Abstract
This study demonstrates that several CC-chemokines, including those that inhibit entry and replication of macrophage-tropic strains of HIV, increase the replication of T cell (T)-tropic strains in CD4+ T cells. Enhancement of T-tropic HIV replication is observed at early stages of replication, requires signaling through inhibitory guanine nucleotide-binding regulatory (Gi) proteins, and is associated with increased cell surface colocalization of CD4 and the T-tropic HIV coreceptor CXCR4. These findings may further our understanding of the factors that influence the replication and spread of T-tropic strains of HIV in vivo and suggest that the use of cell signaling CC-chemokines as therapeutic agents for the purpose of limiting HIV replication in vivo should be approached with caution.
The identification of certain chemokine receptors as strain-specific HIV-1 coreceptors has greatly increased our understanding of the determinants of susceptibility to infection. Macrophage (M)-tropic strains, which are the dominant viral species replicating in vivo at the time of seroconversion and during the asymptomatic stage of disease (1–3), primarily use the CC-chemokine receptor CCR5 as an entry cofactor (4–8). In ≈40–50% of HIV-infected individuals, T cell (T)-tropic strains emerge and replace M-tropic strains as the major viral population; this transition is generally associated with rapid CD4+ T cell decline and disease progression (1–3, 9). T-tropic HIV strains are characterized by the usage of the CXC-chemokine receptor CXCR4 as their major entry cofactor. In addition, numerous primary M-tropic and T-tropic HIV isolates also can use, albeit less efficiently, additional chemokine receptors as entry coreceptors (11–13). The factors that influence the emergence/replication of T-tropic HIV strains in vivo in certain HIV-infected individuals are not well understood.
The inhibitory effects of the natural CCR5 ligands macrophage inflammatory protein (MIP)-1α, MIP-1β and regulated upon activation, normal T cell expressed and secreted (RANTES) on the entry of M-tropic strains of HIV into CD4+ T cells in vitro are well established (4–8, 10) and have led to the consideration of these CC-chemokines as potential therapeutic agents for limiting HIV replication in vivo (14, 15). However, the possibility that CC-chemokines may exert indirect selective pressure in vivo in favor of the replication of T-tropic strains, which do not require CCR5 for entry, has been a source of considerable concern. The present study demonstrates that several CC-chemokines can directly enhance the replication efficiency of T-tropic HIV strains and primary isolates in CD4+ T cells, an effect that is evident only at low inocula of virus. RANTES-mediated enhancement of T-tropic HIV replication can be detected at early stages of the HIV replication cycle, is dependent on signaling through Gi proteins, and is associated with increased colocalization of CD4 and CXCR4 on primary CD4+ T cells. These findings may provide insight regarding the factors that favor the replication and spread of T-tropic strains of HIV in vivo and suggest that the therapeutic use of signal-inducing CC-chemokines in HIV disease should be approached with caution.
MATERIALS AND METHODS
Tissue Culture.
Informed consent in the context of a National Institute of Allergy and Infectious Diseases Institutional Review Board approved protocol was obtained before subjecting HIV-infected and uninfected donors to apheresis. Peripheral blood mononuclear cells (PBMC) were obtained by Ficoll/Hypaque density gradient centrifugation, and CD8-depleted PBMC were obtained by using immunomagnetic beads (Dynal, Great Neck, NY). CD4+ T cells were obtained after 3-days stimulation of CD8-depleted PBMC with anti-CD3 mouse ascites plus IL-2 (10 units/ml; Boehringer Mannheim) by negative selection (depletion of CD14, CD19, and CD16/56 positive cells). Anti-CD3 plus IL-2-stimulated CD8-depleted PBMC or CD4+ T cells from HIV-infected donors were cultured at 106 cells/ml in 48-well plates in media (RPMI medium 1640 supplemented with Hepes buffer, glutamine, and antibiotics plus 10% fetal bovine serum) plus IL-2 in the absence or presence of a mixture of rhMIP-1α, rhMIP-1β, and rhRANTES (100 ng/ml each) (R & D Systems). To determine viral phenotype of isolates obtained from cells of HIV-infected subjects, supernatants harvested at time points of peak viral production were filtered and used to infect MT-2 (16) and U87-hCD4-h chemokine receptor (CR) cells (kind gift of D. Littman, New York University Medical Center, New York) by standard techniques. Anti-CD3 plus IL-2-stimulated CD4+ T cells or CD8-depleted PBMC from uninfected donors were plated in replicates of five in 96-well plates at 5 × 104 cells/well in the absence or presence of various CC-chemokines (100 ng/ml) and infected with dilutions of different HIV-1 strains. The phenotype and chemokine receptor usage (determined by using U87hCD4hCR) of HIV strains used are as follows: Purified HIV-1 strains (Advanced Biotechnologies, Columbia, MD) BaL (M-tropic, CCR5), MN (T-tropic, CXCR4), and IIIB (T cell line-adapted [TCLA], CXCR4); JR-fl and JR-csf (M-tropic, CCR5) (National Institute of Allergy and Infectious Disease AIDS Repository, Bethesda, MD), primary T-tropic HIV-1 isolates SG and #3 (isolated from PBMC of symptomatic HIV-infected subjects; CXCR4, weak CCR5) or molecular HIV-1 clones NL4.3 (TCLA, CXCR4; kind gift from M. Martin, LMM, NIAID), and ELI-1 (T-tropic, CXCR4, weak CCR3; kind gift from Keith Peden, Food and Drug Administration). Supernatants of cell cultures from both HIV-infected and uninfected donors were harvested every 3 days for later analysis of HIV production by reverse transcriptase (RT) assay by using standard [32P]dTTP incorporation techniques (17). Cultures were refed with media plus IL-2 without or with CC-chemokines, and cells from cultures of both HIV-infected and uninfected subjects were removed once a week for analysis of proliferative capacity ([3H]thymidine uptake) and coexpression of cell surface markers CD3 and CD4 and either the activation markers CD25, CD69, and HLA-Dr (Beckton Dickinson) or the T-tropic coreceptor, CXCR4 (analyzed by using the 12G5 mAb) by fluorescence-activated cell sorter.
Single Round HIV Replication by Using env(-) HIV NL4.3 Pseudotyped with Various HIV Envelopes.
Anti-CD3 plus IL-2-stimulated CD4+ T cells (105 cells/well) from HIV-uninfected donors were incubated for 30 min (37°C) with media, which contained either mouse isotype control mAb, RANTES (100 ng/ml) that had been preincubated (30 min, 4°C) with control mAb, or RANTES that had been preincubated with neutralizing anti-RANTES mAb (R & D Systems). Cells were then infected with single round replication competent virus containing an HIV-1 NL4.3-luciferase genome (pNL4.3 Env(−)Luc(+); NIAID AIDS repository) pseudotyped with envelope from either the T-tropic SF33 (pTEJ8-SD-envSF33; a generous gift from Louise Poulin, Quebec, Canada) or M-tropic JR-fl (NIAID AIDS repository) strains. Three days later cells were lysed and 20 μl of extract was assayed for luciferase activity with commercially available reagents (Promega) by using a Dynax MLX luminometer (Chantilly, VA).
Determination of Levels of Early HIV Transcripts.
Anti-CD3 plus IL-2-stimulated CD4+ T cells from HIV seronegative donors were washed twice; 5 × 105 cells were untreated or preincubated with pertussis toxin (300 ng/test) (Calbiochem) for 30 min followed by treatment with RANTES (100 ng/ml), aminooxypentane (AOP)-RANTES (100 ng/ml) or no chemokine. Thirty minutes later cells were infected with RNase-free DNase-treated M-tropic (BaL) strain, a primary T-tropic HIV-1 isolate (SG) or the T-tropic strain MN. Four to six hours after infection cells were washed twice with PBS, and cell pellets were frozen. Control cells were exposed to HIV at 4°C for 10 min before making lysates. Cell lysates were analyzed for early HIV RT products by PCR using the long terminal repeat (LTR) primers M667 and AA55 for 30 cycles by using standard methods (18). Semi-quantitative HIV entry results were confirmed by analysis of the late RT product, gag, by using SK38 and SK39 primers. Products were detected by liquid hybridization with the end-labeled-specific LTR probe CG24 or the gag-specific SK19 probe and quantified by using ACH-2 cells that contain a single proviral copy as standards. β2-microglobulin primers were used to standardize samples for cell numbers.
Determination of CD4 and CXCR4 Colocalization by Confocal Microscopy.
CD4+ T cells (in PBS plus 5% deionized BSA, pH 7.4) were allowed to adhere to glass slides precoated with purified tenascin protein (30 μg/ml) 8-h overnight at 37°C in a humidified atmosphere. Cells were either left untreated or were treated with RANTES (100 ng/ml) for 30 min. Cells were then stained with fluorescein isothiocyanate-conjugated anti-CD4 mAb (Becton Dickinson) and phycoerythrin-conjugated anti-CXCR4 (clone 12G5) mAb (PharMingen) or fluorescein isothiocyanate and phycoerythrin-conjugated isotypic MIgG controls (Becton Dickinson) at 1 μg/test for 30 min at 4°C. Slides were washed twice in PBS plus 5% BSA, fixed with 2% paraformaldehyde for 1 h, washed, and mounted. Laser scanning confocal fluorescence microscopy was performed using a Zeiss LSM 410 microscopic systems built around a Zeiss 135 Axiovert inverted scope. Image processing was done by using the Zeiss lsm 410 (v.3.8) software.
RESULTS
CC-Chemokines Enhance the Replication/Emergence of Endogenous T-Tropic HIV Strains in CD4+ T Cells from HIV-Infected Subjects.
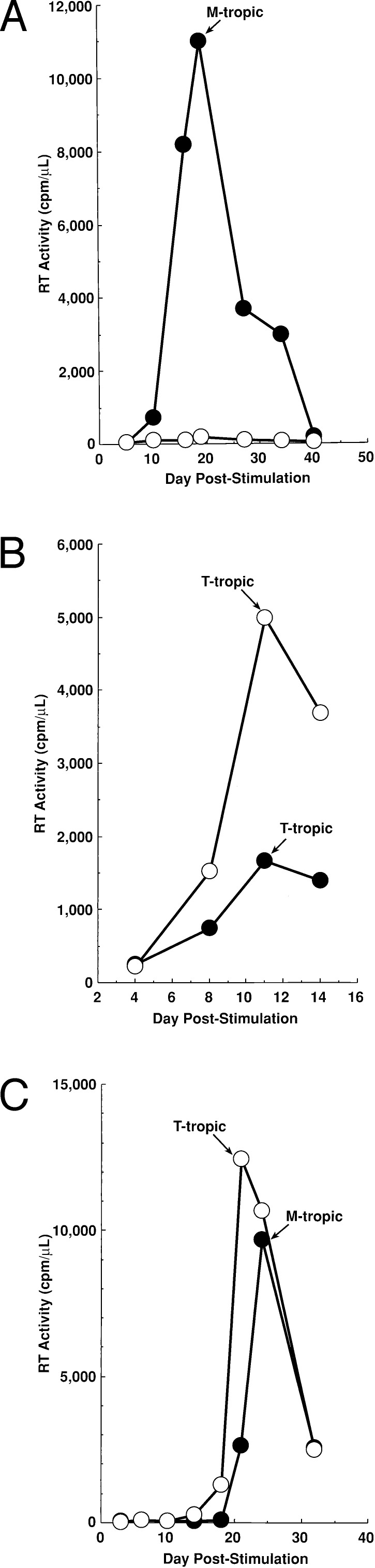
As previously reported (19), HIV replication in cells from the majority of healthy asymptomatic HIV-infected individuals harboring M-tropic strains of HIV was dramatically suppressed in cultures containing CC-chemokines (Fig. 1A). However, HIV replication in cultures of CD4+ T cells from the majority of individuals with more advanced disease, from whom T-tropic strains were isolated, was enhanced by the presence of CC-chemokines (Fig. 1B). Furthermore, in cultures of CD4+ T cells from certain subjects, T-tropic strains of HIV were isolated only if exogenous CC-chemokines were added (Fig. 1C).
Figure 1.
CC-chemokines suppress the replication of M-tropic, but enhance the emergence/replication of T-tropic, HIV quasispecies in CD4+ T cells from HIV-infected individuals. CD4+ T cells from HIV-infected subjects were maintained in the absence (closed symbols) or presence of a mixture of MIP-1α, MIP-1β, and RANTES (open symbols) (100 ng/ml each). Culture supernatants from peak RT time points were used to infect the MT-2 T cell line to determine the presence or absence of T-tropic HIV strains. CD4+ T cells were obtained from (A) an asymptomatic individual with 585 CD4+ T cells/μl; bDNA <500 copies HIV RNA/ml (data are representative of experiments performed with CD4+ T cells obtained from 15 different asymptomatic HIV-infected subjects); (B) a symptomatic subject with 197 CD4+ T cells/μl; bDNA 99,000 copies HIV RNA/ml (data are representative of experiments performed with CD4+ T cells obtained from six different HIV-infected subjects harboring T-tropic strains of HIV-1); and (C) an asymptomatic subject with 660 CD4+ T cells/μl; bDNA 30,000 copies HIV RNA/ml (data are representative of experiments performed with CD4+ T cells obtained from two different HIV-infected subjects).
Effect of CC-Chemokines on the Replication of T-Tropic HIV-1 in CD8-Depleted PBMC from HIV Seronegative Donors Acutely Infected in Vitro.
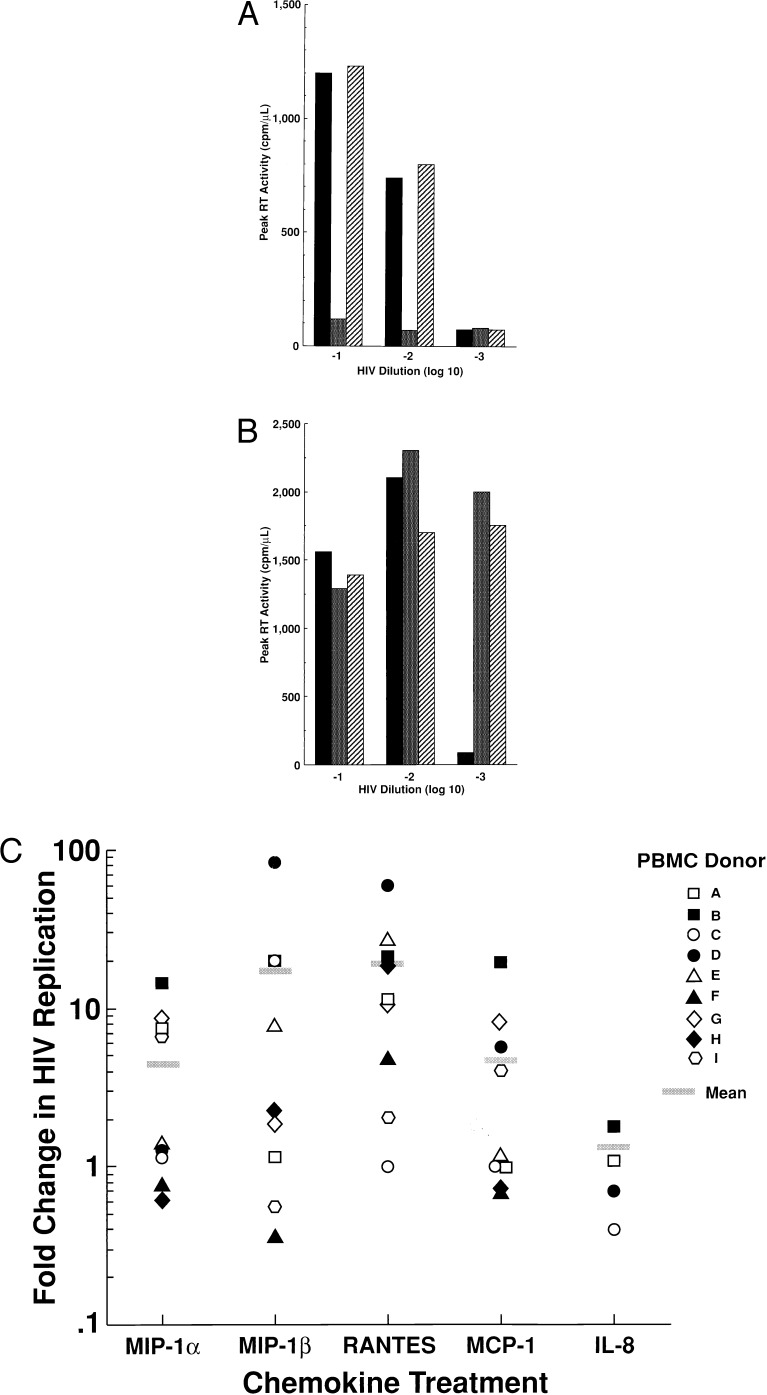
To delineate the parameters necessary to mediate increased replication of T-tropic HIV, RANTES, a CCR5 ligand, and monocyte chemotactic protein (MCP)-1, a CC-chemokine that does not bind CCR5 (20), were compared for their effects on the replication of M-tropic and T-tropic HIV in CD4+ T cells or in CD8-depleted PBMC from HIV-uninfected subjects. As expected, replication of the purified M-tropic JR-fl strain of HIV was inhibited by RANTES but not by MCP-1; no enhancement of virus replication was observed regardless of the concentration of the virus inoculum (Fig 2A). In this regard, MCP-1 failed to significantly (≤2-fold changed compared with untreated culture) alter the replication of the M-tropic HIV-1 strains JR-csf and BaL in CD8-depleted PBMC or CD4+ T cells from four HIV uninfected donors (data not shown). In contrast, the concentration of the primary T-tropic HIV isolate SG that was required to initiate productive infection of CD4+ T cells was decreased tenfold by the addition of either RANTES or MCP-1 (Fig. 2B). Of note, this enhancement of virus replication by RANTES and MCP-1 was observed only at suboptimal concentrations of virus; replication of T-tropic HIV strains was not increased by either CC-chemokine in cultures infected with high concentrations of virus (Fig. 2B). In addition to RANTES and MCP-1, MIP-1α and MIP-1β also were found to increase the replication efficiency of T-tropic HIV in CD8-depleted PBMC from certain HIV-uninfected donors. T-tropic (MN) HIV replication was enhanced by one or more individual CC-chemokines in cells from most donors tested (9 of 11); however, there was considerable donor-to-donor variability with regard to which CC-chemokine(s) mediated this effect (Fig. 2C). The most consistent enhancing effect on T-tropic HIV replication was observed with RANTES and MIP-1β. The CXC-chemokine IL-8 had no significant effect on the replication of either M-tropic or T-tropic strains of HIV-1 (Fig. 2C).
Figure 2.
Dichotomous effect of CC-chemokines on the replication of M-tropic vs. T-tropic HIV-1 strains in CD8-depleted PBMC from HIV seronegative donors infected in vitro. CD8-depleted PBMC from an HIV-uninfected donor were infected with various dilutions of either (A) an M-tropic HIV strain, JR-fl, or (B) a T-tropic primary isolate, SG, without (■) or with the addition of either RANTES (▩) or MCP-1 (▨) (100 ng/ml). Data are representative of three independent experiments. (C) One or more CC-chemokines (100 ng/ml) enhance the replication of a T-tropic HIV-1 strain (MN) in CD8-depleted PBMC or CD4+ T cells from nine different HIV seronegative donors (A–I). The CXC chemokine IL-8 was tested in CD8-depleted PBMC cultures from four different HIV seronegative donors. Data are represented as fold increase in peak viral replication (RT activity) in chemokine treated as compared with untreated cultures.
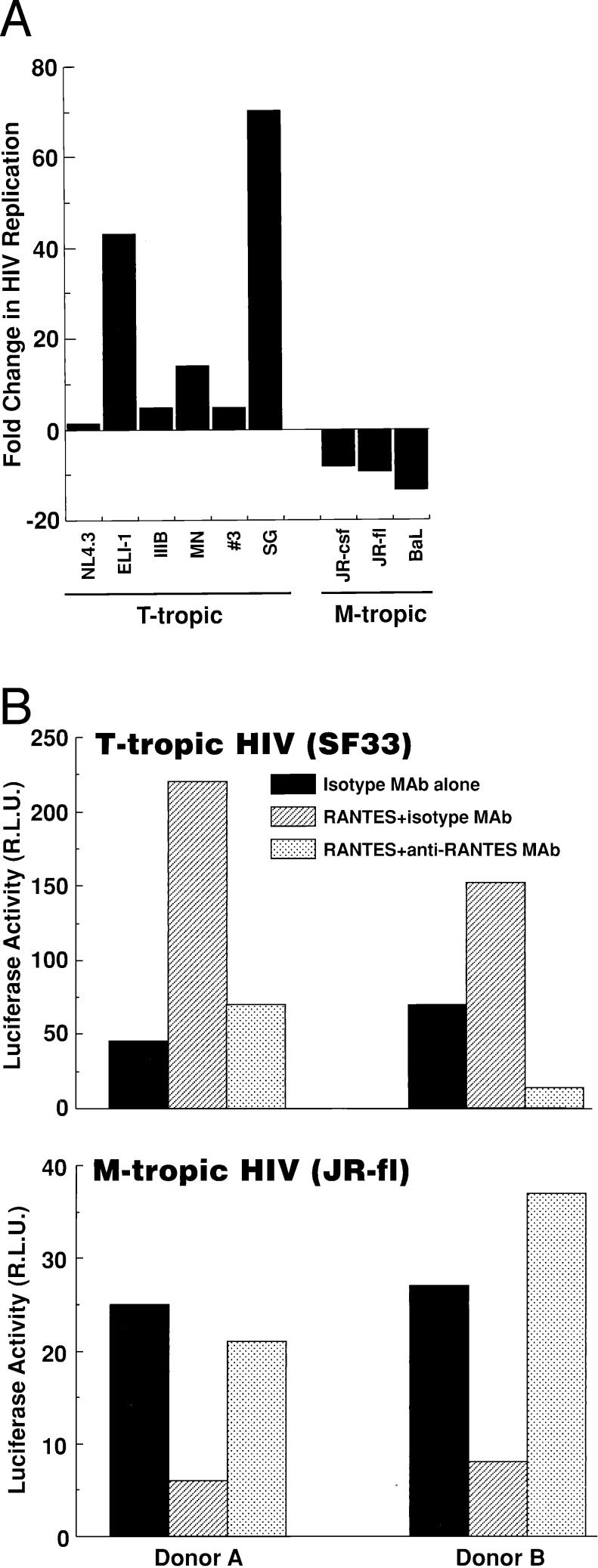
To determine whether CC-chemokine-mediated enhancement of virus replication was common to T-tropic HIV-1 strains, the effect of RANTES on the replication of a panel of M-tropic and T-tropic HIV strains in CD8-depleted PBMC from HIV-uninfected donors was determined. The replication of all M-tropic HIV strains tested was dramatically suppressed by RANTES, whereas the replication of all T-tropic strains thus far tested was enhanced to variable degrees (Fig. 3A). The dichotomous strain-specific effects of RANTES on HIV replication also was demonstrated by using single round replication competent HIV-1-env pseudotyped virus in which early replication events (nef production) are detected by a luciferase reporter. Both the enhancing effect on T-tropic (SF33env) and the inhibitory effect on M-tropic (JR-flenv) HIV “replication” in CD4+ T cells was neutralized by anti-RANTES mAb but not by control mIgG1 isotype control mAb (Fig. 3B).
Figure 3.
(A) RANTES (100 ng/ml) inhibits the replication of M-tropic (JR-fl, JR-csf, or BaL) but enhances the replication of different T-tropic strains (MN, NL4.3, IIIB, and ELI-1) or primary isolates (#3, SG) of HIV-1 in CD8-depleted PBMC from four HIV-uninfected donors. Data are represented as mean fold change in HIV replication of RANTES treated cells as compared with untreated controls. (B) Dichotomous effect of RANTES on single round replication competent HIV pseudotyped with T-tropic or M-tropic HIV envelopes. Neutralizing anti-RANTES mAb abrogates both the enhancing and the inhibitory effect of RANTES on the replication of T-tropic (SF33) and M-tropic (JR-fl) envelope complemented HIV-luc(+) virus, respectively, in CD4+ T cells from two HIV seronegative donors. Data are represented as relative light units (R.L.U.).
CC-chemokines, particularly RANTES, have been demonstrated to increase cellular activation and proliferation (21, 22), and it is well known that productive HIV infection of CD4+ T cells requires cellular activation (18, 23). However, CC-chemokine-mediated enhancement of T-tropic HIV replication in anti-CD3 plus IL-2-stimulated CD4+ T cells did not correlate with significant increases in cellular proliferation ([3H]thymidine uptake) or with an increase in expression of cell surface activation markers (CD25, CD69, and HLA-DR; data not shown).
Effect of RANTES and its Antagonist AOP RANTES on Early Events (Early LTR Transcript Levels) of T-Tropic HIV-1 Replication in CD4+ T Cells: Dependence on Gi Protein Signal Transduction.
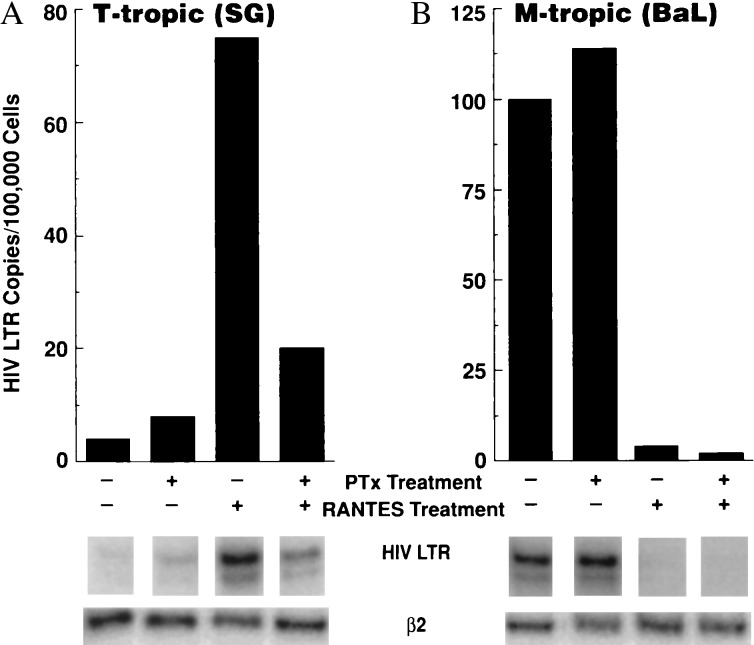
To delineate the mechanism(s) whereby CC-chemokines enhance T-tropic HIV replication, the effect of RANTES on the levels of early HIV RT transcripts in primary CD4+ T cells was determined by semi-quantitative PCR analysis by using LTR primers. A significant increase in the levels of T-tropic HIV LTR transcripts was observed in cells that were treated with RANTES before exposure to low inoculum of virus. This effect was inhibited by prior exposure of cells to pertussis toxin, an inhibitor of Gi protein signal transduction, a major signaling pathway of CC-chemokine receptors (20) (Fig. 4A), as well as by preincubation of RANTES with neutralizing anti-RANTES mAb (data not shown). In contrast, and as previously reported (24, 25), the inhibitory effect of RANTES on the entry of the M-tropic BaL strain was not abrogated by pertussis toxin treatment (Fig. 4B). Parallel cultures were incubated for an additional 14 h, and the late RT product gag was quantitated with similar results (data not shown). Similar experiments were conducted by using the RANTES “antagonist,” AOP-RANTES. This RANTES antagonist is particularly attractive as a candidate therapeutic agent for HIV disease as it inhibits M-tropic HIV replication in macrophages as well as in CD4+ T cells without inducing biological activity such as chemotaxis, even at micromolar concentrations (26). AOP-RANTES did not enhance the levels of T-tropic, yet it dramatically reduced the levels of the M-tropic, early HIV LTR transcripts (data not shown).
Figure 4.
RANTES enhances the entry of a primary T-tropic HIV isolate in CD4+ T cells, and this effect is dependent on Gi protein signal transduction. CD4+ T cells were preincubated with pertussis toxin (300 ng/ml), exposed to RANTES (100 ng/ml), and then infected with a primary T-tropic HIV isolate SG (A), or the M-tropic strain BaL (B). Cells were washed and pelleted after 6 h, and levels of early HIV transcripts were determined by PCR using LTR primers. Data are representative of eight independent experiments.
CC-Chemokine-Mediated Enhancement of T-Tropic HIV-1 Replication Is Associated with Increased Cellular Colocalization of CD4 and CXCR4 but Not with Increases in the Levels of CXCR4 Surface Expression.
The ability of RANTES to up-regulate early events (early LTR transcripts) in the replication of T-tropic HIV suggested that RANTES may alter the expression of the primary T-tropic HIV coreceptor, CXCR4, on CD4+ T cells. However, no increase in the cell surface expression of the T-tropic HIV coreceptor CXCR4 was observed by fluorescence-activated cell sorter analysis upon treatment of cells from HIV seronegative donors with MIP-1β or RANTES (tested at 6 h, day 1, 3, 5, and 7 post-treatment; data not shown).
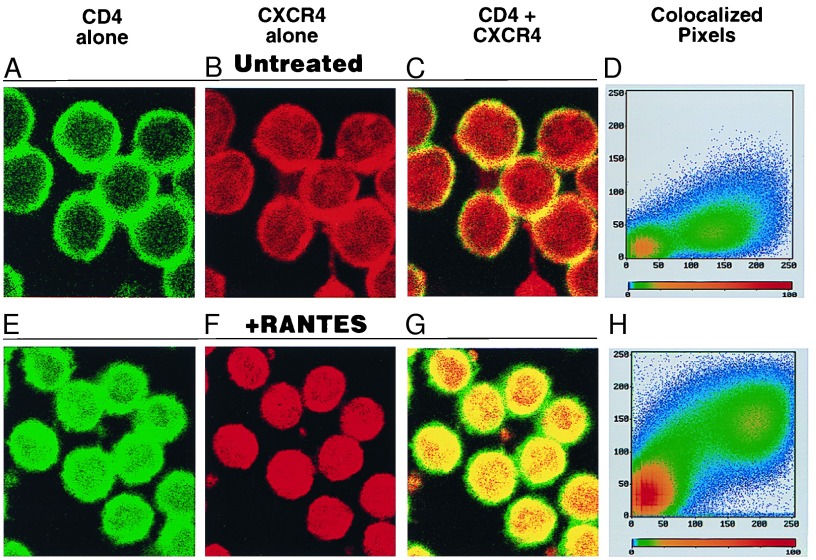
Although some HIV-2 isolates have been reported to use CXCR4 in the absence of CD4 (27), HIV-1 strains appear to require both chemokine receptor and CD4 molecules for efficient entry into CD4+ T cells. The possibility that RANTES increases the association of CD4 and CXCR4, thus elevating the level of efficiency of entry of T-tropic HIV-1, was investigated by using confocal microscopy. Pretreatment of CD4+ T cells with RANTES was found to enhance the colocalization of CD4 and CXCR4 (visualized as gold) as determined by pixel quantitation software (Zeiss sm410 v.3.8) (Fig. 5).
Figure 5.
RANTES increases the colocalization of CD4 and CXCR4 on primary CD4+ T cells. Negatively selected CD4+ T cells immobilized on tenascin-coated slides were untreated (A–D) or treated with RANTES (E–H) for 30 min before staining. Cells were stained with anti-CD4-fluorescein isothiocyanate mAb (A and E, visualized as green) and anti-CXCR4 12G5-phycoerythrin mAb (B and F, visualized as red). Colocalization of CD4 and CXCR4 is illustrated in C and G (visualized as yellow/gold). Quantitation of pixel colocalization is represented in D and H; areas of strong colocalization are depleted in red on the pixelgram. Data are representative of three independent experiments.
DISCUSSION
The present study demonstrates that certain CC-chemokines, including those that inhibit entry of M-tropic HIV, enhance the replication of T-tropic HIV in CD4+ T cells in vitro. This effect is observed only at suboptimal concentrations of virus and can be detected at very early stages of viral replication. Enhancement of T-tropic replication by RANTES is dependent on Gi protein-coupled signal transduction and is associated with increased colocalization of CD4 and CXCR4 on CD4+ T cells.
Although CC-chemokines have been reported to exert a variety of effects on M-tropic HIV replication in macrophages (28, 29), including enhancement (29, 30), initial studies investigating the inhibitory effects of CC-chemokines on the replication of M-tropic strains of HIV-1 in T cells observed no discernible effects on the replication of T-tropic primary isolates or laboratory adapted strains in these cells (4–8, 31, 32). In the present study, CC-chemokine-mediated enhancing effects on T-tropic HIV replication in in vitro-infected CD4+ T cells were clearly viral dose-dependent and were observed only at suboptimal concentrations of virus. A possible explanation for this observation is that HIV envelope gp120 itself can induce CD4-CXCR4 colocalization (34–36) and that chemokine-mediated alterations in the colocalization of viral receptors significantly impact HIV replication only at concentrations of virus below a certain threshold. However, Dolei et al. (33) reported recently that MIP-1α, MIP-1β and RANTES increase T-tropic HIV binding/entry and replication in PHA-stimulated PBMC and the C8166 T cell line irrespective of the multiplicity of infection. Furthermore, in contrast to the observations of the present study, Dolei et al. reported that the CC-chemokine-mediated enhancement of T-tropic HIV replication was due to elevation in the levels of CXCR4 transcripts (cell surface CXCR4 levels were not analyzed) and subsequent increases in virion-cell surface binding, an effect that was dependent on protein tyrosine kinase signal transduction. The basis for the discrepancies between the findings of this previous study and the present study regarding the mechanisms of CC-chemokine-mediated enhancement of T-tropic HIV replication is unclear but may be due to differences in in vitro culture methods.
The ability of certain CC-chemokines to exert a dichotomous effect on the replication of T-tropic vs. M-tropic HIV strains in in vitro-infected CD4+ T cells or CD8-depleted PBMC from HIV-uninfected donors was a consistent observation. Replication of all four M-tropic HIV strains tested [JR-fl, JR-csf, BaL, and ADA (ADA; data not shown)] was inhibited >90% by the addition of RANTES, whereas replication of all T or dual-tropic strains (MN, IIIB, ELI-1, NL4–3, and the pseudotyped SF33 env/NL4–3 HIV-1 virus and 89.6 (89.6, data not shown), or primary T-tropic isolates (SG and no. 3) tested was enhanced to variable degrees by one or more individual CC-chemokines. The variability observed among CC-chemokines in their ability to enhance T-tropic HIV replication may be due, in part, to differences in the levels of endogenous CC-chemokines (and/or other HIV-modulating cytokines) and or to differences in the level of chemokine receptor expression among cells from different donors.
The stimulatory effect of CC-chemokines on the replication of T-tropic HIV strains does not appear to be due to an increase in the overall susceptibility of cells to productive HIV infection independent of cellular tropism. In this regard, cell surface markers of T cell activation and levels of cellular proliferation were not significantly altered upon treatment with CC-chemokines. In addition, MCP-1, which does not bind CCR5 (20) and thus does not block M-tropic HIV infection, failed to enhance the replication of the M-tropic strains JR-fl (Fig. 2A), JR-csf, and BaL (data not shown) in cells from four different donors tested, regardless of the viral concentration used.
The observation that treatment of CD4+ T cells with RANTES increases the association of CXCR4 and CD4, resulting in a more efficient complex for T-tropic viral entry, offers a potential mechanism whereby T-tropic replication is enhanced in the presence of this chemokine. Although CD4-CXCR4 colocalization has been demonstrated to occur upon treatment of cells with HIV gp120 (34–36), which binds both receptors, association of these two molecules by chemokines has not been demonstrated previously. It has, however, been demonstrated that stimulation of T cells with several cytokines or chemokines induces the polarization and colocalization of certain chemokine receptors, an effect that is sensitive to inhibition by pertussis toxin and dependent on the extracellular matrix environment (37). Similarly, the enhancing effect of RANTES on early events (entry/early RT transcripts) of T-tropic HIV replication is dependent on Gi protein-mediated signal transduction as determined by the loss of effect in the presence of pertussis toxin (21). In addition, preliminary studies suggest that pretreatment of cells with pertussis toxin disrupts the pattern of CD4-CXCR4 colocalization induced by RANTES (data not shown). The observation that AOP-RANTES does not enhance T-tropic HIV entry is noteworthy in that this chemokine antagonist binds CCR5 and can stimulate calcium flux (38) but does not induce chemotaxis (26, 38). Of interest, both chemotaxis and, more recently, gp120-mediated colocalization of CD4 and CXCR4 (36) have been demonstrated to be dependent on components of the cytoskeletal network. We currently are investigating the effects of several chemokines on the colocalization of CD4 and other chemokine receptors. The ability of MCP-1, which is the primary ligand of CCR2 and lacks CCR5-binding activity, to enhance T-tropic HIV-1 replication suggests that signaling through several chemokine receptors may influence the association of CD4 and CXCR4. It is possible that, under appropriate conditions, certain chemokines can influence association between CD4 and various other chemokine receptors, such as CCR5, and thus enhance M-tropic HIV entry/replication.
The physiologic relevance of chemokine-mediated enhancement of T-tropic HIV infection efficiency is supported by our observation that CC-chemokines enhanced the emergence or replication of T-tropic strains of HIV in CD4+ T cells from HIV-infected individuals in vitro. The observation that T-tropic HIV replication was detectable in CC-chemokine-treated, but not untreated, CD4+ T cells from certain asymptomatic HIV-infected subjects is of particular interest. These observations suggest that in HIV-infected individuals in whom T-tropic variants are present at very low frequencies, CC-chemokines could result in a significant increase in the representation of T-tropic strains within the viral population.
These findings may have considerable pathophysiologic relevance related to the fact that HIV exists in vivo as a pool of quasi-species (39–41), the composition of which changes over the course of HIV-1 disease. After seroconversion and during the asymptomatic stage of disease, the majority of HIV-infected individuals harbor a viral pool composed virtually exclusively of M-tropic HIV-1 variants; however, in ≈40–50% of HIV-infected individuals, T-tropic strains emerge (1–3, 9), and generally remain, at various frequencies, as components of the replicating viral pool in vivo (40, 41). This shift from exclusively M-tropic to T-tropic (± M-tropic) viral replication in vivo is associated with a rapid decline in CD4+ T cells and disease progression (1–3, 9). CC-chemokines may influence the rate or the magnitude of this shift by simultaneously blocking the replication of M-tropic viruses and enhancing the replication of preexisting T-tropic viruses, thus leading to the emergence of the latter as the predominant species. This possibility must be addressed in any therapeutic strategy that includes the use or manipulation of these proteins for the treatment of HIV-infected individuals (14, 15).
Acknowledgments
We would like to thank Joseph Adelsberger for performing fluorescence-activated cell sorter analyses, Patricia Walsh for her excellent editorial assistance, and Dr. Amanda Proudfoot and Dr. Timothy Wells for their gifts of native RANTES. A.L.K. performed this project in partial fulfillment of the requirements of the Ph.D. program of the Department of Microbiology and Immunology at George Washington University (Washington, DC).
ABBREVIATIONS
- AOP
aminoxypentane
- LTR
long terminal repeat
- T-tropic
T cell-tropic
- M-tropic
macrophage-tropic
- RT
reverse transcriptase
- MIP
macrophage inflammatory protein
- RANTES
regulated upon activation, normal T cell expressed and secreted
- PBMC
peripheral blood mononuclear cells
- MCP
monocyte chemotactic protein
- CR
chemokine receptor
References
- 1. Cheng-Mayer C, Seto D, Tateno M, Levy J A. Science. 1988;240:80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- 2.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E, van Steenwijk R P, Lange J M, Schattenkerk J K, Miedema F, Tersmette M. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tersmette M, Lange J M, de Goede R E, de Wolf F, Eeftink-Schattenkerk J K, Schellekens P T, Coutinho R A, Huisman J G, Goudsmit J, et al. Lancet. 1989;1:983–985. doi: 10.1016/s0140-6736(89)92628-7. [DOI] [PubMed] [Google Scholar]
- 4.Alkhatib G, Combadiere C, Broder C, Feng Y, Kennedy P, Murphy P, Berger E A. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 5.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, et al. Nature (London) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 6.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, et al. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 7.Dragic T, Litwin V, Allaway G, Martin S, Huang Y, Nagashima K, Cayanan C, Maddon P J, Koup R A, Moore J P, et al. Nature (London) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 8.Doranz B J, Rucker J, Yi Y, Smyth R, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 9.Schellekens P T, Tersmette M, Roos M T, Keet R P, de Wolf F, Coutinho R A, Miedema F. AIDS. 1992;6:665–669. doi: 10.1097/00002030-199207000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Cocchi F, DeVico A, Garzino-Demo A, Arya S, Gallo R, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 11.Feng Y, Broder C C, Kennedy P E, Berger E A. Science. 1996;272:872–877. [Google Scholar]
- 12.Simmons G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, et al. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Huang Y, He T, Cao Y, Ho D D. Nature (London) 1996;383:768. doi: 10.1038/383768a0. [DOI] [PubMed] [Google Scholar]
- 14.Fauci A S. Nat Med. 1996;2:966–967. doi: 10.1038/nm0996-966. [DOI] [PubMed] [Google Scholar]
- 15.Wells T N, Proudfoot A E, Power C A, Marsh M. Chem Biol. 1996;3:603–609. doi: 10.1016/s1074-5521(96)90126-x. [DOI] [PubMed] [Google Scholar]
- 16.Koot M, Vos A H, Keet R P, de Goede R E, Dercksen M W, Terpstra F G, Coutinho R A, Miedema F. AIDS. 1992;6:49–54. doi: 10.1097/00002030-199201000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Willey R L, Smith D H, Lasky L A, Theodore T S, Earl P L, Moss B, Capon D J, Martin M A. J Virol. 1988;62:139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 19.Kinter A L, Ostrowski M, Goletti D, Oliva A, Weissman D, Gantt K, Hardy E, Jackson R, Ehler L, Fauci A S. Proc Natl Acad Sci USA. 1996;93:14076–14081. doi: 10.1073/pnas.93.24.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy P M. Cytokine Growth Factor Rev. 1996;7:47–64. doi: 10.1016/1359-6101(96)00009-3. [DOI] [PubMed] [Google Scholar]
- 21.Taub D D, Turcovski-Corrales S M, Key M L, Longo D L, Murphy W J. J Immunol. 1996;156:2095–2103. [PubMed] [Google Scholar]
- 22.Bacon K B, Premack B A, Gardner P, Schall T J. Science. 1996;269:1727–1730. doi: 10.1126/science.7569902. [DOI] [PubMed] [Google Scholar]
- 23.Virelizier J L. Curr Opin Immunol. 1989;2:409–413. doi: 10.1016/0952-7915(89)90151-9. [DOI] [PubMed] [Google Scholar]
- 24.Oravecz T, Pall M, Norcross M A. J Immunol. 1996;157:1329–1332. [PubMed] [Google Scholar]
- 25.Cocchi F, DeVico A L, Garzino-Demo A, Cara A, Gallo R C, Lusso P. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 26.Simmons G, Clapham P R, Picard L, Offord R E, Rosenkilde M M, Schwartz T W, Buser R, Wells T N C, Proudfoot A E. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 27.Endres M J, Clapham P R, Marsh M, Ahuja M, Turner J D, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, et al. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 28.Arenzana-Seisdedos F, Virelizier J L, Rousset D, Clark-Lewis I, Loetscher P, Moser B, Baggiolini M. Nature (London) 1996;383:400. doi: 10.1038/383400a0. (lett.). [DOI] [PubMed] [Google Scholar]
- 29.Schmidtmayerova H, Sherry B, Bukrinsky M. Nature (London) 1996;382:767. doi: 10.1038/382767a0. (lett.). [DOI] [PubMed] [Google Scholar]
- 30.Kelly M D, Naif H M, Adams S L, Cunningham A L, Lloyd A R. J Immunol. 1998;160:3091–3095. [PubMed] [Google Scholar]
- 31.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jansson M, Popovic M, Karlsson A, Cocchi F, Rossi P, Albert J, Wigzell H. Proc Natl Acad Sci USA. 1996;93:15382–15387. doi: 10.1073/pnas.93.26.15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolei A, Biolchini A, Serra C, Curreli S, Gomes E, Dianzani F. AIDS. 1998;12:183–190. doi: 10.1097/00002030-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Lapham C K, Ouyang J, Chandrasekhar B, Nguyen N Y, Dimitrov D S, Golding H. Science. 1996;274:602–605. doi: 10.1126/science.274.5287.602. [DOI] [PubMed] [Google Scholar]
- 35.Ugolini S, Moulard M, Mondor I, Barois N, Demandoix D, Hoxie J, Brelot A, Alizon M, Davoust J, Sattentau Q J. J Immunol. 1997;159:3000–3008. [PubMed] [Google Scholar]
- 36.Iyengar S, Hildreth J E K, Schwartz D H. J Virol. 1998;72:5251–5255. doi: 10.1128/jvi.72.6.5251-5255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nieto M, Frade J M, Sancho D, Mellado M, Martinez-A C, Sanchez-Madrid F. J Exp Med. 1997;186:153–158. doi: 10.1084/jem.186.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mack M, Luckow B, Nelson P J, Cihak J, Simmons G, Clapman P R, Signoret N, Marsh M, Stangassinger M, Borlat F, et al. J Exp Med. 1998;187:1215–1224. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wain-Hobson S. Curr Top Microbiol Immunol. 1992;176:181–193. doi: 10.1007/978-3-642-77011-1_12. [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi Y, Gojobori T. Proc Natl Acad Sci USA. 1997;94:1264–1269. doi: 10.1073/pnas.94.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ida S, Gatanaga H, Shioda T, Nagai Y, Kobayashi N, Shimada K, Kimura S, Iwamoto A, Oka S. AIDS Res Hum Retroviruses. 1997;13:1597–1608. doi: 10.1089/aid.1997.13.1597. [DOI] [PubMed] [Google Scholar]