Abstract
The uptake and lysosomal degradation of collagen by fibroblasts constitute a major pathway in the turnover of connective tissue. However, the molecular mechanisms governing this pathway are poorly understood. Here, we show that the urokinase plasminogen activator receptor–associated protein (uPARAP)/Endo180, a novel mesenchymally expressed member of the macrophage mannose receptor family of endocytic receptors, is a key player in this process. Fibroblasts from mice with a targeted deletion in the uPARAP/Endo180 gene displayed a near to complete abrogation of collagen endocytosis. Furthermore, these cells had diminished initial adhesion to a range of different collagens, as well as impaired migration on fibrillar collagen. These studies identify a central function of uPARAP/Endo180 in cellular collagen interactions.
Keywords: cell adhesion; integrin; matrix internalization; matrix metalloproteinase; uPAR
Introduction
The capacity of multicellular organisms to remodel the ECM is essential for development, homeostasis, and postnatal tissue remodeling, but also for the progression of many degenerative and proliferative diseases, including cancer. Both physiological and pathological matrix remodeling depend on an intricate interplay between cell motility factors, cell matrix adhesion receptors, and cell surface–associated proteases (Murphy and Gavrilovic, 1999). The interstitial collagens are the most abundant protein constituents of connective tissues and they, like other matrix components, are undergoing continuous synthesis and degradation. The pathway proposed to be the major clearance mechanism for collagen under steady-state conditions involves the specific binding of collagen fibrils to the cell surface, followed by the cellular uptake and degradation of the internalized collagen in the lysosomal compartment (Everts et al., 1996). However, unlike the alternative turnover mechanisms that are dominated by extracellular collagen degradation, this pathway is still poorly understood. It is believed to be primarily operative in fibroblasts, and results in the degradation of denatured collagen by proteases within the acidified lysosomal environment (Kielty et al., 1993; Lee et al., 1996; Segal et al., 2001). However, the molecular mechanisms behind the cellular internalization of the collagen substrate are largely unknown.
The urokinase plasminogen activator receptor–associated protein (uPARAP)*/Endo180 is a novel multi-domain transmembrane glycoprotein that was identified through its specific interaction with receptor-bound pro-urokinase plasminogen activator on the surface of certain cultured cells (Behrendt et al., 2000) and, independently, as a constitutive endocytic recycling glycoprotein (Endo180) that is capable of internalizing mAbs directed against it (Howard and Isacke, 2002; Sheikh et al., 2000). uPARAP/Endo180 is a member of the macrophage mannose receptor family of type I transmembrane glycoproteins (Engelholm et al., 2001a). The distinct and highly conserved domain structure that characterizes this protein family includes an NH2-terminal, cysteine-rich ricin B lectin-like domain, a fibronectin type II (FN-II) domain, a series of 8–10 domains related to C-type carbohydrate recognition domains, a transmembrane domain, and a short COOH-terminal cytoplasmic tail (Sonnhammer et al., 1998; Bateman et al., 1999; Engelholm et al., 2001a). uPARAP/Endo180 is highly expressed on osteoblasts and osteocytes at sites of endochondral and intramembranous ossification during development (Engelholm et al., 2001b). The postnatal expression of uPARAP/Endo180 is restricted to specific subsets of fibroblasts, macrophages, and endothelial cells (Sheikh et al., 2000). The biochemical functions of uPARAP/Endo180 have yet to be elucidated. However, several conspicuous properties of the receptor suggest that uPARAP/Endo180 may be involved in the remodeling of the ECM and/or modulation of the localization or availability of soluble ligands in the pericellular environment (Hanasaki and Arita, 1999; Martinez-Pomares et al., 1999; Sheikh et al., 2000; Engelholm et al., 2001a). Of particular interest, initial studies on uPARAP/Endo180 revealed that the uPAR-dependent binding of pro-urokinase plasminogen activator to this receptor is blocked efficiently by low concentrations of collagen type V, suggesting a direct interaction with this constituent of the ECM. This function is likely to be mediated by the FN-II domain because this domain type is typically associated with collagen binding (Ancian et al., 1995). In this work, we generated a targeted deletion in the uPARAP/Endo180 gene and show that uPARAP/Endo180 is essential for the uptake of collagen by fibroblasts, and that it has important functions in fibroblast adhesion and migration on fibrillar collagen matrices.
Results and discussion
Targeted inactivation of the uPARAP/Endo180 gene in mice
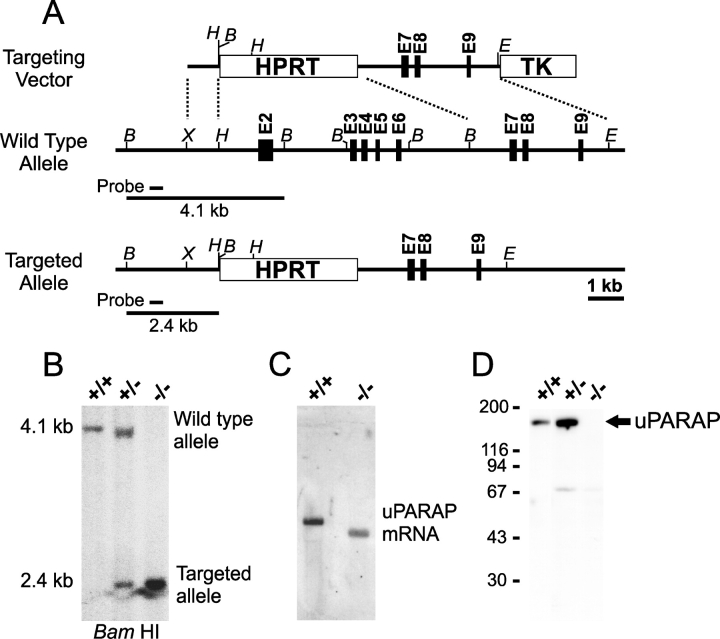
We targeted the uPARAP/Endo180 gene in mice by replacing exons 2–6 with an HPRT expression cassette (Fig. 1 A). The correct targeting of the uPARAP/Endo180 gene was confirmed by Southern hybridization (Fig. 1 B) and by the presence of a truncated uPARAP/Endo180 transcript in mice homozygous for the insertion (uPARAP/Endo180−/− mice), as determined by Northern blot hybridization (Fig. 1 C). Western blot analysis of extracts of cultured fibroblasts from uPARAP/Endo180−/− mice, using an antibody directed against an epitope (residue 809–829) located outside of the introduced deletion, confirmed the absence of intact uPARAP/Endo180 (Fig. 1 D). A weak band with an apparent Mr of 70 kD was observed in Western blots of homozygously targeted as well as wild-type and heterozygous mice (unpublished data). This product was seen in all genotypes, suggesting that it was due to a weak cross-reactivity of the antibody rather than a putative truncated uPARAP/Endo180 gene product. However, it should be noted that even though formation of truncated uPARAP/Endo180 in the targeted mice could not be formally excluded, any such product would be devoid of the FN-II domain due to the targeting strategy (Fig. 1 A, legend). uPARAP/Endo180−/− mice were born in a Mendelian ratio and were outwardly normal, viable, and able to reproduce. The observations documenting these conclusions are summarized in Fig. S1 and legend (available at http://www.jcb.org/cgi/content/full/jcb.200211091/DC1).
Figure 1.
Generation of uPARAP/Endo180-targeted mice. (A) Diagram of the targeting strategy showing the structure of the uPARAP/Endo180-targeting vector (top), wild-type uPARAP/Endo180 allele (middle), and targeted uPARAP/Endo180 allele (bottom). Exons 2–6 of the uPARAP/Endo180 gene (encoding the cysteine-rich, the FN-II, and the first carbohydrate recognition domains) were replaced by the HPRT selection cassette. Exons are indicated with boxes and introns with a solid line. B, BamHI; E, EcoRI; H, HindIII; X, XhoI. (B) Southern blot of BamHI-digested mouse tail DNA from mice genotyped by PCR as uPARAP/Endo180+/+ (left lane), uPARAP/Endo180+/− (middle lane), and uPARAP/Endo180−/− (right lane). The hybridization probe (solid line below the wild-type allele in A) was located upstream of the targeted area. The expected DNA fragments of the wild-type (4.1 kb) and targeted (2.4 kb) alleles that hybridize to the probe are indicated with solid lines in A. (C) Northern blot of total RNA isolated from cultured fibroblasts. RNA from fibroblasts of mice genotyped as uPARAP/Endo180+/+ (left lane) and uPARAP/Endo180−/− (right lane) was hybridized with a cDNA probe complementary to the 3′ end of the uPARAP/Endo180 mRNA. (D) Western blot of lysates of cultured dermal fibroblasts from neonates genotyped as uPARAP/Endo180+/+ (left lane), uPARAP/Endo180+/− (middle lane), and uPARAP/Endo180−/− (right lane). The blot was probed with a murine anti–uPARAP/Endo180 peptide antibody prepared as described in Materials and methods. The positions of molecular mass markers (kD) are indicated left.
uPARAP/Endo180 is required for collagen internalization
Dermal fibroblasts abundantly express uPARAP/Endo180 (Engelholm et al., 2001b). Therefore, we generated matched pairs of uPARAP/Endo180−/− and uPARAP/Endo180+/+ littermate control fibroblasts, providing an ideal tool for studying the function of uPARAP/Endo180 in cell matrix interactions and the association of uPARAP/Endo180 with extracellular ligands. We investigated if uPARAP/Endo180 serves directly as an internalization receptor for collagen. The capacity of uPARAP/Endo180−/− fibroblasts to internalize 125I-labeled collagen was determined and compared with the internalization of 125I-labeled holotransferrin, matrix metalloproteinase (MMP)-13, and fibronectin as independent controls.
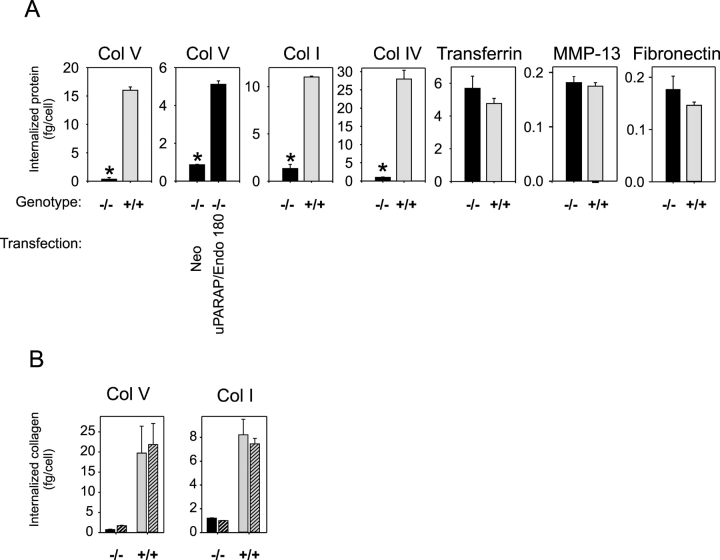
Strikingly, the uPARAP/Endo180-deficient fibroblasts displayed a virtually complete abrogation of the cellular uptake of collagen types I, IV, and V (Fig. 2 A, panels 3, 4, and 1, respectively). This deficiency in collagen internalization was observed with several independent isolates of uPARAP/Endo180−/− fibroblasts and could be alleviated by the reintroduction of uPARAP/Endo180 by transient transfection of the cells with a uPARAP/Endo180 cDNA expression vector (Fig. 2 A, panel 2; unpublished data). In contrast, the cellular uptake of holotransferrin, MMP-13, and fibronectin was unaffected (Fig. 2 A, panels 5–7). The internalization of MMP-13 has been shown previously to be uPARAP/Endo180-independent (Bailey et al., 2002), but depends on the low density lipoprotein receptor–related protein (LRP; Barmina et al., 1999). Therefore, as an additional test of our assay system, we included samples with LRP-deficient cells (LRP−/− and matched controls) in the same manner to study the internalization of 125I-labeled MMP-13. This experiment confirmed the essential role of LRP in this process (P < 0.0007), whereas LRP deficiency had no effect on the cellular uptake of 125I-labeled collagen, examined as described for uPARAP/Endo180−/− cells (unpublished data). These data show that uPARAP/Endo180 has a critical and specific role in the uptake of collagen by fibroblasts.
Figure 2.
uPARAP/Endo180 is required for collagen internalization. (A) Representative examples of the ability of dermal fibroblasts from uPARAP/Endo180−/− (black bars) or littermate uPARAP/Endo180+/+ (gray bars) neonates to internalize the 125I-labeled ligands indicated above each panel. Col I, IV, and V designate collagen subtypes. In panel 2, uPARAP/Endo180−/− fibroblasts were transiently transfected with a uPARAP/Endo180 expression plasmid, or with a neomycin resistance gene control expression plasmid, as indicated. The transfection efficiency was ∼10%. The data are expressed as the total amount of 125I-labeled ligand internalized per cell within a 3-h period at 37°C. Error bars indicate SDs. Asterisk in Col V, P < 0.000001; Col V in transfected cells, P < 0.000003; Col I, P < 0.00000001; and Col IV, P < 0.00005. (B) Cells with the genotype indicated were preincubated in the presence (hatched columns) or absence (unhatched columns) of a blocking antibody against β1 integrins, and the internalization of collagen V and I was measured as above.
Fibroblasts are a dominant cell type in collagen degradation (Everts et al., 1996), but the underlying molecular pathway is still poorly understood and only a few molecules critical to the process have been identified. Integrin α2β1 is known to be involved in cellular collagen interactions and has been proposed to play a role in the initial phase of the internalization process (Kielty et al., 1993; Lee et al., 1996; Segal et al., 2001). Therefore, we studied the effect of antibody-mediated blocking of β1 integrins on collagen internalization by wild-type and uPARAP/Endo180−/−cells (Fig. 2 B). Strikingly, anti-β1 antibody had little or no effect on internalization (hatched bars), even though the same mAb was indeed capable of efficiently blocking β1 integrin function as shown in cell adhesion studies (see next section). Thus, uPARAP/Endo180-dependent internalization of collagen can occur in the absence, or at least with only very low levels, of accessible β1 integrin on the cell surface.
The cellular uptake of collagen has been reported to be insensitive to inhibitors of MMPs, serine proteases, and cysteine proteases, and extensive degradation of collagen before the internalization step does not seem to be needed (Everts et al., 1988, 1989). The major degradation processes in this pathway occur after the cellular uptake event, by the fusion of collagen-containing intracellular vesicles with lysosomes and the degradation of acid-denatured collagen by lysosomal proteases (Everts et al., 1988, 1996; Everts and Beertsen, 1992). Our current demonstration of a crucial function of uPARAP/Endo180 in the actual cellular internalization of collagen thus addresses a central step in this series of events.
uPARAP/Endo180-deficient cells display delayed adhesion to collagen matrices
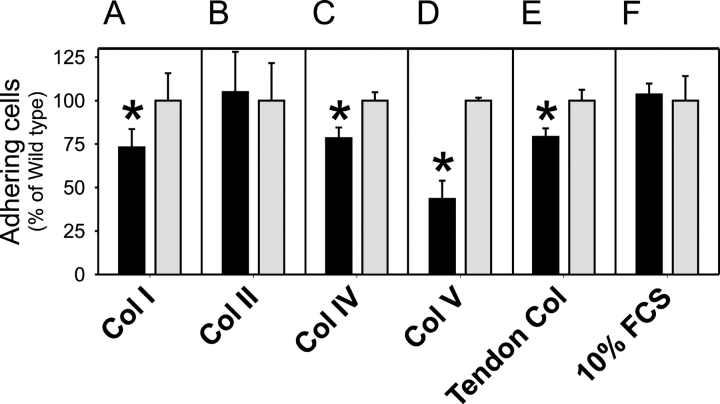
We used a standard cell adhesion assay, in which cells are allowed to attach briefly to immobilized collagens, to investigate if the lack of uPARAP/Endo180 could directly influence cell adhesion. Loss of uPARAP/Endo180 resulted in a 50% reduction in the adhesion of fibroblasts to type V collagen-coated surfaces (Fig. 3 D). This impairment was observed in comparisons of several independent isolates of uPARAP/Endo180−/− fibroblasts with littermate control uPARAP/Endo180+/+ fibroblasts (unpublished data). Interestingly, uPARAP/Endo180−/− cells also demonstrated a reduced ability to adhere to other immobilized collagens, including purified types I and IV collagen and total tendon collagen (Fig. 3, A, C, and E), although the reduction in these cases was less dramatic. In contrast, uPARAP/Endo180 deficiency did not affect the adhesion of fibroblasts to a noncollagen substrate such as fibronectin (Fig. S2 C) or a surface coated with total serum proteins (Fig. 3 F). A time-course study of cell adhesion to collagen V and collagen I showed that the effect of uPARAP/Endo180 deficiency was limited to the initial phase (15–30 min after plating), and wild-type and targeted cells reached the same level of adhesion after 1–2 h (unpublished data).
Figure 3.
uPARAP/Endo180 promotes cell adhesion to collagen. Dermal fibroblasts from uPARAP/Endo180−/− (black bars) or littermate uPARAP/Endo180+/+ neonates (gray bars) were allowed to attach for 30 min at 37°C to tissue culture wells coated with collagens type I (A), type II (B), type IV (C), type V (D), total tendon collagen (E), or serum proteins from 10% FCS (F). The number of cells adhering to the different matrices was determined by an MTT assay and expressed as the percentage of adherent uPARAP/Endo180+/+ cells. Error bars indicate SDs of quadruplicate determinations. The data are representative of results obtained with three different sets of uPARAP/Endo180−/− and littermate control fibroblasts. Asterisk in A, P < 0.03; C, P < 0.002; D, P < 0.0004; and E, P < 0.002.
Because α2β1 integrin is considered important in various cellular interactions with collagen as stated in the previous section, it was important to determine if the role of uPARAP/Endo180 in these adhesion processes was dependent on additional integrin-mediated interactions. Therefore, we performed a new series of adhesion experiments in the presence of the same blocking antibody against β1 as used above in the internalization studies, or an irrelevant control antibody (anti-TNP). This experiment (Fig. S2) confirmed the central function of β1 in fibroblast adhesion to collagen, as well as to fibronectin used as a positive control. Adhesion to collagen I, collagen V, and fibronectin-coated surfaces was reduced to levels close to baseline by the anti-β1 antibody, irrespective of whether uPARAP/Endo180−/− or uPARAP/Endo180+/+ fibroblasts were used. The effect of uPARAP/Endo180 deficiency on collagen adhesion observed in the absence of anti-β1 antibody, however, was not due to a down-regulation of this integrin on the targeted cells. Thus, flow cytometry analysis (Fig. S3) showed that β1 expression levels on wild-type and uPARAP−/− cells were identical or closely similar. The two cell types also had indistinguishable morphologies after plating on various matrices (Fig. S4).
These experiments revealed that uPARAP/Endo180 has an early modulatory function in fibroblast adhesion to collagen matrices, whereas β1 integrins appear to be indispensable for this process. Thus, our observations make it tempting to speculate that uPARAP/Endo180 is critical for fully effective initial cellular interactions with collagen, this role having an impact on collagen adhesion, beyond its crucial role in collagen internalization. A simple cell binding assay with solubilized 125I-labeled collagen V at 4°C supported this notion, as a >50% reduction in binding was observed with uPARAP/Endo180−/− cells (unpublished data).
uPARAP/Endo180 deficiency impairs the migration of fibroblasts on collagen fibrils
Cell migration is intimately linked with adhesion to the ECM (Murphy and Gavrilovic, 1999). To directly test if uPARAP is important for cellular migration on collagen, we performed single-cell, parallel time-lapse video microscopy of matched pairs of primary dermal uPARAP/Endo180−/− and littermate control fibroblasts on a mixed fibrillar collagen matrix (Fig. 4, A and B). uPARAP/Endo180−/− cells demonstrated a significant and reproducible impairment in their migration, displaying a >30% reduction in the average migration rate. The mechanistic details of this effect await further studies. One role of uPARAP/Endo180 in cell migration may be directly related to increasing cellular adhesion. However, uPARAP/Endo180-mediated collagen internalization may also promote migration by increasing adhesion site turnover, thereby facilitating cell spreading. Finally, the interplay between uPARAP/Endo180 and integrins may influence integrin-mediated signaling.
Figure 4.
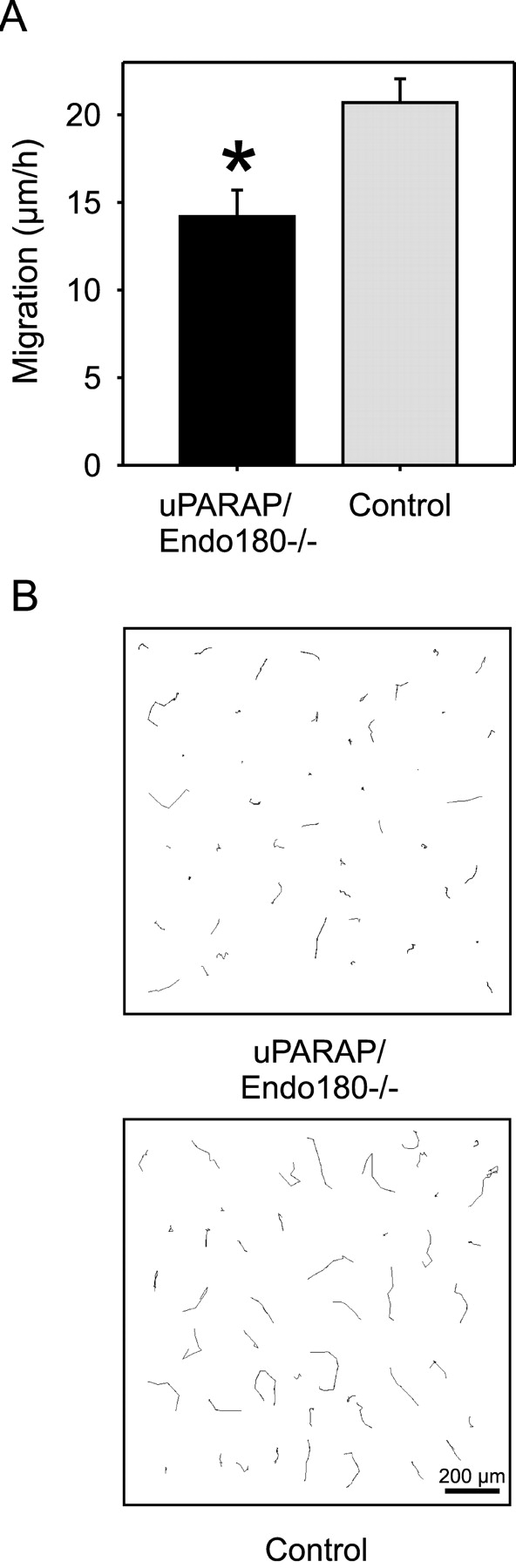
uPARAP/Endo180 promotes cell migration on collagen. (A) The migration of dermal fibroblasts from uPARAP/Endo180−/− (black bar) or littermate control neonates (gray bar) on total tendon collagen was tracked for 6 h by time-lapse video microscopy. Data from individual cells of each genotype were collected in parallel recordings, and the rate of migration was calculated by pooling four independent sets of experiments, each showing similar results, that included cells from a total of four different uPARAP/Endo180−/− mice and five littermate control mice. A total of 52 uPARAP/Endo180−/− cells and 82 control cells were included in the measurements. Error bars indicate the standard error of the migration rates. Asterisk, P < 0.0016. (B) Migration paths on tendon collagen from 46 individual uPARAP/Endo180−/− and 46 individual control fibroblasts selected randomly. The cells were tracked for 6 h by time-lapse microscopy. Size bar is indicated in the lower right corner of the control cell panel.
In summary, we have shown that uPARAP/Endo180, a mesenchymal cell surface receptor, has a pivotal function in collagen internalization, facilitates the initial adhesion of fibroblasts to collagen, and accelerates the migration of fibroblasts on a fibrillar collagen matrix. The fact that uPARAP/Endo180-targeted mice do not present an obvious, aberrant phenotype makes it clear that uPARAP/Endo180 deficiency is tolerable in the absence of external challenges. Several pathways for collagen turnover operate in parallel (Holmbeck et al., 1999), and apparently these mechanisms can functionally compensate for the loss of uPARAP/Endo180 to an extent that prevents a robust presentation of a phenotype. The generation of mice with combined deficiencies in several collagen degradation pathways may provide valuable information as to the interplay and functional overlap between the pericellular and endocytic pathways of collagen degradation, and may shed more light on the specific functions of uPARAP/Endo180 in vivo.
Materials and methods
uPARAP/Endo180 gene targeting
uPARAP/Endo180 gene targeting was accomplished by replacement of exons 2–6 with an HPRT cassette in embryonic stem cells, followed by the generation of chimeric mice and interbreeding of chimeric offspring carrying the disrupted uPARAP/Endo180 allele (Fig. 1 A). uPARAP/Endo180−/− mice were derived from two independently targeted embryonic stem cell clones (see supplemental materials and methods for further details).
Western blot analysis
Synthesis of peptide 809–829 of the murine uPARAP/Endo180 sequence (GenBank/EMBL/DDBJ accession no. AAC52729), immunization of rabbits, affinity purification of antibodies and Western blotting of cell lysates were performed as described previously (Schnack Nielsen et al., 2002) using the antibody at a final concentration of 2 μg/ml.
Ligand internalization assay
Primary skin fibroblasts were isolated from neonates as described previously (Holmbeck et al., 1999). 20 μg type I collagen, type IV collagen, type V collagen, MMP-13, human holotransferrin (all from Calbiochem-Novachem), and human plasma fibronectin (Sigma-Aldrich) were labeled with 125I as described previously (Behrendt et al., 1996). Cellular ligand internalization assays were performed as described previously (Hahn-Dantona et al., 2001). In brief, samples of 105 cells were seeded in 24-well tissue culture plates and cultured in DME, 10% FCS until near confluence. The cells were washed gently in DME at 37°C and were then cultured for at least 1 h in binding buffer (DME, 20 mM Hepes, pH 7.4, 15 mg/ml BSA, and 1× Nutridoma-[SP] serum substitute; Roche). The medium was removed and replaced by binding buffer with 125I-labeled protein ranging in concentration from 0.5 to 5 nM, followed by incubation at 37°C. In some experiments, the cells were preincubated for 30 min at 4°C with 10 μg/ml anti-β1 antibody (HA2/5) or 10 μg/ml isotype-matched control anti-TNP antibody (G235–1; both from BD Biosciences) before the addition of labeled ligand. The cells were then washed twice with ice-cold PBS, and incubated <2 min at 4°C with 50 μg/ml trypsin, 50 μg/ml proteinase K, and 0.53 mM EDTA in HBSS. The detached cells were centrifuged at 3,000 rpm for 5 min at 4°C, and the radioactivity in the pellet (internalized material) and supernatant (surface-released material) was measured in a gamma counter. Statistical significance was calculated by two-tailed t test.
Transient transfection of primary fibroblasts
Transfection of cultured fibroblasts was performed essentially as described previously (Kjøller and Hall, 2001), using the vector pcDNA3-uPARAP/Endo180 (provided by Dr. Clare Isacke, Chester Beatty Laboratories, London, UK; Sheikh et al., 2000). The transfection efficiency, estimated by determination of the percentage of GFP-expressing cells after cotransfection with pEGF-P1 (CLONTECH Laboratories, Inc.) was ∼10%.
Cell adhesion assay
96-well plates were coated for 1 h at 37°C with either 10% FCS in DME, or with rat tail tendon collagen (provided by Dr. Jack Windsor, University of Indianapolis, Indianapolis, IN), human type I collagen, murine type II collagen, human type IV collagen, or human type V collagen (all from Calbiochem-Novabiochem), at 20 μg/ml in 10 mM acetic acid. The coated wells were washed three times with PBS, and the residual binding sites were blocked by incubation with 0.2% BSA in PBS for 1 h at 37°C, followed by three additional washes with PBS at RT. Primary fibroblasts were detached by mild trypsinization, washed, resuspended in serum-free medium with 0.2% BSA, and incubated at 37°C for 30 min with gentle rotation to recover from trypsinization. Cell viability was determined by Trypan blue exclusion. 5 × 104 cells were added to each well and were allowed to attach for 30 min at 37°C. Nonadherent cells were removed by two gentle washes with 200 μl PBS, and the number of adherent cells was determined by MTT analysis (Liu et al., 2001). In some experiments, cells were preincubated with anti-β1 antibody or control antibody as specified above. Statistical significance was calculated by two-tailed t test.
Cell migration assay
Tissue culture plates with a glass coverslip bottom (MatTek Corporation) were coated with rat tail tendon collagen as described above. The dishes were washed twice with PBS, and incubated for 1 h at 37°C with 2% heat-denatured BSA in PBS to block residual binding sites. The integrity of the collagen fibrils was verified by incubation with purified trypsin and MMP-9 (Netzel-Arnett et al., 2002). uPARAP/Endo180−/− or uPARAP/Endo180-expressing (uPARAP/Endo180+/+ or +/−) littermate control fibroblasts (1,000 cells/well) were seeded overnight on the collagen layer in DME containing 10% FCS. FCS was included during seeding and microscopy due to the long duration of the experiments. Cell migration was analyzed by time-lapse video microscopy as described previously (Gu et al., 1999). In brief, cell movements were recorded using inverted microscopes (Carl Zeiss MicroImaging, Inc.), collecting video images with tube cameras (Newvicon model 2400; Hamamatsu Photonics) at 10-min intervals for at least 6 h. From the individual cell tracks, cell velocities were calculated using MetaMorph® 4.6 software (Universal Imaging Corporation). A one-way ANOVA and a Bonferroni multiple comparisons post-test were performed using InStat® software (GraphPad Software, Inc.) to determine the statistical significance between samples. The experiment was repeated with four independently isolated pairs of uPARAP/Endo180−/− and littermate-matched uPARAP/Endo180+ fibroblasts, and included a total of 52 and 82 individual measurements, respectively.
Flow cytometry
Cells were detached in PBS with 5 mM EDTA, 5 mg/ml BSA, washed twice in binding buffer (PBS with 0.1 mg/ml CaCl2, 0.05 mg/ml MgCl2, 1 mg/ml NaN3, and 5 mg/ml BSA), and incubated for 30 min at 4°C with either FITC-conjugated anti-β1 integrin antibody (HA2/5) or FITC-conjugated isotype-matched control anti-TNP antibody (G235–1), using 5 μg of antibody for 106 cells in 200 μl binding buffer. After incubation, cells were washed twice in ice-cold binding buffer and fixed in 1% PFA in binding buffer without NaN3. Flow cytometry analysis was then performed using a FACsort™ instrument (Becton Dickinson).
Online supplemental material
Supplemental materials and methods, and additional primary data are available at (http://www.jcb.org/cgi/content/full/jcb.200211091/DC1).
Supplemental Material
Acknowledgments
We thank the National Institute of Dental and Craniofacial Research gene targeting core for blastocyst injections, Drs. Mary Jo Danton and Silvio Gutkind for critically reading the manuscript, Dr. Arne Holm (The Royal Veterinary and Agricultural University, Copenhagen, Denmark) for peptide synthesis, Dr. Joachim Herz (University of Texas, Southwestern Medical Center, Dallas, TX) for LRP-deficient cells, and Jette K. Christiansen and Anders Sørensen for excellent technical assistance.
This work was supported by grants from the Danish Cancer Society, the Danish Cancer Research Foundation, the Danish Research Council, and the Danish Biotechnology Program (to L.H. Engelholm, K. Dano, and N. Behrendt).
The online version of this article includes supplemental material.
Footnotes
Abbreviations used in this paper: FN-II, fibronectin type II; MMP, matrix metalloproteinase; uPARAP, urokinase plasminogen activator receptor–associated protein.
References
- Ancian, P., G. Lambeau, and M. Lazdunski. 1995. Multifunctional activity of the extracellular domain of the M-type (180 kDa) membrane receptor for secretory phospholipases A2. Biochemistry. 34:13146–13151. [DOI] [PubMed] [Google Scholar]
- Bailey, L., D. Wienke, M. Howard, V. Knauper, C.M. Isacke, and G. Murphy. 2002. Investigation of the role of Endo180/urokinase-type plasminogen activator receptor-associated protein as a collagenase 3 (matrix metalloproteinase 13) receptor. Biochem. J. 363:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmina, O.Y., H.W. Walling, G.J. Fiacco, J.M. Freije, C. Lopez-Otin, J.J. Jeffrey, and N.C. Partridge. 1999. Collagenase-3 binds to a specific receptor and requires the low density lipoprotein receptor-related protein for internalization. J. Biol. Chem. 274:30087–30093. [DOI] [PubMed] [Google Scholar]
- Bateman, A., E. Birney, R. Durbin, S.R. Eddy, R.D. Finn, and E.L. Sonnhammer. 1999. Pfam 3.1: 1313 multiple alignments and profile HMMs match the majority of proteins. Nucleic Acids Res. 27:260–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt, N., E. Rønne, and K. Danø. 1996. Domain interplay in the urokinase receptor. Requirement for the third domain in high affinity ligand binding and demonstration of ligand contact sites in distinct receptor domains. J. Biol. Chem. 271:22885–22894. [DOI] [PubMed] [Google Scholar]
- Behrendt, N., O.N. Jensen, L.H. Engelholm, E. Mørtz, M. Mann, and K. Danø. 2000. A urokinase receptor-associated protein with specific collagen binding properties. J. Biol. Chem. 275:1993–2002. [DOI] [PubMed] [Google Scholar]
- Engelholm, L.H., B.S. Nielsen, K. Danø, and N. Behrendt. 2001. a. The urokinase receptor associated protein (uPARAP/endo180): a novel internalization receptor connected to the plasminogen activation system. Trends Cardiovasc. Med. 11:7–13. [DOI] [PubMed] [Google Scholar]
- Engelholm, L.H., B.S. Nielsen, S. Netzel-Arnett, H. Solberg, X.D. Chen, J.M. Lopez Garcia, C. Lopez-Otin, M.F. Young, H. Birkedal-Hansen, K. Danø, et al. 2001. b. The urokinase plasminogen activator receptor-associated protein/endo180 is coexpressed with its interaction partners urokinase plasminogen activator receptor and matrix metalloprotease-13 during osteogenesis. Lab. Invest. 81:1403–1414. [DOI] [PubMed] [Google Scholar]
- Everts, V., and W. Beertsen. 1992. Phagocytosis of collagen fibrils by periosteal fibroblasts in long bone explants. Effect of concanavalin A. Tissue Cell. 24:935–941. [DOI] [PubMed] [Google Scholar]
- Everts, V., W. Beertsen, and R. Schroder. 1988. Effects of the proteinase inhibitors leupeptin and E-64 on osteoclastic bone resorption. Calcif. Tissue Int. 43:172–178. [DOI] [PubMed] [Google Scholar]
- Everts, V., R.M. Hembry, J.J. Reynolds, and W. Beertsen. 1989. Metalloproteinases are not involved in the phagocytosis of collagen fibrils by fibroblasts. Matrix. 9:266–276. [DOI] [PubMed] [Google Scholar]
- Everts, V., E. van der Zee, L. Creemers, and W. Beertsen. 1996. Phagocytosis and intracellular digestion of collagen, its role in turnover and remodelling. Histochem. J. 28:229–245. [DOI] [PubMed] [Google Scholar]
- Gu, J., M. Tamura, R. Pankov, E.H. Danen, T. Takino, K. Matsumoto, and K.M. Yamada. 1999. Shc and FAK differentially regulate cell motility and directionality modulated by PTEN. J. Cell Biol. 146:389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn-Dantona, E., J.F. Ruiz, P. Bornstein, and D.K. Strickland. 2001. The low density lipoprotein receptor-related protein modulates levels of matrix metalloproteinase 9 (MMP-9) by mediating its cellular catabolism. J. Biol. Chem. 276:15498–15503. [DOI] [PubMed] [Google Scholar]
- Hanasaki, K., and H. Arita. 1999. Biological and pathological functions of phospholipase A(2) receptor. Arch. Biochem. Biophys. 372:215–223. [DOI] [PubMed] [Google Scholar]
- Holmbeck, K., P. Bianco, J. Caterina, S. Yamada, M. Kromer, S.A. Kuznetsov, M. Mankani, P.G. Robey, A.R. Poole, I. Pidoux, et al. 1999. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 99:81–92. [DOI] [PubMed] [Google Scholar]
- Howard, M.J., and C.M. Isacke. 2002. The C-type lectin receptor Endo180 displays internalization and recycling properties distinct from other members of the mannose receptor family. J. Biol. Chem. 277:32320–32331. [DOI] [PubMed] [Google Scholar]
- Kielty, C.M., M. Lees, C.A. Shuttleworth, and D. Woolley. 1993. Catabolism of intact type VI collagen microfibrils: susceptibility to degradation by serine proteinases. Biochem. Biophys. Res. Commun. 191:1230–1236. [DOI] [PubMed] [Google Scholar]
- Kjøller, L., and A. Hall. 2001. Rac mediates cytoskeletal rearrangements and increased cell motility induced by urokinase-type plasminogen activator receptor binding to vitronectin. J. Cell Biol. 152:1145–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, W., J. Sodek, and C.A. McCulloch. 1996. Role of integrins in regulation of collagen phagocytosis by human fibroblasts. J. Cell. Physiol. 168:695–704. [DOI] [PubMed] [Google Scholar]
- Liu, S., T.H. Bugge, and S.H. Leppla. 2001. Targeting of tumor cells by cell surface urokinase plasminogen activator-dependent anthrax toxin. J. Biol. Chem. 276:17976–17984. [DOI] [PubMed] [Google Scholar]
- Martinez-Pomares, L., P.R. Crocker, R. Da Silva, N. Holmes, C. Colominas, P. Rudd, R. Dwek, and S. Gordon. 1999. Cell-specific glycoforms of sialoadhesin and CD45 are counter-receptors for the cysteine-rich domain of the mannose receptor. J. Biol. Chem. 274:35211–35218. [DOI] [PubMed] [Google Scholar]
- Murphy, G., and J. Gavrilovic. 1999. Proteolysis and cell migration: creating a path? Curr. Opin. Cell Biol. 11:614–621. [DOI] [PubMed] [Google Scholar]
- Netzel-Arnett, S., D.J. Mitola, S.S. Yamada, K. Chrysovergis, K. Holmbeck, H. Birkedal-Hansen, and T.H. Bugge. 2002. Collagen dissolution by keratinocytes requires cell surface plasminogen activation and matrix metalloproteinase activity. J. Biol. Chem. 277:45154–45161. [DOI] [PubMed] [Google Scholar]
- Schnack Nielsen, B., F. Rank, L.H. Engelholm, A. Holm, K. Danø, and N. Behrendt. 2002. Urokinase receptor-associated protein (uPARAP) is expressed in connection with malignant as well as benign lesions of the human breast and occurs in specific populations of stromal cells. Int. J. Cancer. 98:656–664. [DOI] [PubMed] [Google Scholar]
- Segal, G., W. Lee, P.D. Arora, M. McKee, G. Downey, and C.A. McCulloch. 2001. Involvement of actin filaments and integrins in the binding step in collagen phagocytosis by human fibroblasts. J. Cell Sci. 114:119–129. [DOI] [PubMed] [Google Scholar]
- Sheikh, H., H. Yarwood, A. Ashworth, and C.M. Isacke. 2000. Endo180, an endocytic recycling glycoprotein related to the macrophage mannose receptor is expressed on fibroblasts, endothelial cells and macrophages and functions as a lectin receptor. J. Cell Sci. 113:1021–1032. [DOI] [PubMed] [Google Scholar]
- Sonnhammer, E.L., S.R. Eddy, E. Birney, A. Bateman, and R. Durbin. 1998. Pfam: multiple sequence alignments and HMM-profiles of protein domains. Nucleic Acids Res. 26:320–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.