Abstract
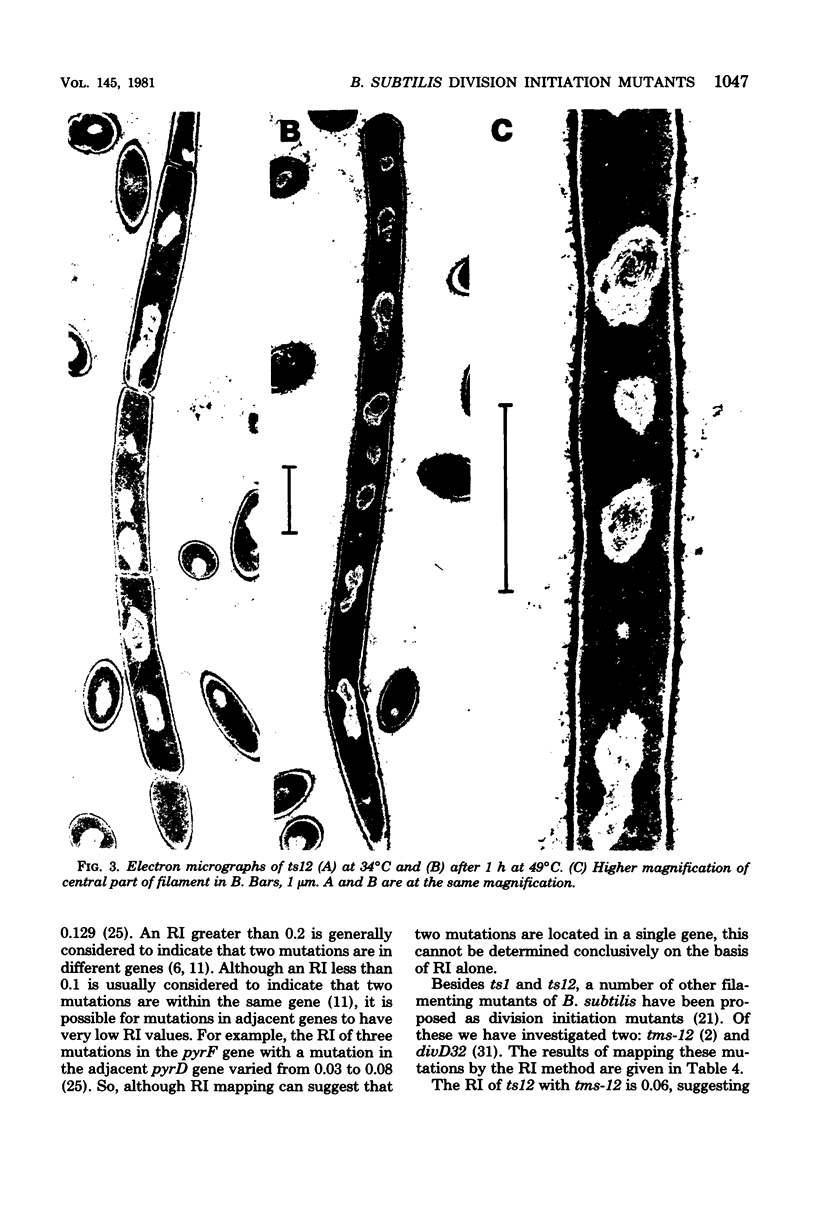
Two temperature-sensitive, filamenting mutants of Bacillus subtilis (ts1 and ts12) have been shown to be defective in the initiation of septation. Recombination index mapping showed that these mutations mapped in two different but closely linked genes. A third proposed initiation mutation, tms-12, probably maps in the same gene as ts12. Another proposed initiation mutation was not linked with these genes by transformation, indicating that there was a minimum of three genes involved in the initiation of division. PBS1 transduction mapping located these three genes close to the pyr cluster.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakefield X. O., Landman O. E. Temperature-sensitive divisionless mutant of Bacillus subtilis defective in the initiation of septation. J Bacteriol. 1973 Feb;113(2):985–998. doi: 10.1128/jb.113.2.985-998.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Septum formation in Escherichia coli: characterization of septal structure and the effects of antibiotics on cell division. J Bacteriol. 1974 Jul;119(1):303–324. doi: 10.1128/jb.119.1.303-324.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callister H., Wake R. G. Completed chromosomes in thymine-requiring Bacillus subtilis spores. J Bacteriol. 1974 Nov;120(2):579–582. doi: 10.1128/jb.120.2.579-582.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
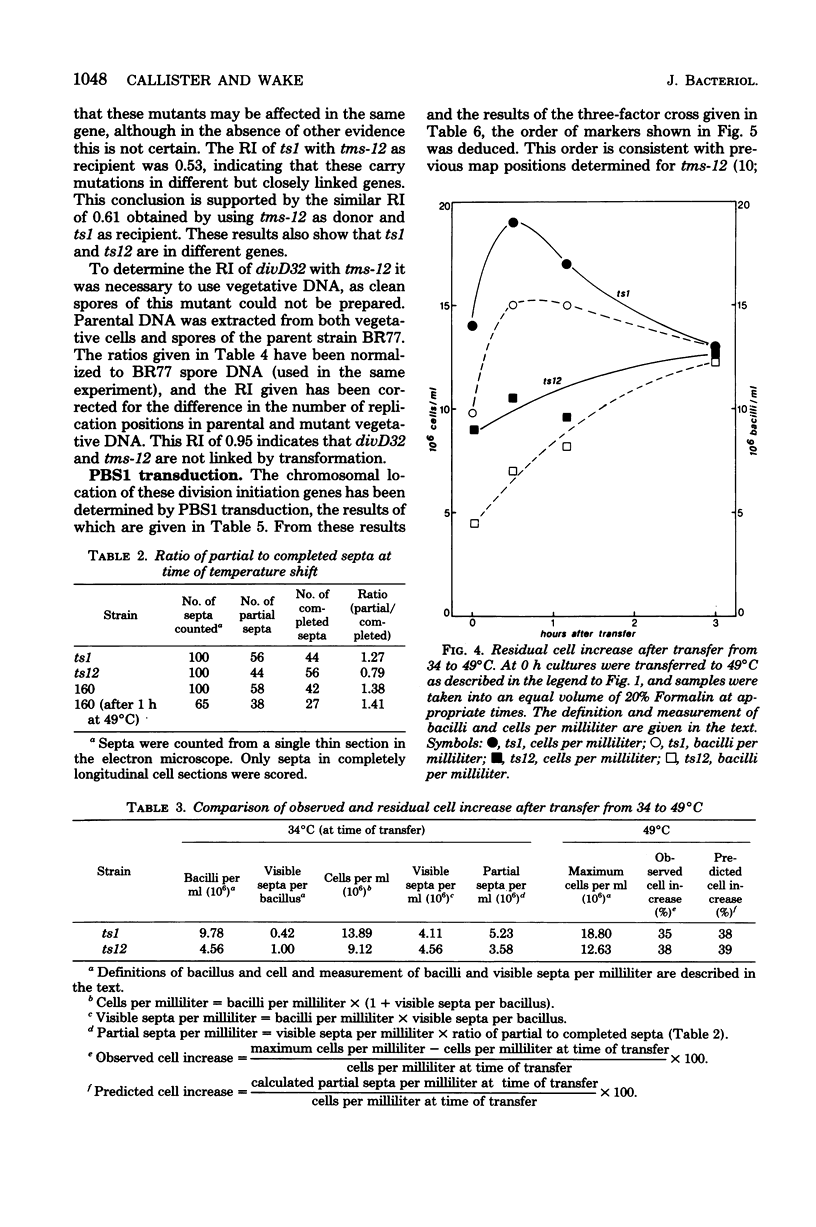
- Callister H., Wake R. G. Completion of the replication and division cycle in temperature-sensitive DNA initiation mutants of Bacillus subtilis 168 at the non-permissive temperature. J Mol Biol. 1977 Nov 25;117(1):71–84. doi: 10.1016/0022-2836(77)90023-7. [DOI] [PubMed] [Google Scholar]
- Carlton B. C. Fine-structure mapping by transformation in the tryptophan region of Bacillus subtilis. J Bacteriol. 1966 May;91(5):1795–1803. doi: 10.1128/jb.91.5.1795-1803.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote J. G. Sporulation in Bacillus subtilis. Genetic analysis of oligosporogenous mutants. J Gen Microbiol. 1972 Jun;71(1):17–27. doi: 10.1099/00221287-71-1-17. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Goldthwaite C., Smith I., Marmur J. Genetic mapping in Bacillus subtilis. J Mol Biol. 1967 Jul 14;27(1):163–185. doi: 10.1016/0022-2836(67)90358-0. [DOI] [PubMed] [Google Scholar]
- Henner D. J., Hoch J. A. The Bacillus subtilis chromosome. Microbiol Rev. 1980 Mar;44(1):57–82. doi: 10.1128/mr.44.1.57-82.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada S., Carroll L. E., Sueoka N. Genetic mapping of a group of temperature-sensitive dna initiation mutants in Bacillus subtilis. Genetics. 1980 Apr;94(4):809–823. doi: 10.1093/genetics/94.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamata D., Gross J. D. Isolation and genetic analysis of temperature-sensitive mutants of B. subtilis defective in DNA synthesis. Mol Gen Genet. 1970;108(3):277–287. doi: 10.1007/BF00283358. [DOI] [PubMed] [Google Scholar]
- Karamata D., McConnell M., Rogers H. J. Mapping of rod mutants of Bacillus subtilis. J Bacteriol. 1972 Jul;111(1):73–79. doi: 10.1128/jb.111.1.73-79.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher R. J., Jr, Gooder H. Genetics and biochemistry of pyrimidine biosynthesis in Bacillus subtilis: linkage between mutations resulting in a requirement for uracil. J Bacteriol. 1973 Nov;116(2):577–581. doi: 10.1128/jb.116.2.577-581.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper J. Gene order and co-transduction in the leu-ara-fol-pyrA region of the Salmonella typhimurium linkage map. J Bacteriol. 1974 Jan;117(1):94–99. doi: 10.1128/jb.117.1.94-99.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. F., Wolf-Watz H., Donachie W. D. Organization of genes in the ftsA-envA region of the Escherichia coli genetic map and identification of a new fts locus (ftsZ). J Bacteriol. 1980 May;142(2):615–620. doi: 10.1128/jb.142.2.615-620.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinness T., Wake R. G. Completed Bacillus subtilis nucleoid as a doublet structure. J Bacteriol. 1979 Nov;140(2):730–733. doi: 10.1128/jb.140.2.730-733.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H. Cell division suppression in the Bacillus subtilis div IC-A1 minicell-producing mutant. J Bacteriol. 1975 Mar;121(3):1166–1172. doi: 10.1128/jb.121.3.1166-1172.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Tamura G. Mutant of Escherichia coli with thermosensitive protein in the process of cellular division. J Bacteriol. 1972 Nov;112(2):959–966. doi: 10.1128/jb.112.2.959-966.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Norlander L., Grundström T., Bloom G. D., Boquet P., Frelat G. Septum formation-defective mutant of Escherichia coli. J Bacteriol. 1976 Oct;128(1):401–412. doi: 10.1128/jb.128.1.401-412.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukushina J. I., Ikeda Y. Genetic analysis of the developmental processes during germination and outgrowth of Bacillus subtilis spores with temperature-sensitive mutants. Genetics. 1969 Sep;63(1):63–74. doi: 10.1093/genetics/63.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin B. W., Kelleher R. J., Jr, Gooder H. Pyrimidine biosynthetic pathway of Baccillus subtilis. J Bacteriol. 1975 Aug;123(2):604–615. doi: 10.1128/jb.123.2.604-615.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Mendelson N. H., Coyne S. I., Hallock L. L., Cole R. M. Minicells of Bacillus subtilis. J Bacteriol. 1973 May;114(2):860–873. doi: 10.1128/jb.114.2.860-873.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M., Schaechter M. Control of cell division in bacteria. Bacteriol Rev. 1974 Jun;38(2):199–221. doi: 10.1128/br.38.2.199-221.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N., Yoshikawa H. The chromosome of Bacillus subtilis. I. Theory of marker frequency analysis. Genetics. 1965 Oct;52(4):747–757. doi: 10.1093/genetics/52.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara H., Yamane K., Maruo B. Thermosensitive, extracellular neutral proteases in Bacillus subtilis: isolation, characterization, and genetics. J Bacteriol. 1979 Aug;139(2):583–590. doi: 10.1128/jb.139.2.583-590.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upcroft P., Dyson H. J., Wake R. G. Characteristics of a Bacillus subtilis W23 mutant temperature sensitive for initiation of chromosome replication. J Bacteriol. 1975 Jan;121(1):121–127. doi: 10.1128/jb.121.1.121-127.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alstyne D., Simon M. I. Division mutants of Bacillus subtilis: isolation and PBS1 transduction of division-specific markers. J Bacteriol. 1971 Dec;108(3):1366–1379. doi: 10.1128/jb.108.3.1366-1379.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake R. G. A study of the possible extent of synthesis of repair DNA during germination of Bacillus subtilis spores. J Mol Biol. 1967 Apr 28;25(2):217–234. doi: 10.1016/0022-2836(67)90139-8. [DOI] [PubMed] [Google Scholar]
- Walker J. R., Kovarik A., Allen J. S., Gustafson R. A. Regulation of bacterial cell division: temperature-sensitive mutants of Escherichia coli that are defective in septum formation. J Bacteriol. 1975 Aug;123(2):693–703. doi: 10.1128/jb.123.2.693-703.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Watz H., Normark S. Evidence for a role of N-acetylmuramyl-L-alanine amidase in septum separation in Escherichia coli. J Bacteriol. 1976 Nov;128(2):580–586. doi: 10.1128/jb.128.2.580-586.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. Genetic mapping of sporulation operons in Bacillus subtilis using a thermosensitive sporulation mutant. J Bacteriol. 1975 Jun;122(3):1109–1116. doi: 10.1128/jb.122.3.1109-1116.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusman D. R., Inouye M., Pardee A. B. Cell division in Escherichia coli: evidence for regulation of septation by effector molecules. J Mol Biol. 1972 Aug 14;69(1):119–136. doi: 10.1016/0022-2836(72)90027-7. [DOI] [PubMed] [Google Scholar]