Abstract
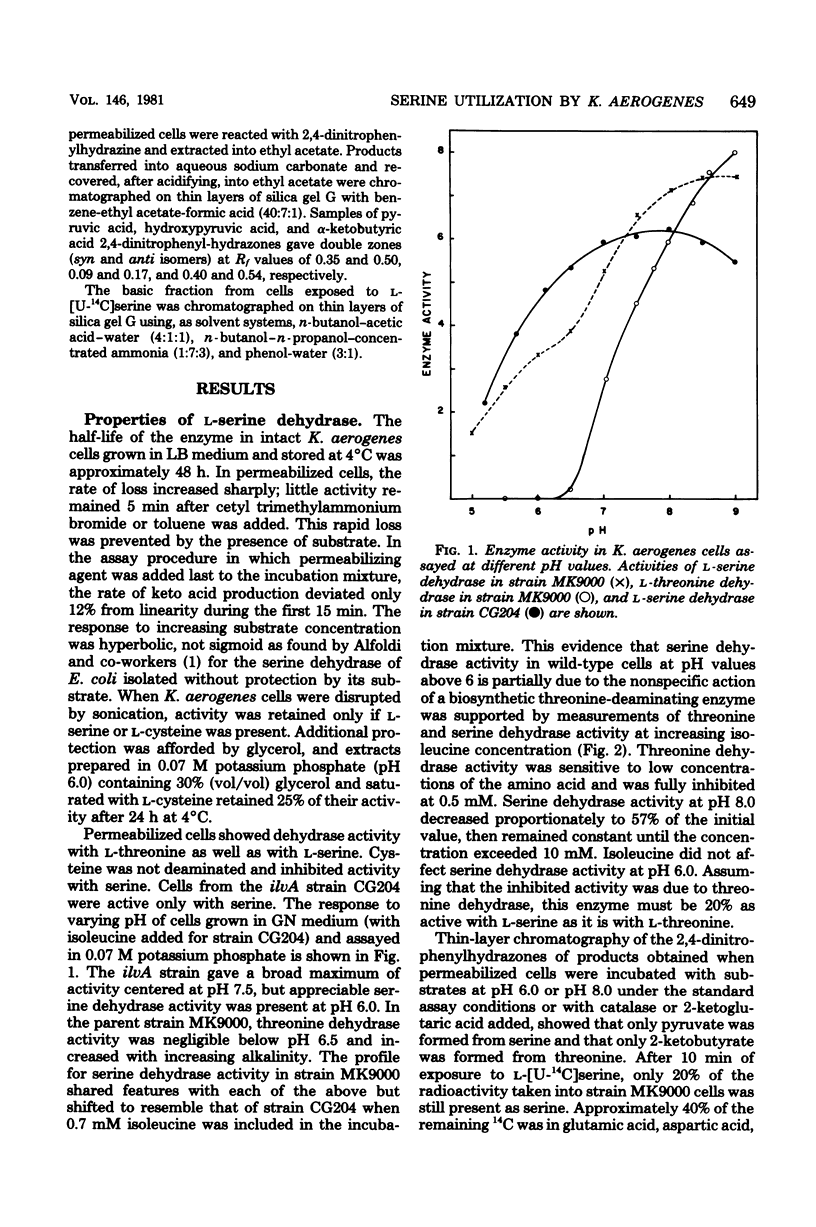
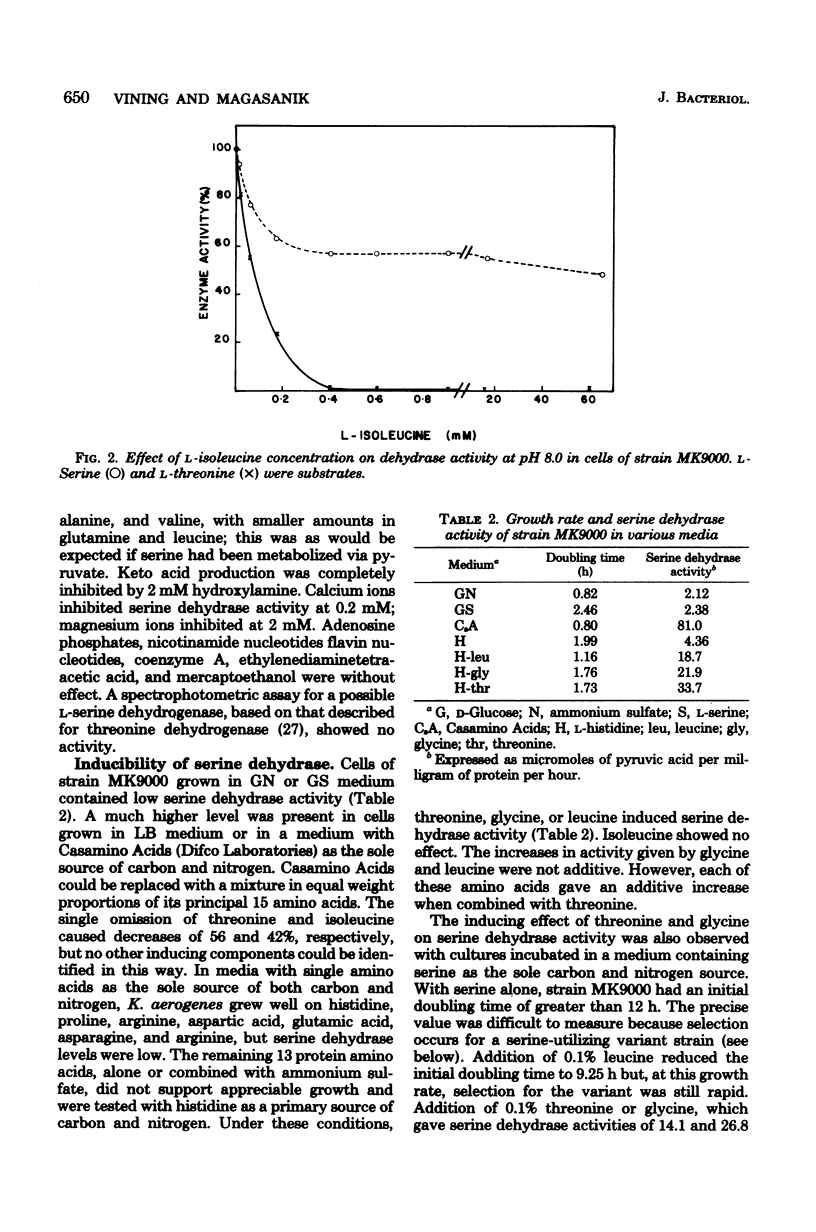
Klebsiella aerogenes was found to contain a specific L-serine dehydrase that was induced by threonine, glycine or leucine, but not by its substrate. Cellular concentrations were sensitive to carbon rather than nitrogen sources in the growth medium. A nonspecific isoleucine-sensitive L-threonine dehydrase supplemented the specific L-serine dehydrase activity. K. aerogenes also contains a leucine-inducible L-threonine dehydrogenase which probably initiated a threonine-utilization pathway in which the serine-specific dehydrate participated. Strains that were altered in their ability to metabolize serine differed in either L-serine dehydrase or L-threonine dehydrase activity. Thus, K. aerogenes growing on L-serine as a sole nitrogen source relies upon two enzymes that metabolize the amino acid as subsidiary functions.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARTMAN M., MARKENSON J. Studies on serine and threonine deaminases of Escherichia coli and the action of dihydrostreptomycin thereupon. Enzymologia. 1958 Jan 31;19(1):9–15. [PubMed] [Google Scholar]
- Alföldi L., Raskó I., Kerekes E. L-serine deaminase of Escherichia coli. J Bacteriol. 1968 Nov;96(5):1512–1518. doi: 10.1128/jb.96.5.1512-1518.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENZIMAN M., SAGERS R. D., GUNSALUS I. C. L-serine specific dehydrase from Clostridium acidi-urici. J Bacteriol. 1960 Apr;79:474–479. doi: 10.1128/jb.79.4.474-479.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S. C., Turner J. M. Bacterial catabolism of threonine. Threonine degradation initiated by L-threonine-NAD+ oxidoreductase. Biochem J. 1976 May 15;156(2):449–458. doi: 10.1042/bj1560449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S. C., Turner J. M. Bacterial catabolism of threonine. Threonine degradation initiated by l-threonine hydrolyase (deaminating) in a species of Corynebacterium. Biochem J. 1977 Jun 15;164(3):579–587. doi: 10.1042/bj1640579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R. A., Janssen K. A., Resnick A. D., Blumenberg M., Foor F., Magasanik B. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J Bacteriol. 1977 Feb;129(2):1001–1009. doi: 10.1128/jb.129.2.1001-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R. A., Macaluso A., Magasanik B. Glutamate dehydrogenase: genetic mapping and isolation of regulatory mutants of Klebsiella aerogenes. J Bacteriol. 1976 Jul;127(1):141–148. doi: 10.1128/jb.127.1.141-148.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore M. A., Turner J. M. Threonine metabolism via two-carbon compounds by Pseudomonas oxalaticus. J Gen Microbiol. 1971 Aug;67(2):243–246. doi: 10.1099/00221287-67-2-243. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brenchley J. E., Prival M. J., Magasanik B. Regulation of the synthesis of enzymes responsible for glutamate formation in Klebsiella aerogenes. J Biol Chem. 1973 Sep 10;248(17):6122–6128. [PubMed] [Google Scholar]
- Deleo A. B., Magasanik B. Identification of the structural gene for glutamine synthetase in Klebsiella aerogenes. J Bacteriol. 1975 Jan;121(1):313–319. doi: 10.1128/jb.121.1.313-319.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan R. M., Phillips A. T. Requirements for induction of the biodegradative threonine dehydratase in Escherichia coli. J Bacteriol. 1977 Nov;132(2):370–376. doi: 10.1128/jb.132.2.370-376.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foor F., Cedergren R. J., Streicher S. L., Rhee S. G., Magasanik B. Glutamine synthetase of Klebsiella aerogenes: properties of glnD mutants lacking uridylyltransferase. J Bacteriol. 1978 May;134(2):562–568. doi: 10.1128/jb.134.2.562-568.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J., Newman E. B. Derivation of glycine from threonine in Escherichia coli K-12 mutants. J Bacteriol. 1975 Jun;122(3):810–817. doi: 10.1128/jb.122.3.810-817.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Magasanik B. Urease of Klebsiella aerogenes: control of its synthesis by glutamine synthetase. J Bacteriol. 1977 Aug;131(2):446–452. doi: 10.1128/jb.131.2.446-452.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Magasanik B. Utilization of arginine by Klebsiella aerogenes. J Bacteriol. 1978 Feb;133(2):680–685. doi: 10.1128/jb.133.2.680-685.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillardin C. M., Magasanik B. Involvement of the product of the glnF gene in the autogenous regulation of glutamine synthetase formation in Klebsiella aerogenes. J Bacteriol. 1978 Mar;133(3):1329–1338. doi: 10.1128/jb.133.3.1329-1338.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. L., Elliott W. H. The enzymic formation of aminoacetone from threonine and its further metabolism. Biochem J. 1964 Sep;92(3):537–549. doi: 10.1042/bj0920537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg S., Newman E. B. Studies on L-serine deaminase in Escherichia coli K-12. J Bacteriol. 1974 Apr;118(1):53–58. doi: 10.1128/jb.118.1.53-58.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen K. A., Magasanik B. Glutamine synthetase of Klebsiella aerogenes: genetic and physiological properties of mutants in the adenylylation system. J Bacteriol. 1977 Feb;129(2):993–1000. doi: 10.1128/jb.129.2.993-1000.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN S. M., SAGERS R. D. Intermediary metabolism of Diplococcus glycinophilus. II. Enzymes of the acetategenerating system. J Bacteriol. 1962 Jan;83:121–126. doi: 10.1128/jb.83.1.121-126.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessie T. G., Whiteley H. R. Properties of threonine deaminase from a bacterium able to use threonine as sole source of carbon. J Bacteriol. 1969 Nov;100(2):878–889. doi: 10.1128/jb.100.2.878-889.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B., Prival M. J., Brenchley J. E., Tyler B. M., DeLeo A. B., Streicher S. L., Bender R. A., Paris C. G. Glutamine synthetase as a regulator of enzyme synthesis. Curr Top Cell Regul. 1974;8(0):119–138. doi: 10.1016/b978-0-12-152808-9.50010-9. [DOI] [PubMed] [Google Scholar]
- McGilvray D., Morris J. G. Utilization of L-threonine by a species of Arthrobacter. A novel catabolic role for "aminoacetone synthase". Biochem J. 1969 May;112(5):657–671. doi: 10.1042/bj1120657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E. B., Batist G., Fraser J., Isenberg S., Weyman P., Kapoor V. The use of glycine as nitrogen source by Escherichia coli K12. Biochim Biophys Acta. 1976 Jan 14;421(1):97–105. doi: 10.1016/0304-4165(76)90173-2. [DOI] [PubMed] [Google Scholar]
- Newman E. B., Kapoor V., Potter R. Role of L-threonine dehydrogenase in the catabolism of threonine and synthesis of glycine by Escherichia coli. J Bacteriol. 1976 Jun;126(3):1245–1249. doi: 10.1128/jb.126.3.1245-1249.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. Induced formation of serine and threonine deaminases by Escherichia coli. J Bacteriol. 1955 Dec;70(6):667–674. doi: 10.1128/jb.70.6.667-674.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahel G., Zelenetz A. D., Tyler B. M. gltB gene and regulation of nitrogen metabolism by glutamine synthetase in Escherichia coli. J Bacteriol. 1978 Jan;133(1):139–148. doi: 10.1128/jb.133.1.139-148.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prival M. J., Brenchley J. E., Magasanik B. Glutamine synthetase and the regulation of histidase formation in Klebsiella aerogenes. J Biol Chem. 1973 Jun 25;248(12):4334–4344. [PubMed] [Google Scholar]
- Resnick A. D., Magasanik B. L-Asparaginase of Klebsiella aerogenes. Activation of its synthesis by glutamine synthetase. J Biol Chem. 1976 May 10;251(9):2722–2728. [PubMed] [Google Scholar]
- Smith G. R., Halpern Y. S., Magasanik B. Genetic and metabolic control of enzymes responsible for histidine degradation in Salmonella typhimurium. 4-imidazolone-5-propionate amidohydrolase and N-formimino-L-glutamate formiminohydrolase. J Biol Chem. 1971 May 25;246(10):3320–3329. [PubMed] [Google Scholar]
- Streicher S. L., Bender R. A., Magasanik B. Genetic control of glutamine synthetase in Klebiella aerogenes. J Bacteriol. 1975 Jan;121(1):320–331. doi: 10.1128/jb.121.1.320-331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher S. L., Deleo A. B., Magasanik B. Regulation of enzyme formation in Klebsiella aerogenes by episomal glutamine synthetase of Escherichia coli. J Bacteriol. 1976 Jul;127(1):184–192. doi: 10.1128/jb.127.1.184-192.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B., Magasanik B. Molecular basis of transient repression of beta-galactosidase in Escherichia coli. J Bacteriol. 1969 Feb;97(2):550–556. doi: 10.1128/jb.97.2.550-556.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B. Regulation of the assimilation of nitrogen compounds. Annu Rev Biochem. 1978;47:1127–1162. doi: 10.1146/annurev.bi.47.070178.005403. [DOI] [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Threonine deamination in Escherichia coli. II. Evidence for two L-threonine deaminases. J Bacteriol. 1957 Jan;73(1):105–112. doi: 10.1128/jb.73.1.105-112.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD W. A., GUNSALUS I. C. Serine and threonine desaminaes of Escherichia coli; activators for a cell-free enzyme. J Biol Chem. 1949 Nov;181(1):171–182. [PubMed] [Google Scholar]
- Wong H. C., Lessie T. G. Hydroxy amino acid metabolism in Pseudomonas cepacia: role of L-serine deaminase in dissimilation of serine, glycine, and threonine. J Bacteriol. 1979 Oct;140(1):240–245. doi: 10.1128/jb.140.1.240-245.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui Y., Watanabe Y., Ito S., Shizuta Y., Hayaishi O. Multivalent induction of biodegradative threonine deaminase. J Bacteriol. 1977 Nov;132(2):363–369. doi: 10.1128/jb.132.2.363-369.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


