to the activities measured in these cells. As an internal control, expression of Renilla luciferase driven by the constitutive TK promoter was included. Because only relative luciferase activity levels (firefly/Renilla) were considered, the inhibition of host cell translation by WT FMDV during the course of the experiments was accounted for in the calculations. As seen in Fig. 1A, only leaderless virus infection induces detectable NF-κB-dependent luciferase gene expression. By 6 h postinfection (hpi), 10- and 70-fold increases in luciferase activity were seen when the infections were performed at MOIs of 1 and 10, respectively. No significant increase was observed in WT virus-infected cells. The levels of relative luciferase activity were higher for leaderless virus-infected than for transfected poly(I:C) (25 μg/ml) cells, suggesting a more efficient response of BHK cells to viral infection than to synthetic dsRNA (Fig. 1B). When the cells were infected in the presence of dsRNA poly(I:C) (Fig. 1B), an approximately 30 to 40% reduction in reporter gene activity was observed for WT virus-infected cells. Consistent with the results shown in Fig. 1A, cells treated with poly(I:C) and simultaneously infected with leaderless virus had higher levels of luciferase activity than cells treated with poly(I:C) alone.
FIG. 1.
FMDV blocks NF-κB activity. (A) BHK-21 cells were cotransfected with a reporter plasmid expressing firefly luciferase driven by an NF-κB-dependent promoter (pNF-κB-LUC) and a control plasmid expressing Renilla luciferase constitutively (pRL-TK). Twenty-four hpi, cells were mock treated or infected with FMDV A12-IC (WT) or A12-LLV2 (leaderless) at different MOIs for the indicated times. A positive control with poly(I:C) was included. Relative luciferase activity (firefly/Renilla) was assayed on whole-cell lysates. RU, relative units. (B) Experiments were performed as described above (A), but 24 h prior to viral infection, poly(I:C) was transfected. The values shown represent the means and standard deviations of three independent experiments.
WT FMDV inhibits NF-κB-dependent endogenous gene expression.
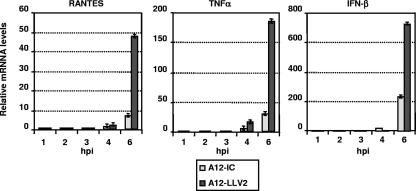
Viral activation of NF-κB is reflected in an increase in the expression of multiple factors involved in the innate and adaptive immune response, including the proinflammatory cytokine TNF-α and the chemokine RANTES (15, 42, 44, 56). In order to corroborate the effect of FMDV on the activation of NF-κB, we measured levels of RANTES and TNF-α mRNA by real-time RT-PCR in primary PK cells. In parallel, we analyzed the expression of IFN-β mRNA. As shown in Fig. 2, by 6 hpi, at an MOI of 10, leaderless virus induced approximately five to six times more RANTES and TNF-α mRNA than WT virus. Expression of IFN-β was about three to four times higher for leaderless than for WT virus. When the infection was performed at an MOI of 1, similar results were obtained for RANTES and TNF-α, while 30- to 50-fold-higher levels of expression were observed for IFN-β (data not shown). At the time of sampling, there was at most only twofold-higher levels of viral RNA in WT than in leaderless virus-infected cells (data not shown). These results indicated that WT virus limits the transcriptional activation of NF-κB-dependent endogenous gene expression.
FIG. 2.
WT FMDV blocks induction of endogenous NF-κB-dependent chemokine and cytokine expression. Expression of RANTES, TNF-α, and IFN-β mRNAs was measured by real-time RT-PCR in primary PK cells infected with WT and leaderless FMDV at an MOI of 10 for the indicated times. Porcine GAPDH was used as an internal control. Results are expressed as increases (n-fold) in gene expression for virus-infected cells with respect to mock-infected cells. The values shown represent the means and standard deviations of three experiments.
FMDV does not inhibit NF-κB translocation.
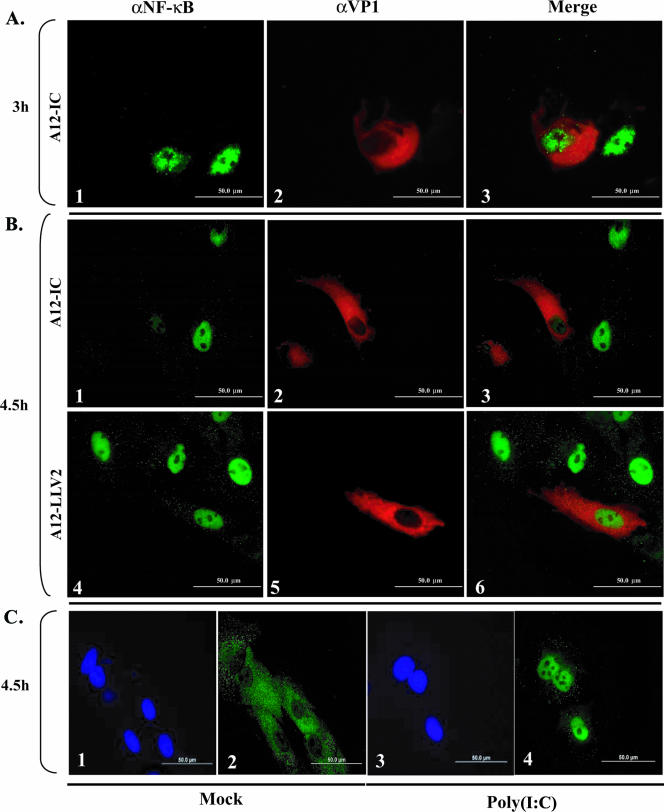
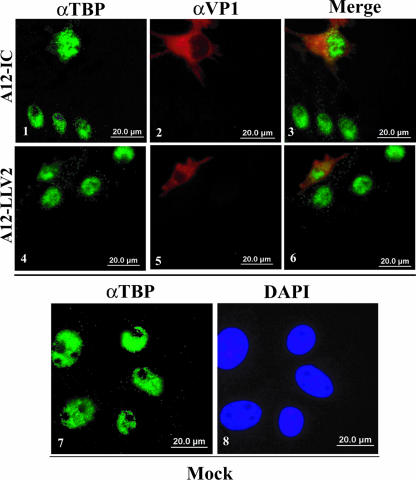
The above-described results did not define the specific step(s) in the activation of the NF-κB pathway that might be inhibited. We initially investigated NF-κB activation in FMDV-infected primary PK cells by directly examining the cellular localization of p65/RelA using a polyclonal antibody raised against a peptide from the C-terminal region of p65/RelA. By 3 hpi, WT virus induced the translocation of p65/RelA to the nucleus of infected cells (Fig. 3A, panels 1 to 3). However, by 4.5 hpi, the nuclear p65/RelA signal was significantly reduced in infected cells (Fig. 3B, panels 1 to 3). While we were unable to detect p65/RelA signal in the nucleus of leaderless virus-infected cells at 3 hpi (data not shown), interestingly, by 4.5 and up to 24 hpi, leaderless virus-infected cells displayed a much stronger p65/RelA nuclear signal than WT virus (Fig. 3B, compare panels 6 and 3, and data not shown). Furthermore, there was an increased number of uninfected bystander cells with p65/RelA nuclear signal in leaderless than in WT virus-infected cultures (Fig. 3B, compare panels 3 and 6). As expected, p65/RelA was localized to the cytoplasm of mock-infected cells (Fig. 3C, panel 2). Transfection of poly(I:C) resulted in p65/RelA nuclear translocation (Fig. 3C, panel 4). Similar results were obtained when NF-κB was detected with a monoclonal antibody that recognized the first 286 amino acids of p65/RelA, although the signal was less intense (data not shown). To verify that the decrease in nuclear staining was p65/RelA specific, cells were stained with antibodies that recognize only the nuclear fraction of TBP. As shown in Fig. 4, no difference in the intensity of TBP staining was detected between WT and leaderless FMDV-infected cells (compare panels 1 and 4). This observation indicated that the decrease in p65/RelA in the nucleus of WT virus-infected cells was specific and not the result of a global effect on nuclear proteins.
FIG. 3.
p65/RelA indirect immunofluorescence analysis during FMDV infection. PK cells were infected at an MOI of 10 with either WT (A and B, panels 1 to 3) or leaderless (B, panels 4 to 6) virus. Parallel mock infection (C, panels 1 and 2) or poly(I:C) treatment (25 μg/ml and Lipofectamine) (C, panels 3 and 4) was performed. p65/RelA was detected using a rabbit polyclonal antibody (Abcam RB-1638) recognizing the C terminus of p65/RelA and an Alexa Fluor 488-conjugated secondary antibody. The viral protein VP1 was detected using mouse monoclonal anti-VP1 antibody (αVP1) (6HC4) and an Alexa Fluor 594-conjugated secondary antibody. Nuclei were stained with DAPI (C, panels 1 and 3).
FIG. 4.
FMDV does not cause nonspecific nuclear protein degradation. IBRS-2 cells were infected with either WT (panels 1 to 3) or leaderless (panels 4 to 6) virus at an MOI of 10 for 4.5 h or mock treated (panels 7 and 8). Nuclear TBP was detected using a mouse monoclonal antibody (ab818) and Alexa Fluor 488-conjugated secondary antibody. The viral protein VP1 was detected as described in the legend of Fig. 3.
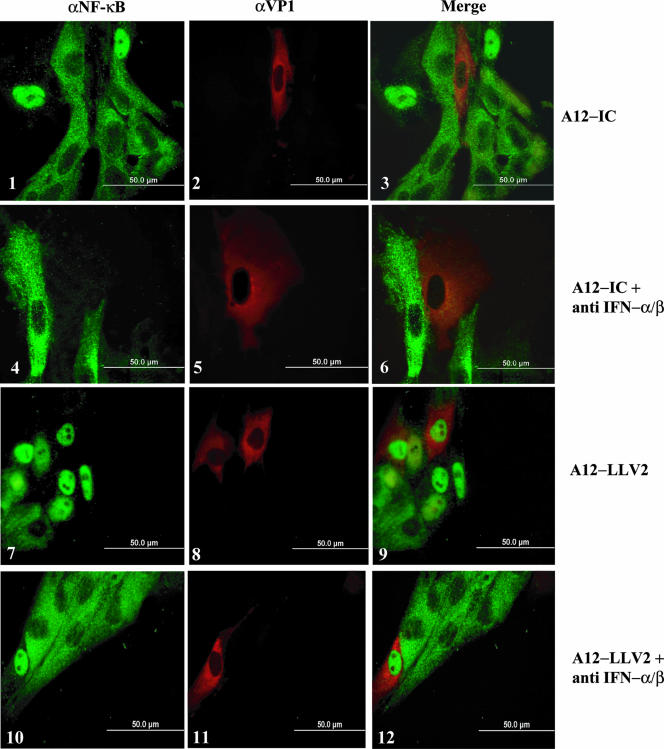
As discussed above, leaderless virus-infected cultures had a higher number of uninfected bystander cells that displayed p65/RelA nuclear localization than WT virus-infected cultures. We hypothesized that the IFN protein secreted into the supernatant of infected PK cells might activate NF-κB in uninfected cells since, as previously reported, leaderless virus infection results in higher levels of secreted IFN protein than WT virus infection (14, 18). As shown in Fig. 5 (panel 9), bystander cells in leaderless virus-infected cultures displayed the brightest fluorescence for nuclear p65/RelA. The addition of anti-IFN-α and anti-IFN-β antibodies to the supernatant of PK cells partially prevented NF-κB nuclear translocation (Fig. 5, compare panel 3 with panel 6 and panel 9 with panel 12), suggesting that the neutralization of IFNs blocks the activation of NF-κB in uninfected cells.
FIG. 5.
Neutralization of type I IFN inhibits NF-κB nuclear translocation in bystander cells. PK cells were infected with either WT or leaderless FMDV at an MOI of 10 for 4.5 h in the absence or presence of neutralizing anti-IFN-α (αIFN) and anti-IFN-β antibodies (see Materials and Methods). p65/RelA and the viral protein VP1 were detected as described in the legend of Fig. 3.
p65/RelA is degraded during FMDV infection.
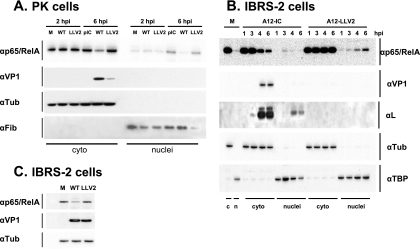
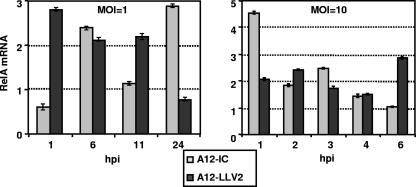
As demonstrated above, NF-κB translocation to the nucleus appeared to be unaffected during FMDV infection, although at late times after infection, the intensity of the signal was weaker in WT virus- than in leaderless virus-infected cells. We analyzed, by Western blotting, the profile of p65/RelA in nuclear and cytoplasmic fractions of infected PK cells and an established porcine cell line, IBRS-2 cells. As shown in Fig. 6A, by 2 hpi, there were comparable amounts of p65/RelA in cytoplasmic and nuclear cell extracts of WT and leaderless virus-infected cultures. However, by 6 hpi, there was a significant reduction in the overall amount of p65/RelA only in extracts of WT virus-infected cells. Loading controls, α-tubulin and fibrillarin, indicated no significant difference among the respective samples. Analysis of viral VP1 indicated that, as expected, WT virus replicated to higher levels than leaderless virus. This observation raised the possibility that another viral gene product could have been involved in p65/RelA degradation. To verify that p65/RelA degradation correlated only with the presence of Lpro, infections were performed using IBRS-2 cells where similar virus yields for WT and leaderless virus can be obtained. Figure 6B shows that the p65/RelA protein profile in these cells exactly resembled the results obtained for primary porcine cells. However, even in this cell line, there was still a significant growth advantage for WT virus in comparison to leaderless virus as reflected by higher amounts of viral VP1. To compensate for this growth difference, we infected IBRS-2 cells with WT virus at an MOI of 10 and leaderless virus at an MOI of 100. Figure 6C shows that for comparable levels of VP1, there were significantly lower levels of p65/RelA only in WT virus-infected cells, a result that suggested a direct correlation between NF-κB disappearance and the presence of Lpro. No p65/RelA degradation products of lower molecular weight could be detected in infected cells using three different NF-κB antibodies even when long sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed (data not shown). To investigate if the disappearance of p65/RelA was the result of the specific down-regulation of the expression of mRNA, we measured the levels of p65/RelA mRNA by real-time RT-PCR. As shown in Fig. 7, no significant decrease in the p65/RelA mRNA level was detected during the course of infection with WT or leaderless virus. These results suggest a direct role of FMDV in p65/RelA protein degradation.
FIG. 6.
WT FMDV infection leads to a loss of p65/RelA. PK (A) or IBRS-2 (B) cells were infected at an MOI of 10 with WT or leaderless FMDV for the indicated times. IBRS-2 (C) cells were infected for 6 h with WT virus (MOI of 10) or leaderless virus (MOI of 100). At the indicated times, cytoplasmic (cyto or c) and nuclear (nuclei or n) extracts (A and B) or whole-cell extracts (C) were prepared and analyzed by Western blotting using rabbit polyclonal-anti p65/RelA antibody (RB-1638), mouse monoclonal anti-α-tubulin antibody (αTub) (Ab-2 MS-581), mouse monoclonal anti-viral VP1 antibody (6HC4), rabbit polyclonal anti-leader antibody, mouse monoclonal anti-fibrillarin antibody (αFib) (ab4566), and mouse monoclonal anti-TBP antibody (ab818). M, molecular weight marker.
FIG. 7.
FMDV infection does not affect p65/RelA mRNA expression. p65/RelA mRNA expression was measured by real-time RT-PCR in primary PK cells infected with WT and leaderless FMDV at an MOI of 10 for the indicated times. Porcine GAPDH was used as an internal control. Results are expressed as increases (n-fold) in gene expression for virus-infected cells with respect to mock-infected cells. The values shown represent the means and standard deviations of three independent experiments.
Disappearance of NF-κB is not caused by apoptosis.
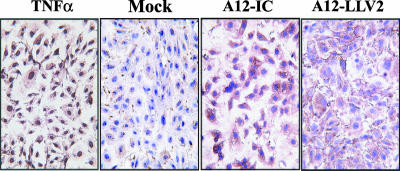
Apoptosis is one of the mechanisms of cell death, and recently, it has been shown that picornaviruses alter the apoptotic homeostasis of infected host cells (8). In order to rule out the possibility that the disappearance of NF-κB in FMDV-infected cells was the result of viral induction of apoptosis or that, alternatively, p65/RelA cleavage resulted in a proapoptotic event during infection, we analyzed infected PK cells by a TUNEL assay. As shown in Fig. 8, no indication of nuclear fragmentation, a signature feature of apoptotic cells, was seen by 6 hpi, despite the disappearance of p65/RelA and the considerable cytopathic effect observed. In contrast, porcine TNF-α treatment for 12 h induced apoptosis in more than 90% of the culture. This result indicates that under our experimental conditions, FMDV-infected cells do not undergo apoptosis-mediated cell death.
FIG. 8.
FMDV does not cause apoptosis. Primary PK cells were infected with WT or leaderless FMDV at an MOI of 10 or mock treated for 6 h. As a positive control, cells were treated with TNF-α (10 ng/ml) for 12 h. Apoptosis was evaluated by TUNEL assay.
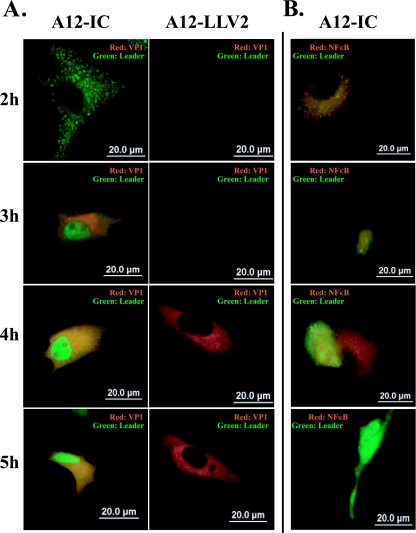
Lpro localizes to the nucleus of infected cells.
Western blot analysis of p65/RelA in WT virus-infected cells showed a decrease in the cellular cytoplasmic and nuclear fractions; however, as shown in Fig. 6, this effect was not noticeable early during infection. Moreover, in some experiments, by 2 hpi, higher levels of p65/RelA were detected in the nuclei of WT than in leaderless virus-infected cells (data not shown). At later time points, the levels of the p65/RelA protein were significantly reduced in the nuclei and cytoplasm of WT-infected cells. In order to determine if there was a correlation between the degradation of p65/RelA and the expression and subcellular localization of Lpro during infection, we examined both proteins by indirect immunofluorescence. Figure 9 shows that by 2 hpi, Lpro was detected as a punctated staining homogenously distributed in the cytoplasm of the infected cell (Fig. 9A, panel 1). However, at 3 hpi and thereafter, Lpro was also found in the nucleus, indicating progressive protein translocation during the course of the infection (Fig. 9A, panels 3, 5, and 7). There was no background signal in leaderless virus-infected cells, confirming the specificity of the observed Lpro reaction (Fig. 9A, panels 2, 4, 6, and 8). Double staining of p65/RelA and Lpro in WT FMDV-infected cells (Fig. 9B, panels 1 to 4) showed that whereas there was significant colocalization by 4 hpi, the p65/RelA signal progressively decreased and became almost nondetectable by 5 hpi.
FIG. 9.
FMDV leader protein translocates to the nucleus. Primary PK cells were infected with WT (A, panels 1, 3, 5, and 7, and B, panels 1 to 4) or leaderless (A, panels 2, 4, 6, and 8) FMDV at an MOI of 10 for the indicated times. Leader protein was detected with rabbit polyclonal anti-leader antibody and Alexa Fluor 488-conjugated secondary antibody, VP1 was detected with mouse monoclonal antibody 6H4C and Alexa Fluor 594 secondary antibody, and NF-κB was detected with rabbit polyclonal anti p65/RelA antibody (RB-1638) and Alexa Fluor 594 secondary antibody.
FMDV Lpro is sufficient for degradation of p65/RelA.
To directly determine if Lpro is the only FMDV protein required for the degradation of p65/RelA, we used a previously constructed chimeric TMEV containing the Lpro coding region of FMDV (48). Initiation of translation of all seven serotypes of the FMDV polyprotein can potentially occur at two alternative, in-frame AUG codons separated by 84 bases, resulting in the synthesis of two leader proteins, Lab and Lb (55). Lb, whose translation starts at the second AUG, is the predominant Lpro found in FMDV-infected cultures (10, 47). In order to study the effect of Lpro independently of the other FMDV products, we used TMEV-Lb, in which the dispensable TMEV leader protein was replaced by FMDV Lb (48).
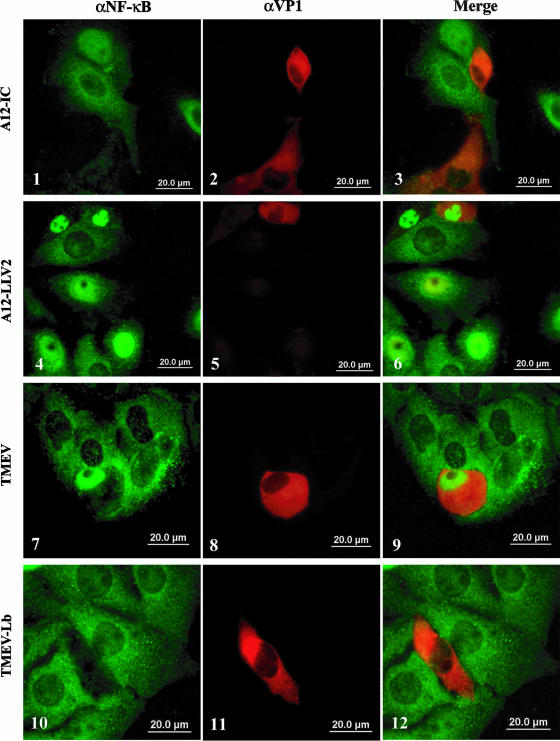
TMEV is a murine picornavirus, but given that all our previous experiments were performed using porcine cells, a natural host for FMDV, we initially assessed the permissiveness of IBRS-2 cells to TMEV by end-point titration of infected cultures. Although not optimal, these cells supported an increase of at least 100-fold in the viral titer when infected with TMEV and an increase of six- to sevenfold when infected with TMEV-Lb by 24 hpi, thereby making them suitable for our experiments. Infected IBRS-2 cells were examined at 24 hpi by indirect immunofluorescence. As shown in Fig. 10, p65/RelA translocates to the nucleus of cells infected with TMEV (Fig. 10, panels 7 to 9), resembling the results obtained using leaderless virus-infected cells (Fig. 10, panels 4 to 6). However, p65/RelA staining is practically absent in the nuclei of TMEV-Lb-infected cells (Fig. 10, panels 10 to 12). Likewise, as described above, there is almost no p65/RelA staining in WT FMDV-infected cells (Fig. 10, panels 1 to 3). At early times during TMEV-Lb infection (approximately 6 hpi), a weak TMEV VP1 signal correlated with relatively strong nuclear p65/RelA staining (data not shown), but as seen in Fig. 10, at later time points (24 hpi), a stronger signal for VP1 correlated with the disappearance of the p65/RelA signal from infected cells. Interestingly, the decrease of p65/RelA nuclear staining in TMEV-Lb-infected cells was concurrent with the translocation of Lb to the nucleus (data not shown). These results demonstrated that the loss of the p65/RelA signal is directly dependent on the presence of FMDV Lb in the nucleus of infected cells.
FIG. 10.
FMDV Lpro is sufficient for p65/RelA degradation. IBRS-2 cells were infected with WT or leaderless FMDV, TMEV, and chimeric TMEV-Lb at an MOI of 10. Twenty-four hpi, p65/RelA and viral proteins were visualized by indirect immunofluorescence. p65/RelA was detected with rabbit polyclonal antibody (RB-1638) and Alexa Fluor 488-conjugated secondary antibody. TMEV VP1 and FMDV VP1 were detected with monoclonal antibodies DAmAb2 and 6HC4, respectively, and both were visualized with Alexa Fluor 594-conjugated secondary antibody.
DISCUSSION
Upon viral infection, activation of the transcription factors NF-κB, IRF3/7, and AP-1 lead to the induction of IFN-α/β mRNA and the establishment of a robust antiviral innate immune response (31). To escape or overcome this early host response, viruses have evolved mechanisms that inhibit one or more steps involved in the induction of IFN and/or the function of ISGs (16, 25). For FMDV, we have previously shown that WT virus infection results in both the shutoff of cap-dependent host protein synthesis, including IFN-α/β, and the inhibition of IFN-α/β mRNA induction (11, 18). Previous work demonstrated that Lpro is directly responsible for the cleavage of translation initiation factor eIF-4G (20, 32).
In the current study, we demonstrate that Lpro is directly associated with the degradation of p65/RelA, thus affecting the NF-κB activity required for the full expression of IFN-β and other inflammatory cytokines.
Virus infection can block NF-κB activity, thus antagonizing the host immune response. In most cases, irreversible binding of viral proteins to the IκB inhibitor or interference with IκB degradation results in the inhibition of p65/RelA nuclear translocation and therefore a decrease in NF-κB-dependent gene expression (22, 30). Our results show that WT FMDV utilizes a different mechanism to counteract NF-κB activity. WT FMDV does not prevent the activation or translocation of p65/RelA into the nucleus. Furthermore, we did not observe any alteration in the nucleocytoplasmic trafficking of a reporter green fluorescent protein containing a nuclear localization signal upon infection with FMDV (data not shown). Similar results were found for cells infected with a recombinant mengovirus containing the FMDV Lpro coding region (F. J. van Kuppeveld, personal communication). Our experiments show that the p65/RelA protein is degraded during WT FMDV infection. Interestingly, p65/RelA integrity is preserved when cells are infected with leaderless FMDV, suggesting a direct involvement of Lpro in the degradation process. However, since leaderless virus is an attenuated strain and results in lower virus yields in cell culture, it is possible that other FMDV products could be involved in the degradation of p65/RelA. Moreover, recent studies with poliovirus have shown that the viral 3C protease cleaves p65/RelA during infection (43). To address this issue, we performed two sets of experiments. First, IBRS-2 cells were infected with leaderless virus at a high MOI (MOI of 100 versus 10 for WT) to compensate for the leaderless virus growth disadvantage. Under these conditions, we observed a significant reduction in levels of the NF-κB protein only in WT virus-infected cells when equivalent amounts of viral VP1 were present for both viruses. Second, a chimeric TMEV containing only the Lpro coding region of FMDV was used to evaluate the function of this protein in the absence of other FMDV products (48). Our results showed that whereas WT TMEV induced the translocation and accumulation of NF-κB in the nucleus of infected cells, the chimeric TMEV-Lb induced the translocation of NF-κB to the nucleus early during infection, but at later time points, p65/RelA staining completely disappeared when Lb was detected in the nucleus of infected cells. These results suggest that FMDV Lpro is both necessary and sufficient for this effect.
We predict that p65/RelA can be cleaved by Lpro at specific recognition sites since this viral product is a protease. Analysis of the amino acid sequence of p65/RelA indicates the presence of one putative Lpro cleavage site close to its amino terminus. However, no discrete p65/RelA degradation products were detected by Western blot analysis even when several commercially available antibodies were tested. Nevertheless, further studies are needed to confirm this observation.
NF-κB has been reported to be an antiapoptotic factor (3). However, other studies have shown that NF-κB can overcome apoptotic inhibition when p65/RelA is cleaved at the C terminus, creating a dominant negative inhibitor protein that can promote apoptosis (38). We could not detect apoptosis in WT or leaderless virus-infected cells. However, we do not know if cleavage of NF-κB by FMDV could result in apoptosis if sufficient time were permitted during the course of infection.
The absence of defined degradation products indicated that p65/RelA apoptotic cleavage did not occur during FMDV infection. In addition, these results suggested that complete protein degradation occurred. One of the most studied mechanisms that mediate protein degradation involves ubiquitination and proteasomal targeting (28). Moreover, recent studies have shown that p65/RelA bound to promoter sequences can be ubiquitinated and degraded in the nucleus of the cell (53, 54). Recently, it has also been shown that the Npro product of pestiviruses, including bovine viral diarrhea virus and classical swine fever virus, induces the ubiquitination and degradation of IRF3 (1, 29). It is possible that FMDV uses an analogous mechanism to degrade NF-κB. In our experiments, the timing of p65/RelA degradation coincided with the nuclear translocation of FMDV Lpro, and furthermore, the addition of the proteasome inhibitor MG132 partially prevented p65/RelA degradation (data not shown). Studies to evaluate the ubiquitination state of p65/RelA during FMDV infection as well as to determine if Lpro directly degrades NF-κB are planned.
A unique observation resulting from our studies is that a significant portion of Lpro rapidly translocates to the nucleus. In the picornavirus family, only cardioviruses, in addition to FMDV, contain an L protein, although cardiovirus L proteins are not proteinases. Similar to FMDV L, encephalomyocarditis virus (EMCV) and TMEV L proteins are associated with a block in the induction of IFN-α/β upon viral infection (34, 60, 61). Previous studies demonstrated that these proteins can alter the general nucleocytoplasmic trafficking affecting the intracellular sublocalization of multiple factors required for the establishment of an antiviral state (17, 39, 49). Recently, it has been shown that EMCV L localizes near the cytoplasmic side of the nuclear pore complex and disrupts the RanGDP-GTP gradient, thereby blocking nucleocytoplasmic transport during infection (49). Our results show that in contrast to EMCV L, FMDV Lpro enters the nucleus of the infected cell without interfering with intracellular trafficking. Moreover, nuclear localization of FMDV Lpro correlates with the disappearance of NF-κB in the absence of any other FMDV protein. It is possible, however, that FMDV Lpro might affect the trafficking of other proteins, but further studies are required to test this hypothesis.
Our observations add a new factor to the complexity of the interaction between FMDV and the host. The leader proteinase of FMDV has a major role in antagonizing the innate immune response at multiple levels, including the inhibition of transcription and translation. Our current emphasis is on utilizing genomics and reverse genetics to identify regions within Lpro that may be responsible for the various functions of this protein. A more detailed knowledge of Lpro and other viral proteins that may be involved in counteracting the host innate response should help us develop more effective disease control strategies.
Acknowledgments
We thank Ray Roos for providing monoclonal antibody DAmAb2 against TMEV VP1 and Frank van Kuppeveld for providing plasmid NLS-GFP. We also thank Lindomar Pena for technical support.
This research was supported in part by the Plum Island Animal Disease Research Participation Program administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Department of Agriculture (appointment of Fayna Diaz-San Segundo) and by CRIS project number 1940-32000-048-00D, ARS, USDA (Marvin J. Grubman).
Footnotes
Published ahead of print on 19 September 2007.
REFERENCES
- 1.Bauhofer, O., A. Summerfield, Y. Sakoda, J.-D. Tratschin, M. A. Hofmann, and N. Ruggli. 2007. Classical swine fever virus Npro interacts with interferon regulatory factor 3 and induces its proteasomal degradation. J. Virol. 81:3087-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baxt, B., D. O. Morgan, B. H. Robertson, and C. A. Timpone. 1984. Epitopes on foot-and-mouth disease virus outer capsid protein VP1 involved in neutralization and cell attachment. J. Virol. 51:298-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beg, A. A., and D. Baltimore. 1996. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science 274:782-784. [DOI] [PubMed] [Google Scholar]
- 4.Belsham, G. J., and J. K. Brangwyn. 1990. A region of the 5′ noncoding region of foot-and-mouth disease virus RNA directs efficient internal initiation of protein synthesis within cells: involvement with the role of L protease in translational control. J. Virol. 64:5389-5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biron, C. A., and G. C. Sen. 2001. Interferons and other cytokines, p. 321-351. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 6.Bonizzi, G., and M. Karin. 2004. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25:280-288. [DOI] [PubMed] [Google Scholar]
- 7.Brown, C. C., M. E. Piccone, P. W. Mason, T. S. McKenna, and M. J. Grubman. 1996. Pathogenesis of wild-type and leaderless foot-and-mouth disease virus in cattle. J. Virol. 70:5638-5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buenz, E. J., and C. L. Howe. 2006. Picornaviruses and cell death. Trends Microbiol. 14:28-36. [DOI] [PubMed] [Google Scholar]
- 9.Callahan, J. D., F. Brown, F. A. Osorio, J. H. Sur, E. Kramer, G. W. Long, J. Lubroth, S. J. Ellis, K. S. Shoulars, K. L. Gaffney, D. L. Rock, and W. M. Nelson. 2002. Use of a portable real-time reverse transcriptase-polymerase chain reaction assay for rapid detection of foot-and-mouth disease virus. J. Am. Vet. Med. Assoc. 220:1636-1642. [DOI] [PubMed] [Google Scholar]
- 10.Cao, X., I. E. Bergmann, R. Fullkrug, and E. Beck. 1995. Functional analysis of the two alternative translation initiation sites of foot-and-mouth disease virus. J. Virol. 69:560-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chinsangaram, J., M. Koster, and M. J. Grubman. 2001. Inhibition of L-deleted foot-and-mouth disease virus replication by alpha/beta interferon involves double-stranded RNA-dependent protein kinase. J. Virol. 12:5498-5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinsangaram, J., P. W. Mason, and M. J. Grubman. 1998. Protection of swine by live and inactivated vaccines prepared from a leader proteinase-deficient serotype A12 foot-and-mouth disease virus. Vaccine 16:1516-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinsangaram, J., M. P. Moraes, M. Koster, and M. J. Grubman. 2003. Novel viral disease control strategy: adenovirus expressing alpha interferon rapidly protects swine from foot-and-mouth disease. J. Virol. 77:1621-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chinsangaram, J., M. E. Piccone, and M. J. Grubman. 1999. Ability of foot-and-mouth disease virus to form plaques in cell culture is associated with suppression of alpha/beta interferon. J. Virol. 73:9891-9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collart, M. A., P. Baeuerle, and P. Vassalli. 1990. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four κB-like motifs and of constitutive and inducible forms of NF-κB. Mol. Cell. Biol. 10:1498-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conzelmann, K. K. 2005. Transcriptional activation of alpha/beta interferon genes: interference by nonsegmented negative-strand RNA viruses. J. Virol. 79:5241-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delhaye, S., V. van Pesch, and T. Michiels. 2004. The leader protein of Theiler's virus interferes with nucleocytoplasmic trafficking of cellular proteins. J. Virol. 78:4357-4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de los Santos, T., S. de Avila Botton, R. Weiblen, and M. J. Grubman. 2006. The leader proteinase of foot-and-mouth disease virus inhibits the induction of beta interferon mRNA and blocks the host innate immune response. J. Virol. 80:1906-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demarchi, F., M. I. Gutierrez, and M. Giacca. 1999. Human immunodeficiency virus type 1 Tat protein activates transcription factor NF-κB through the cellular interferon-inducible, double-stranded RNA-dependent protein kinase, PKR. J. Virol. 73:7080-7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devaney, M. A., V. N. Vakharia, R. E. Lloyd, E. Ehrenfeld, and M. J. Grubman. 1988. Leader protein of foot-and-mouth disease virus is required for cleavage of the p220 component of the cap-binding protein complex. J. Virol. 62:4407-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-κB and Rel proteins: evolutionary conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 22.Gilmore, T. D., and M. Herscovitch. 2006. Inhibitors of NF-kappaB signaling: 785 and counting. Oncogene 25:6887-6899. [DOI] [PubMed] [Google Scholar]
- 23.Grubman, M. J., and B. Baxt. 2004. Foot-and-mouth disease. Clin. Microbiol. Rev. 17:465-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grubman, M. J., and J. Chinsangaram. 2000. Foot-and-mouth disease virus: the role of the leader proteinase in viral pathogenesis. Recent Res. Dev. Virol. 2:123-134. [Google Scholar]
- 25.Haller, O., G. Kochs, and F. Weber. 2006. The interferon response circuit: induction and suppression by pathogenic viruses. Virology 344:119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayden, M. S., and S. Ghosh. 2004. Signaling to NF-kappaB. Genes Dev. 18:2195-2224. [DOI] [PubMed] [Google Scholar]
- 27.Helin, E., S. Matikainen, I. Julkunen, J. Heino, T. Hyypia, and R. Vainionpaa. 2002. Measles virus enhances the expression of cellular immediate-early genes and DNA-binding of transcription factor AP-1 in lung epithelial A549 cells. Arch. Virol. 147:1721-1732. [DOI] [PubMed] [Google Scholar]
- 28.Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67:425-479. [DOI] [PubMed] [Google Scholar]
- 29.Hilton, L., K. Moganeradj, G. Zhang, Y. H. Chen, R. E. Randall, J. W. McCauley, and S. Goodbourn. 2006. The NPro product of bovine viral diarrhea virus inhibits DNA binding by interferon regulatory factor 3 and targets it for proteasomal degradation. J. Virol. 80:11723-11732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiscott, J., T. L. Nguyen, M. Arguello, P. Nakhaei, and S. Paz. 2006. Manipulation of the nuclear factor-kappaB pathway and the innate immune response by viruses. Oncogene 25:6844-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honda, K., H. Yanai, A. Takaoka, and T. Taniguchi. 2006. Regulation of the type I IFN induction: a current view. Int. Immunol. 17:1367-1378. [DOI] [PubMed] [Google Scholar]
- 32.Kirchweger, R., E. Ziegler, B. J. Lamphear, D. Waters, H. D. Liebig, W. Sommergruber, F. Sobrino, C. Hohenadl, D. Blaas, R. E. Rhoads, and T. Skern. 1994. Foot-and-mouth disease virus leader proteinase: purification of the Lb form and determination of its cleavage site on eIF-4γ. J. Virol. 68:5677-5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleina, L. G., and M. J. Grubman. 1992. Antiviral effects of a thiol protease inhibitor on foot-and-mouth disease virus. J. Virol. 66:7168-7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong, W. P., G. D. Ghadge, and R. P. Roos. 1994. Involvement of cardiovirus leader in host cell-restricted virus expression. Proc. Natl. Acad. Sci. USA 91:1796-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kowalik, T. F., B. Wing, J. S. Haskill, J. C. Azizkhan, A. S. Baldwin, Jr., and E. S. Huang. 1993. Multiple mechanisms are implicated in the regulation of NF-kappa B activity during human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 90:1107-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhn, R., N. Luz, and E. Beck. 1990. Functional analysis of the internal translation initiation site of foot-and-mouth disease virus. J. Virol. 64:4625-4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langland, J. O., J. M. Cameron, M. C. Heck, J. K. Jancovich, and B. L. Jacobs. 2006. Inhibition of PKR by RNA and DNA viruses. Virus Res. 119:100-110. [DOI] [PubMed] [Google Scholar]
- 38.Levkau, B., M. Scatena, C. M. Giachelli, R. Ross, and E. W. Raines. 1999. Apoptosis overrides survival signals through a caspase-mediated dominant-negative NF-kappa B loop. Nat. Cell Biol. 1:227-233. [DOI] [PubMed] [Google Scholar]
- 39.Lidsky P. V., S. Hato, M. V. Bardina, A. G. Aminev, A. C. Palmenberg, E. V. Sheval, V. Y. Polyakov, F. J. van Kuppeveld, and V. I. Agol. 2006. Nucleocytoplasmic traffic disorder induced by cardioviruses. J. Virol. 80:2705-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mason, P. W., M. E. Piccone, T. S. McKenna, J. Chinsangaram, and M. J. Grubman. 1997. Evaluation of a live-attenuated foot-and-mouth disease virus as a vaccine candidate. Virology 227:96-102. [DOI] [PubMed] [Google Scholar]
- 41.Medina, M., E. Domingo, J. K. Brangwyn, and G. J. Belsham. 1993. The two species of the foot-and-mouth disease virus leader protein, expressed individually, exhibit the same activities. Virology 194:355-359. [DOI] [PubMed] [Google Scholar]
- 42.Moriuchi, M., H. Moriuchi, and A. S. Fauci. 1997. Nuclear factor kappa-B potently up-regulates the promoter activity of RANTES, a chemokine that blocks HIV infection. J. Immunol. 158:3483-3491. [PubMed] [Google Scholar]
- 43.Neznanov, N., K. M. Chumakov, L. Neznanova, A. Almasan, A. K. Banerjee, and A. V. Gudkov. 2005. Proteolytic cleavage of the p65-RelA subunit of NF-kappaB during poliovirus infection. J. Biol. Chem. 280:24153-24158. [DOI] [PubMed] [Google Scholar]
- 44.Pahl, H. L. 1999. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18:6853-6866. [DOI] [PubMed] [Google Scholar]
- 45.Palma, J. P., D. Kwon, N. A. Clipstone, and B. S. Kim. 2003. Infection with Theiler's murine encephalomyelitis virus directly induces proinflammatory cytokines in primary astrocytes via NK-kappaB activation: potential role for the initiation of demyelinating disease. J. Virol. 77:6322-6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papi, A., and S. L. Johnston. 1999. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-κB-mediated transcription. J. Biol. Chem. 274:9707-9720. [DOI] [PubMed] [Google Scholar]
- 47.Piccone, M. E., E. Rieder, P. W. Mason, and M. J. Grubman. 1995. The foot-and-mouth disease virus leader proteinase gene is not required for viral replication. J. Virol. 69:5376-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piccone, M. E., H.-H. Chen, R. R. Roos, and M. J. Grubman. 1996. Construction of a chimeric Theiler's murine encephalomyelitis virus containing the leader gene of foot-and-mouth disease virus. Virology 226:135-139. [DOI] [PubMed] [Google Scholar]
- 49.Porter, F. W., Y. A. Bochkov, A. J. Albee, C. Wiese, and A. C. Palmenberg. 2006. A picornavirus protein interacts with Ran-GTPase and disrupts nucleocytoplasmic transport. Proc. Natl. Acad. Sci. USA 3:12417-12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rieder, E., T. Bunch, F. Brown, and P. W. Mason. 1993. Genetically engineered foot-and-mouth disease viruses with poly(C) tracts of two nucleotides are virulent in mice. J. Virol. 67:5139-5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts, P. J., and G. J. Belsham. 1995. Identification of critical amino acids within the foot-and-mouth disease virus leader protein, a cysteine protease. Virology 213:140-146. [DOI] [PubMed] [Google Scholar]
- 52.Rueckert, R. R. 1996. Picornaviridae: the viruses and their replication, p. 609-654. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, PA.
- 53.Ryo, A., F. Suizu, Y. Yoshida, K. Perrem, Y.-C. Liou, G. Wulf, R. Rottapel, S. Yamaoka, and K. P. Lu. 2003. Regulation of NF-κB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol. Cell 12:1413-1426. [DOI] [PubMed] [Google Scholar]
- 54.Saccani, S., I. Marazzi, A. A. Beg, and G. Natoli. 2004. Degradation of promoter-bound p65/RelA is essential for prompt termination of the nuclear factor κB response. J. Exp. Med. 200:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sangar, D. V., S. E. Newton, D. J. Rowlands, and B. E. Clarke. 1987. All foot and mouth disease virus serotypes initiate protein synthesis at two separate AUGs. Nucleic Acids Res. 15:3305-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shakhov, A. N., M. A. Collart, P. Vassalli, S. A. Nedospasov, and C. V. Jongeneel. 1990. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J. Exp. Med. 171:35-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skern, T., I. Fita, and A. Guarne. 1998. A structural model of picornavirus leader proteinases based on papain and bleomycin hydrolase. J. Gen. Virol. 79:301-307. [DOI] [PubMed] [Google Scholar]
- 58.Strebel, K., and E. Beck. 1986. A second protease of foot-and-mouth disease virus. J. Virol. 58:893-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takeuchi, O., and S. Akira. 2007. Signaling pathways activated by microorganisms. Curr. Opin. Cell Biol. 19:185-191. [DOI] [PubMed] [Google Scholar]
- 60.van Pesch, V., O. van Eyll, and T. Michiels. 2001. The leader protein of Theiler's virus inhibits immediate-early alpha/beta interferon production. J. Virol. 75:7811-7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zoll, J., W. J. Melchers, J. M. Galama, and F. J. van Kuppeveld. 2002. The mengovirus leader protein suppresses alpha/beta interferon production by inhibition of the iron/ferritin-mediated activation of NF-κB. J. Virol. 76:9664-9672. [DOI] [PMC free article] [PubMed] [Google Scholar]