Abstract
The molecular mechanisms underlying early/recycling endosomes-to-TGN transport are still not understood. We identified interactions between the TGN-localized putative t-SNAREs syntaxin 6, syntaxin 16, and Vti1a, and two early/recycling endosomal v-SNAREs, VAMP3/cellubrevin, and VAMP4. Using a novel permeabilized cell system, these proteins were functionally implicated in the post-Golgi retrograde transport step. The function of Rab6a' was also required, whereas its closely related isoform, Rab6a, has previously been implicated in Golgi-to-endoplasmic reticulum transport. Thus, our study shows that membrane exchange between the early endocytic and the biosynthetic/secretory pathways involves specific components of the Rab and SNARE machinery, and suggests that retrograde transport between early/recycling endosomes and the endoplasmic reticulum is critically dependent on the sequential action of two members of the Rab6 subfamily.
Keywords: shiga toxin; early/recycling endosomes; SNARE; Rab protein; retrograde transport
Introduction
The existence of two connecting routes between the endocytic and the biosynthetic/secretory pathway has been suggested (Rohn et al., 2000). The first one is linking late endosomes and the trans-Golgi network (TGN)*. A series of studies revealed the molecular mechanisms driving mannose 6-phosphate receptor retrieval to the TGN via this pathway. It is regulated by the small GTPase Rab9 and its effector p40 (Lombardi et al., 1993) and facilitated by α-SNAP, a component of the SNARE machinery (Itin et al., 1997). The recently identified protein TIP47 interacts with the cytosolic domain of mannose 6-phosphate receptors, and is also implicated in late endosomes-to-TGN trafficking (Carroll et al., 2001).
Morphological and biochemical evidence indicates that the receptor-binding, nontoxic B-subunit of Shiga toxin (STxB) traffics via an alternative pathway directly from the early endosome (EE) to the TGN, bypassing the late endocytic pathway (Mallard et al., 1998). This newly described pathway may also involve the recycling endosome (RE) and the GTPase Rab11 (Wilcke et al., 2000). Similar observations were made studying TGN38, a cellular protein known to recycle between the TGN and the plasma membrane (Ghosh et al., 1998). The molecular mechanisms underlying EE/RE-to-TGN transport are still largely unexplored. Our observation that internalized STxB colocalized with γ-adaptin and clathrin on EE/RE implied that AP1/clathrin coats might regulate membrane dynamics at the EE/RE-TGN interface (Mallard et al., 1998). Based on functional studies, a role for AP1 and the AP1 interactor PACS-1 was revealed in retrograde transport to the TGN of furin and MPR (Meyer et al., 2000; Crump et al., 2001; Fölsch et al., 2001), suggesting that these molecules may at least in part also use the EE/RE-to-TGN pathway. Importantly, this direct pathway may allow signaling molecules such as CD19 (Khine et al., 1998), interferon α/β receptors (Khine and Lingwood, 2000), or GPI-anchored receptors, e.g., CD14 (Thieblemont and Wright, 1999), to escape the degradative environment of late endosomes to reach internal sites where they may interact with their intracellular targets.
Another group of proteins with key functions in membrane transport are small N-ethylmaleimide (NEM)-sensitive fusion factor (NSF) attachment protein (SNAP) receptors (SNAREs) (Rothman and Wieland, 1996; Hay and Scheller, 1997; Johannes and Galli, 1998). These tail-anchored molecules are located on specific membranes inside the cell where they form stable complexes that can be dissociated by the ATPase NSF/SNAP (Söllner et al., 1993). The current model suggests that t-SNAREs on target membranes, and v-SNAREs on transport vesicles cooperate with other proteins to form trans-complexes that bring vesicle- and target-membranes in close proximity to promote membrane fusion (Weber et al., 1998). However, the details of the fusion reaction and the exact role of the SNAREs remain to be established (Mayer, 1999). After fusion, the cis-SNARE complexes are dissociated by NSF/SNAP activity and SNAREs can reenter a new functional cycle.
Here, we identified the central elements of the molecular machinery of post-Golgi retrograde transport, thus establishing, by functional means, the existence of a regulated and efficient transport connection between the early endocytic pathway and the TGN.
Results
A novel experimental system to measure STxB transport from EE/RE to the TGN
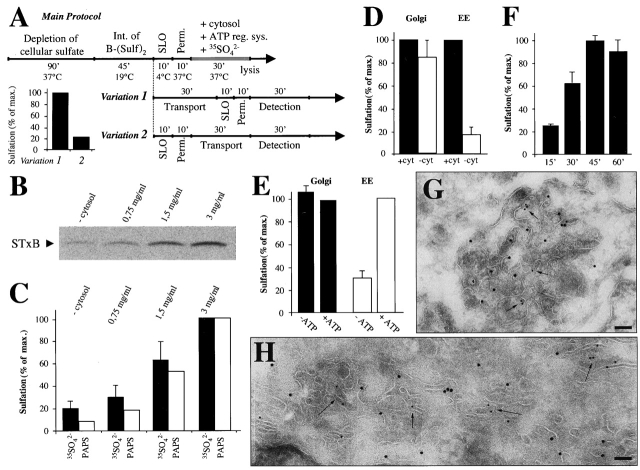
In the absence of a molecular understanding of membrane dynamics at the interface between the endocytic and the biosynthetic/secretory pathways, the existence of EE/RE-to-TGN transport has remained controversial. We have therefore reconstituted transport of STxB from EE/RE to the TGN in streptolysin O (SLO)-permeabilized HeLa cells. A previously constructed recombinant modified STxB with two COOH-terminal sulfation sites, termed STxB-Sulf2, was chosen as a reporter molecule because its sulfation by TGN-localized sulfotransferase allows detection and quantification of arrival in the TGN (Mallard et al., 1998). STxB-Sulf2 was accumulated in EE/RE of intact HeLa cells by continuous incubation at low temperatures (Mallard et al., 1998) (Fig. 1 A, main protocol). The cells were then subjected to SLO permeabilization, and transport to the TGN was assayed in the presence of [35S]sulfate (Fig. 1 B). The SLO permeabilization technique was particularly adapted to our studies because only the plasma membrane is permeabilized following binding of SLO to cells on ice.
Figure 1.
Characterization of the experimental permeabilized cell system. (A) Generic protocols used to reconstitute STxB transport from the EE to the TGN. (Perm.) Permeabilization in the absence of exogenous cytosol. (Inset) Variations 1 and 2 were used to compare transport efficiencies in intact or permeabilized HeLa cells (means of two experiments). (Detection) Incubation of permeabilized cells with [35S]sulfate and ATP-regenerating system (ATP reg. sys.) in the absence of cytosol. (B and C) STxB transport to the TGN depends on the presence of cytosol in a dose-dependent manner. A representative gel is shown in B, and the corresponding quantification in C. Means of two [35S]-PAPS (PAPS) or three [35S]sulfate (35SO4 2-) experiments (± SEM). Note that similar responses are observed when [35S]sulfate or [35S]-PAPS are used as sulfuryl donors (the total amounts of sulfated STxB-Sulf2 obtained in both conditions are comparable). (D) Sulfation as such is not cytosol dependent. STxB-Sulf2 was preaccumulated, either in the Golgi apparatus at 37°C, or in the EE at 19.5°C before permeabilization and incubated with or without cytosol. (E) STxB transport to the TGN is ATP dependent. As in D, STxB-Sulf2 was accumulated in the EE or the Golgi apparatus before permeabilization and incubation with complete or ATP-depleted cytosol in the presence of 35S-PAPS to render the sulfation reaction as such ATP independent. (F) Kinetics of STxB transport to the TGN. Permeabilized cells were continuously incubated with [35S]sulfate for the indicated times. (G and H) STxB transport to the TGN in permeabilized cells detected by electron microscopy. STxB was internalized into the EE, and the cells were permeabilized and then incubated for 30 min at 37°C before fixation and cryosectioning. Cryosections were labeled for STxB (15-nm gold particles) and (G) TGN46 (10-nm gold particles) or (H) GalT (10-nm gold particles). Bars, 100 nm.
In the absence of exogenous cytosol, transport was 19% (± 5.8%, n = 55) of that detected in the presence of 3 mg/ml of cytosol (Fig. 1, B, C, and D, condition EE). The sulfation reaction per se was not dependent on exogenous cytosol because STxB-Sulf2, preaccumulated in the Golgi apparatus of intact cells (Fig. 1 D, Golgi), was sulfated, after permeabilization, in the same manner in the presence or absence of exogenous cytosol. In addition, the same dose-dependence on exogenous cytosol was observed when [35S]-labeled 3′-phosphoadenosine 5′-phosphosulfate (PAPS) instead of [35S]sulfate was used as a direct sulfuryl donor (Fig. 1 C).
To determine whether STxB transport to the TGN was energy dependent, we examined both complete and ATP-depleted cytosol (Fig. 1 E). These experiments were done with [35S]-labeled PAPS to render the sulfation reaction itself ATP independent. Under these conditions, TGN-localized STxB-Sulf2 was still efficiently sulfated, independent of the addition of an ATP regeneration system (Fig. 1 E Golgi, black bars). However, STxB transport to the TGN from the EE was strongly inhibited in the absence of ATP (Fig. 1 E, EE, white bars).
STxB-Sulf2 was transported to the TGN with comparable kinetics in permeabilized and intact cells. In fact, maximal sulfation was reached after 45 min in permeabilized cells (Fig. 1 F), as in intact cells (Mallard et al., 1998). Furthermore, we found that the efficiency of transport in permeabilized cells was 25% of that in intact cells (Fig. 1 A, insert), comparable to other in vitro systems that reconstitute coupled budding and fusion reactions. Throughout this manuscript, this percentage was set to 100% for comparison purposes. Finally, electron microscopical studies established that in SLO-permeabilized cells, a significant part of internalized STxB (Fig. 1, G–H, 15 nm) gained access to structures labeled by the TGN markers TGN46 (Fig. 1 G, 10-nm gold particles, arrows) and galactosyl-transferase (Fig. 1 H, 10-nm particles, arrows), as previously described in intact cells (Johannes et al., 1997; Mallard et al., 1998). Morphologically identifiable Golgi stacks were also marked under these conditions (Fig. 1 H). In the absence of cytosol, no STxB transport to the Golgi could be detected (unpublished data).
Taken together, these results show that STxB transport from EE/RE to the TGN was efficiently reconstituted in SLO-permeabilized cells. The process exhibited the hallmarks characteristics of in vivo transport, and revealed canonical biochemical requirements observed for other in vitro reconstituted transport steps.
t-SNARE proteins in EE/RE-to-TGN transport
SNAREs are key regulators of vesicular membrane traffic. To test whether EE/RE-to-TGN transport was SNARE dependent, SNARE activity was inhibited using the dominant-negative α-SNAP mutant L294A that is unable to stimulate the ATPase activity of NSF (Barnard et al., 1997). When added to permeabilized cells, recombinant α-SNAP(L294A) inhibited STxB transport in a dose-dependent manner (Fig. 2 A). Transport could also be slightly stimulated by the addition of low concentrations of wild-type α-SNAP (Fig. 2 A). These data strongly indicated a role for SNARE proteins in EE/RE-to-TGN transport.
Figure 2.
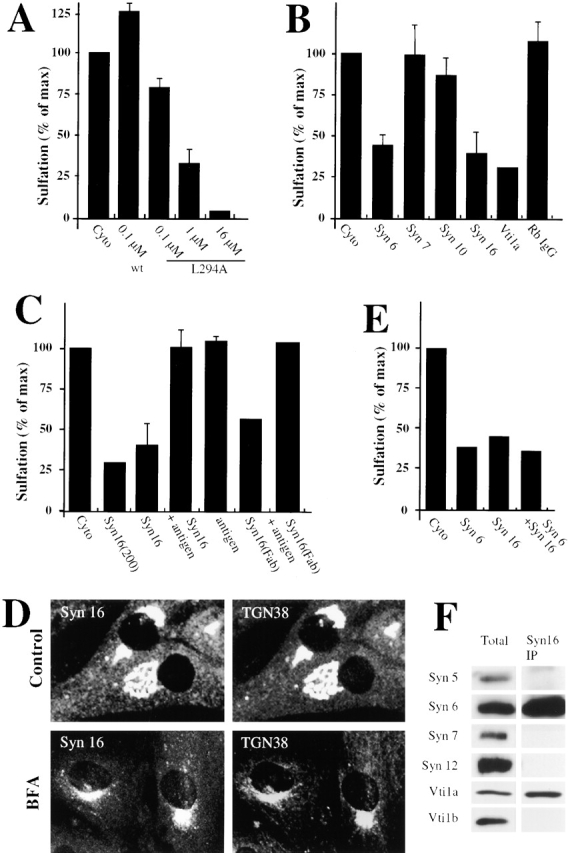
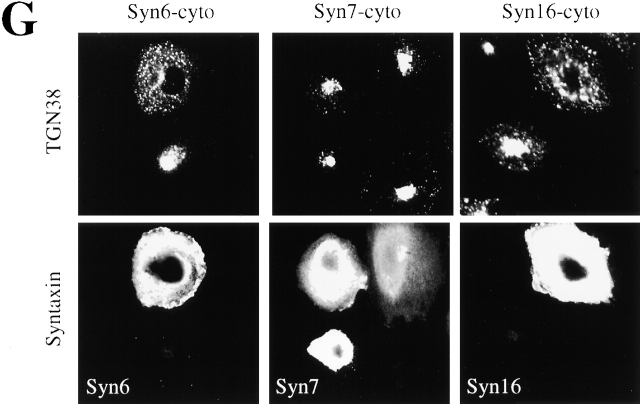
Retrograde transport to the TGN is mediated by the t-SNAREs Syn6, Syn16, and Vti1a. An experimental protocol as shown in Fig. 1 A was used. (A) STxB-Sulf2 transport to the TGN was assayed by sulfation analysis in the presence of the indicated concentrations of recombinant wild- type α-SNAP (wt) or a dominant negative α-SNAP mutant (L294A). As in the following parts of the figure, means (± SEM) of two to six experiments are shown. (B) 25–50 μg/ml of anti-Syn6, 7, 10, 16, or anti-Vti1a antibodies were continuously present from permeabilization on. Rb IgG, rabbit control IgG. The experiments with Syn6 were performed both with a monoclonal and a polyclonal antibody. (C) Anti-Syn16 antibody and Fab fragments generated from this antibody (Syn16[Fab]) had comparable inhibitory effects on STxB-Sulf2 transport to the TGN. Inhibition could be reversed by prebinding of the antibodies to recombinant His-tagged Syn16. Higher doses of anti-Syn16 (200 μg/ml; Syn16[200]) did not significantly increase the inhibitory effect. (D) Syn16 localization in the TGN. Note that upon BFA treatment, the perinuclear staining of TGN38 and Syn16 collapsed into a microtubule organizing center-like staining, a characteristic of TGN proteins. (E) Antibodies against Syn6 and Syn16 had no additive inhibitory effects on STxB-Sulf2 transport to the TGN, suggesting that both proteins function in the same molecular complex. (F) Antibody against Syn16 coimmunoprecipitated Syn6 and Vti1a, but not Vti1b, the cis-Golgi Syn5 or the endosomal Syn7 or Syn12. (G) Expression of Syn6-cyto and Syn16-cyto, but not of Syn7-cyto in Tac-TGN38–transfected CHO cells, specifically inhibited transport of internalized anti-Tac antibodies to the TGN.
We then set out to use the permeabilized cell system to identify the t-SNAREs that would function in the fusion process involving EE/RE-derived STxB-containing transport intermediates. Syn6, Syn10, Syn16, and Vti1a were chosen for our studies because of their localization in the Golgi apparatus (Bock et al., 1997; Simonsen et al., 1998; Tang et al., 1998a, 1998b; Xu et al., 1998), and Syn7 as a negative control for its exclusive localization on endosomes (Nakamura et al., 2000). Syn16 appeared of particular interest because of its extensive colocalization with the trans-Golgi marker TGN38 (Fig. 2 D, top panel), which persisted upon BFA treatment (Fig. 2 D, bottom panel). As shown in Fig. 2 B, antibodies against Syn6, Syn16, and Vti1a potently inhibited transport, while anti-Syn7, anti-Syn10, or an irrelevant rabbit control IgG mixture had no significant effect. The use of anti-Syn16 at higher doses did not significantly increase inhibition (Fig. 2 C, Syn16[200]), indicating that either the antibody itself was only partially inhibitory, or that Syn16 containing complexes are not the only ones controlling STxB transport to the TGN. Prebinding of anti-Syn16 to recombinant His-tagged Syn16 completely abolished the inhibitory effect of the antibody (Fig. 2 C). The anti-Syn16 effect was not due to cross-linking of Syn16 on target membranes, since monovalent Fab fragments generated from the antibody also potently inhibited transport (Fig. 2 C). Prebinding of Fab fragments to His-tagged Syn16 resulted in the loss of inhibition (Fig. 2 C). Taken together, these results suggest that Syn16, Syn6, and Vti1a participate in STxB transport to the TGN.
Interestingly, we observed that the inhibitory effects of anti-Syn6 and anti-Syn16 antibodies were not additive (Fig. 2 E), indicating that their respective target molecules function in the same transport pathway, possibly within the same molecular complex. The latter indication was tested by coimmunoprecipitation. Syn16 was immunoprecipitated from Triton X-100 lysates of HeLa cells. The precipitate was then probed by immunoblot for the presence of a cis-Golgi SNARE, Syn5 (Dascher et al., 1994; Rowe et al., 1998), the Golgi/TGN and endosomal SNAREs Syn6, Vti1a, and Vti1b (Bock et al., 1997; Advani et al., 1998; Xu et al., 1998), and the endosomal SNAREs Syn7 and Syn12 (Tang et al., 1998c; Nakamura et al., 2000). Syn6 was coimmunoprecipitated with Syn16 (Fig. 2 F), confirming their physical association. Vti1a was also found in the Syn16 immunoprecipitate (Fig. 2 F), an observation that is consistent with the inhibitory effect of the anti-Vti1a antibody on transport to the TGN (Fig. 2 B). In a separate experiment, both Vti1a and Syn16 were also coimmunoprecipitated with Syn6 (unpublished data).
We used an overexpression approach to further confirm the role of Syn6 and Syn16 in retrograde transport to the TGN in intact cells. Previous studies had indicated that the cellular TGN marker protein TGN38, like STxB, cycles from EE/RE to the TGN (Ghosh et al., 1998; Mallard et al., 1998). As syntaxins are tail-anchored proteins with a majority of the polypeptide exposed to the cytosol, the cytosolic domains (cyto) of these molecules are expected to function as dominant negative mutants. We therefore tested whether expression of Syn6-cyto and Syn16-cyto would have an effect on the trafficking of TGN38 in CHO cells stably expressing a Tac epitope–tagged version of the protein (Ghosh et al., 1998). In most untransfected cells or cells expressing Syn7-cyto, anti-Tac antibody, added from the outside of the cell, was found to accumulate in the perinuclear Golgi area (Fig. 2 G). In contrast, >50% of cells expressing Syn16-cyto or Syn6-cyto did not display Golgi-accumulation (Fig. 2 G), indicating that the soluble cytosolic domains of Syn6 and Syn16 blocked TGN38 transport to the TGN. Similar results were obtained when STxB EE/RE-to-TGN transport was followed in cytosolic domain expressing cells (unpublished data). In conclusion, the same Golgi/TGN-localized t-SNAREs appeared to regulate the retrograde transport of two markers, STxB and TGN38, to the TGN.
Identification of v-SNAREs in EE-to-TGN transport
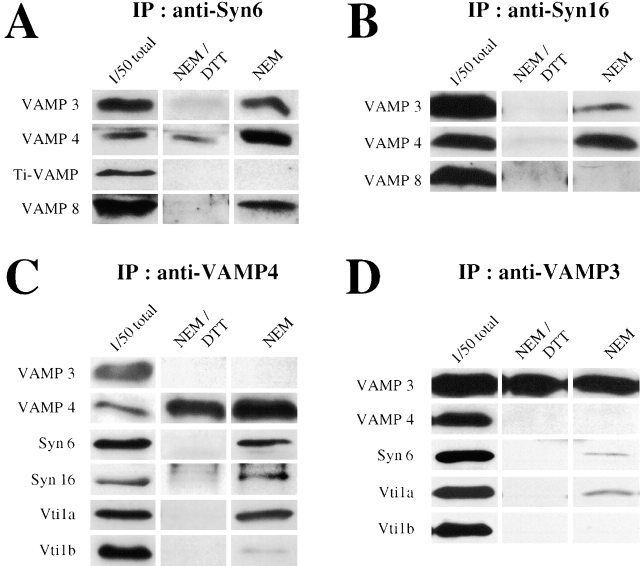
A SNARE-mediated EE/RE-to-TGN transport model would implicate an interaction between the TGN-localized Syn6/Syn16/Vti1a t-SNAREs and putative early endosomal v-SNAREs. Therefore, anti-Syn6 or anti-Syn16 immunoprecipitates were analyzed for the presence of four VAMP family proteins known to be localized to endosomes: VAMP3/cellubrevin (McMahon et al., 1993; Galli et al., 1994), VAMP4 (Steegmaier et al., 1999), VAMP7/TI-VAMP (Galli et al., 1998), and VAMP8/endobrevin (Wong et al., 1998). Intact cells were treated before lysis either with NEM to accumulate cognate SNARE complexes, or with DTT-inactivated NEM as a control. An increase of SNARE complex recovery after NEM treatment indicates that the complexes did not form after lysis and dilution (Galli et al., 1998). VAMP3/cellubrevin, VAMP4, and VAMP8/endobrevin coimmunoprecipitated with Syn6 (Fig. 3 A). Of these, only VAMP4, and to a lesser extent VAMP3/cellubrevin coimmunoprecipitated with Syn16 (Fig. 3 B). Association of VAMP3/cellubrevin and VAMP4 with Syn6 and Syn16 implied that they were candidate v-SNAREs for the regulation of EE-to-TGN transport.
Figure 3.
Identification of v-SNAREs in STxB transport from the EE to the TGN. Cells were treated either with NEM, or NEM quenched with DTT (NEM/DTT) before lysis in immunoprecipitation buffer. IP, immunoprecipitation. (A) VAMP3/cellubrevin, VAMP4, and VAMP8/endobrevin were coimmunoprecipitated with Syn6, but not VAMP7/TI-VAMP. (B) Among the VAMPs that interacted with Syn6, only VAMP3/ cellubrevin and VAMP4 were also coimmunoprecipitated with Syn16. (C) Antibodies to VAMP4 coimmunoprecipitated Syn6, Syn16, and Vti1a, and immunoprecipitated VAMP4 itself. Vti1b could also be detected, but to a much lesser extent than Vti1a. (D) Antibodies to VAMP3/cellubrevin immunoprecipitated VAMP3/cellubrevin itself, and coimmunoprecipitated Syn6 and Vti1a, but not Vti1b. The presence of Syn16 on blots could not be resolved due to its close proximity to the heavy chains of anti-VAMP3/cellubrevin antibody used for the immunoprecipitation. Note that anti-VAMP4 did not coimmunoprecipitate VAMP3/cellubrevin (C), and vice versa (D).
Antibodies to VAMP4 or VAMP3/cellubrevin also coimmunoprecipitated Syn6 and Vti1a (Fig. 3, C–D). Again, VAMP4 was more efficient than VAMP3/cellubrevin, suggesting that VAMP4 may be more important for retrograde transport at the EE/RE-TGN-interface than VAMP3/cellubrevin. Syn16 was clearly detected in the anti-VAMP4 immunoprecipitate (Fig. 3 C), whereas a smear from the heavy chain prohibited its detection in the anti-VAMP3/cellubrevin immunoprecipitate (unpublished data). A small amount of Vti1b could be found in the anti-VAMP4 immunoprecipitate (Fig. 3 C), much less than for Vti1a. Importantly, antibody to VAMP4 did not coimmunoprecipitate VAMP3/cellubrevin, and vice versa (Fig. 3, C–D). This observation suggests that both v-SNAREs, VAMP4 and VAMP3/cellubrevin, interacted independently with the same t-SNAREs.
To study the function of VAMP4, an antibody was made against a peptide from the NH2 terminus of the protein (residues 1–15) with maximal sequence divergence compared with other VAMPs. Anti-VAMP4 inhibited EE/RE-to-TGN transport in a dose dependent manner, and inhibition was lost when the antibody was prebound to its antigen (Fig. 4 A). We furthermore observed that purified VAMP4-cyto inhibited STxB transport to the TGN on permeabilized HeLa cells, whereas VAMP8-cyto had no effect (Fig. 4 A). As shown for Syn6 and Syn16 (Fig. 2 G), overexpression of VAMP4-cyto also inhibited TGN38 cycling to the TGN, whereas VAMP7-cyto was without effect (Fig. 4 B). Thus, VAMP4 function was necessary not only for retrograde transport of STxB, but also for that of TGN38.
Figure 4.
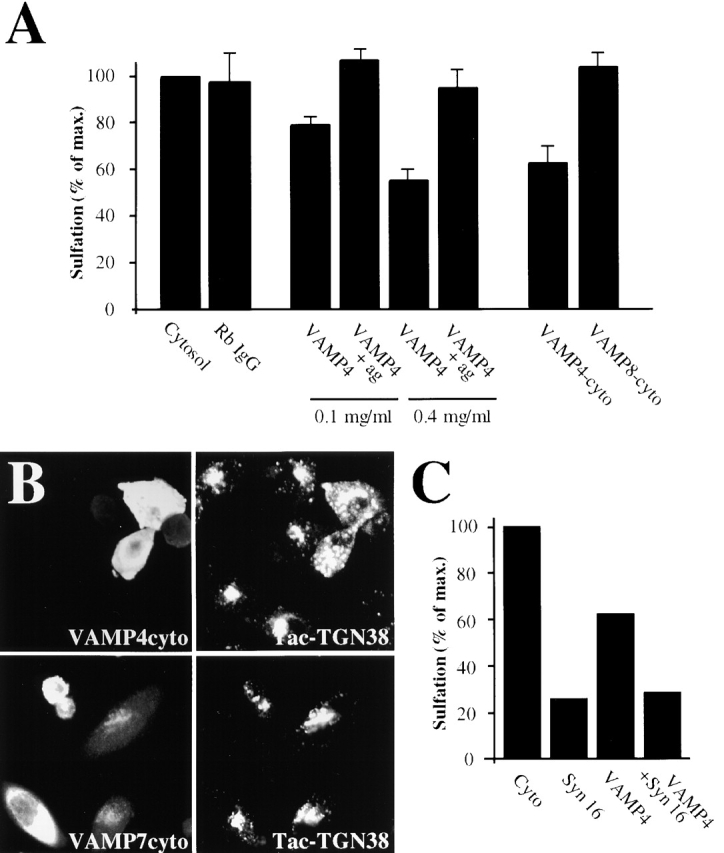
Functional implication of VAMP4 in EE-to-TGN transport. An experimental protocol as shown in Fig. 1 A was used. (A) Permeabilized HeLa cells were incubated in the continuous presence of 0.1 or 0.4 mg/ml of anti-VAMP4 antibody, 0.4 mg/ml of control Rb IgG, or 0.25 mg/ml recombinant cytosolic fragments of VAMP4 (VAMP4-cyto) or VAMP8 (VAMP8-cyto). STxB-Sulf2 transport from EE/RE to the TGN was sampled by sulfation analysis. The inhibitory anti-VAMP4 effect was reversed by prebinding of the antibody to its peptide antigen. Peptide antigen alone had no effect on transport (unpublished data). Means of three to seven experiments (± SEM) are shown. (B) Expression of VAMP4-cyto inhibited anti-Tac antibody transport to the TGN in CHO cells expressing Tac-TGN38, whereas expression of VAMP7-cyto had no effect. (C) Antibodies against VAMP4 and Syn16 had no additive inhibitory effects on STxB-Sulf2 transport from EE/RE to the TGN, suggesting that both molecules function within the same molecular complex. One experiment representative of two is shown.
VAMP4 and Syn16 appeared to interact physically and functionally. Indeed, antibodies against Syn16 and VAMP4, when combined, had no additive inhibitory effects (Fig. 4 C), suggesting that these two molecules functioned in the same molecular complex, as described above for Syn6 and Syn16 (Fig. 2, E–F).
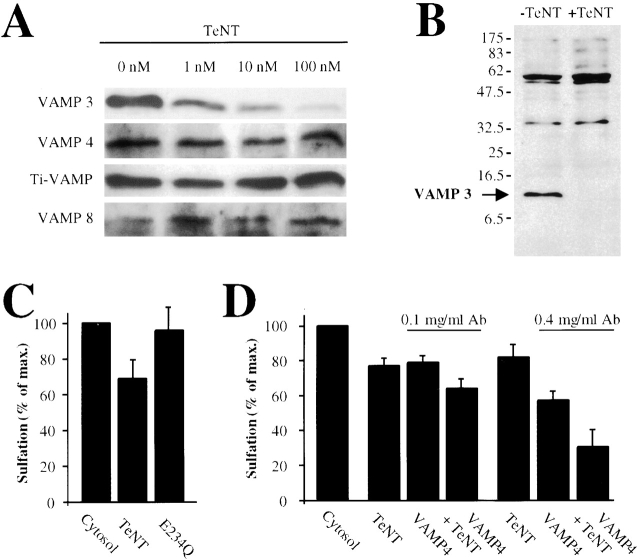
Our anti-peptide antibody against the NH2 terminus of VAMP3/cellubrevin was without effect, even at high concentrations (unpublished data). Because it has been reported that VAMP3/cellubrevin is cleaved by tetanus neurotoxin (TeNT) (Galli et al., 1994), we tested whether TeNT would modulate transport. As shown in Fig. 5 A, TeNT cleaved VAMP3/cellubrevin (Galli et al., 1994), but not VAMP7/TI-VAMP (Galli et al., 1998), VAMP4, or VAMP8/endobrevin. Furthermore, an antibody that recognizes all synaptobrevin-like and TeNT-sensitive VAMPs (clone 10.1) (McMahon et al., 1993) revealed that VAMP3/cellubrevin was the only member of this group to be expressed in HeLa cells (Fig. 5 B). At TeNT concentrations above 100 nM, a condition under which most VAMP3/cellubrevin was degraded (Fig. 5 A), EE/RE-to-TGN transport was inhibited by 25%, whereas an inactive mutant of TeNT, TeNT (E234Q), was without effect (Fig. 5 C). As a whole, these data suggest that the TeNT effect was due to modulation of VAMP3/cellubrevin activity.
Figure 5.
Putative role of VAMP3/cellubrevin in EE/RE-to-TGN transport. (A) TeNT was added at the indicated doses to SLO-permeabilized HeLa cells. Lysates from these cells were blotted for the indicated v-SNAREs. Note that among the tested proteins, only VAMP3/cellubrevin was cleaved. (B) TeNT-treated and control HeLa cell extracts were probed with an antibody (10.1) which recognizes the synaptobrevin-like members of the VAMP family. (Arrow) Migration of VAMP3/cellubrevin on a parallel blot. (C) The incubation of permeabilized cells with TeNT led to a partial inhibition of STxB-Sulf2 transport to the TGN, whereas the TeNT mutant E234Q was without effect. (D) When anti-VAMP4 antibody and TeNT were used in the same reactions, additive inhibition of STxB-Sulf2 transport was observed, suggesting that VAMP4 and the TeNT targets function in different molecular complexes or in parallel pathways. In C and D, an experimental protocol as shown in Fig. 1 A was used. Means of three to nine experiments (± SEM) are shown.
Importantly, when TeNT and anti-VAMP4 antibody were added together, transport inhibition was additive (Fig. 5 D). This observation is consistent with the above-mentioned hypothesis that both v-SNAREs interact independently with the Syn6/Syn16/Vti1a t-SNAREs (Fig. 3). The level of residual transport (Fig. 5 D) was comparable to that observed in the presence of anti-Syn6, anti-Syn16, or anti-Vti1a antibodies (Fig. 3 B), again confirming that these molecules act in the same transport step.
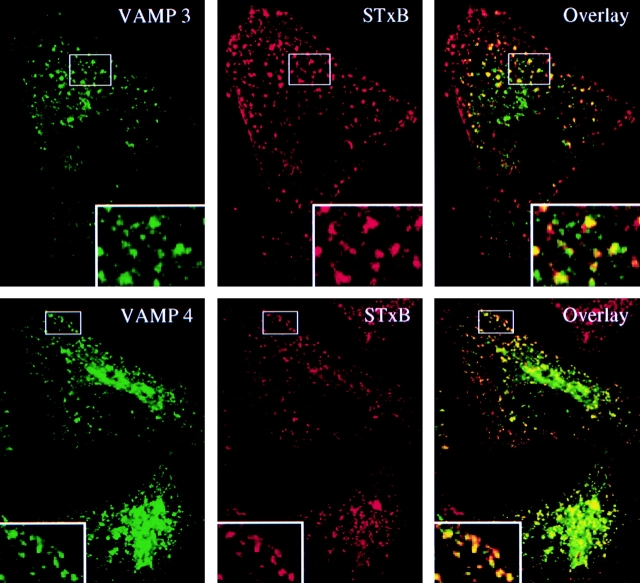
To further investigate the potential role of VAMP3/cellubrevin and VAMP4 in EE/RE-to-TGN transport, the distribution of both proteins was compared with that of STxB internalized at low temperatures into the EE (Fig. 6). Many of the STxB containing early endosomal structures were also labeled with VAMP3/cellubrevin. Using anti-VAMP4 (unpublished data) or GFP-coupled VAMP4 (Fig. 6), similar observations were made.
Figure 6.
VAMP3/cellubrevin and GFP-VAMP4 colocalized with STxB on membranes of EE/RE. Cy3-labeled STxB was internalized at low temperatures into EE/RE of untransfected (VAMP3) or GFP-VAMP4–transfected HeLa cells. The cells were then fixed and stained with anti-VAMP3, where indicated. A subset of STxB containing structures were also labeled for VAMP3/cellubrevin or VAMP4.
The role of Rab6a′ in EE/RE-to-TGN transport
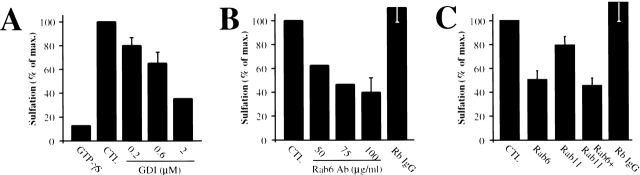
The addition of the nonhydrolyzable GTP-analogue GTPγS to the permeabilized cell assay resulted in a strong inhibition of EE/RE-to-TGN transport (Fig. 7 A), implicating a function for GTPases. We further showed the involvement of proteins of the Rab family of small GTPases (Zerial and McBride, 2001), as their removal from membranes by preincubation of SLO-permeabilized cells with recombinant Rab-GDI (GDP-dissociation inhibitor) resulted in a strong inhibition of transport (Fig. 7 A). An antibody against Golgi-localized Rab6 potently inhibited EE/RE-to-TGN transport (Fig. 7 B). The inhibition obtained with the anti-Rab6 antibody was markedly stronger than that observed with an antibody to Rab11 (Fig. 7 C), which we had previously implicated in the regulation of membrane dynamics at the RE-TGN interface (Wilcke et al., 2000). The combined use of both antibodies did not lead to a significant increase in inhibition (Fig. 7 C).
Figure 7.
The Rab6 and Rab11 regulate EE/RE-to-TGN transport. (A) Permeabilized HeLa cells were incubated either continuously with GTPγS (1 mM), or pretreated with the indicated concentrations of recombinant Rab-GDI. (B) The indicated concentrations of anti-Rab6 antibody or control rabbit IgG (0.1 mg/ml) were continuously present from permeabilization on. (C) Anti-Rab6 (75 μg/ml) and anti-Rab11 (100 μg/ml) antibodies did not have additive inhibitory effects when added at the same time to the permeabilized cell assay.
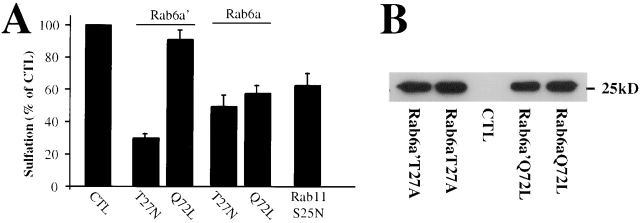
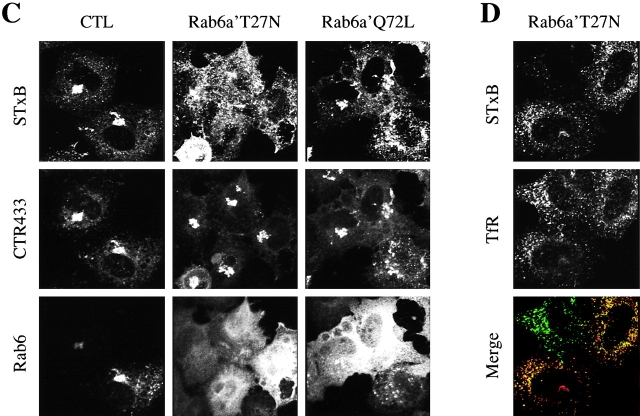
Recently it has become clear that mammalian cells express two Rab6 isoforms, termed Rab6a and Rab6a′ (Echard et al., 2000). Rab6a and Rab6a′ differ in only three amino-acids, and available anti-Rab6 antibodies recognize both proteins equally well (Echard et al., 2000). To discriminate between Rab6a and Rab6a′ function, mutants were overexpressed in HeLa cells (Fig. 8 A). A dominant-negative Rab6a′ mutant (Rab6a′T27N) was found to be a potent inhibitor of EE/RE-to-TGN transport, as measured by the sulfation assay (Fig. 8 A) or immunofluorescence analysis in transfected cells (Fig. 8 C). In cells overexpressing Rab6a′T27N, STxB accumulated in transferrin receptor containing EE/RE (Fig. 8 D). In contrast, the GTPase deficient mutant Rab6a′Q72L only weakly affected transport (Fig. 8 A), an effect that probably coincided with a mild alteration of Golgi morphology (Fig. 8 C). These findings thus suggested that Rab6a′ regulates retrograde transport to the TGN.
Figure 8.
Specific role of the Rab6a' isoform in EE/RE-to-TGN transport. (A) Sulfation analysis on intact cells overexpressing the indicated Rab6a and Rab6a′ mutants or a dominant negative Rab11 mutant. Note that dominant negative Rab6a′T27N strongly inhibited retrograde transport to the TGN. (B) Western blot analysis of the expression levels of the indicated Rab6 mutants. All mutants were equally well expressed. CTL, empty vector transfected cells. (C) Cy3-labeled STxB was internalized for 45 min at 37°C into cells expressing the indicated proteins. The cells were then fixed and stained with antibodies to the medial Golgi marker CTR433 or Rab6. (D) In Rab6a′T27N–expressing cells, STxB (red) accumulated in transferrin receptor (TfR, green) containing EE/RE.
On the other hand, both Rab6aT27N and Rab6aQ72L inhibited transport by ∼50% (Fig. 8 A). The block induced by Rab6aQ72L was actually expected, as this mutant causes redistribution of Golgi membranes (Martinez et al., 1997) including the TGN-localized t-SNAREs Syn6, Syn16, and Vti1a (unpublished data). The inhibitory effect of Rab6aT27N, although less pronounced than that of Rab6a′T27N, was more surprising in the light of its function in Golgi-to-ER transport (Martinez et al., 1997). However, it should be pointed out that all Rab6-binding proteins identified so far interact with both Rab6a and Rab6a′. The only noticeable exception is the Rab6a interacting protein Rabkinesin-6 (Echard et al., 2000), a molecular motor likely to be involved in the long-range movement of Rab6a positive structures between the Golgi and the ER (Echard et al., 1998; White et al., 1999). Therefore, overexpression of Rab6aT27N might titrate molecules required for Rab6a′ function, resulting in an indirect inhibition of EE/RE-to-TGN transport.
All Rab6 mutants that were used in this study were expressed to similar levels (Fig. 8 B). The specificity of our observations is illustrated by the fact that overexpression under identical conditions as those described above of a dominant negative Rab4 mutant did not affect post-Golgi retrograde transport (unpublished data). Furthermore, recently published data suggests that dominant negative Rab5 does not interfere with STxB transport to the Golgi apparatus (Nichols et al., 2001). In view of the potent and specific inhibition of retrograde transport by Rab6a′T27N, we therefore conclude that Rab6a′ function is required in this transport step.
Discussion
STxB and TGN38 are both transported from EE/RE directly to the TGN (Ghosh et al., 1998; Mallard et al., 1998), bypassing the late endocytic pathway taken by mannose 6-phosphate receptors (Lombardi et al., 1993). Through this direct pathway, both proteins efficiently escape the route to lysosomes. Furthermore, STxB only marginally recycles (Mallard et al., 1998), raising the question of the molecular mechanisms that are responsible for its efficient transport to the TGN. To address this question, we reconstituted STxB transport from EE/RE to the TGN in SLO-permeabilized HeLa cells. Transport in permeabilized cells conserved the hallmarks of transport in intact cells. First, it occurred with comparable kinetics in intact and permeabilized cells and was cytosol- and energy-dependent, as expected for a classical vesicular transport process. Second, we could show by electron microscopy that in permeabilized cells, STxB accumulated in a cytosol-dependent manner in TGN46- and GalT-positive Golgi compartments, as in intact cells. Third, modulating the activity of specific syntaxins, VAMP and Rab proteins had the same effects on EE/RE-to-TGN transport in both, permeabilized and intact cells. Taken together, these data indicate that EE/RE-to-TGN transport is efficiently reconstituted in SLO-permeabilized HeLa cells.
Specific SNAREs regulate EE/RE-to-TGN transport
In recent years, it has become clear that SNARE proteins are key regulators of membrane fusion (Rothman and Wieland, 1996; Hay and Scheller, 1997; Johannes and Galli, 1998). As each SNARE has a precise subcellular distribution, it has been suggested that selective interactions between SNAREs contribute to the specificity of membrane exchanges between intracellular compartments. This concept has recently been further documented by in vitro (McNew et al., 2000) and permeabilized cell (Scales et al., 2000) studies. Our data is in accordance with this concept of specific SNARE interactions. In fact, we show by coimmunoprecipitation that Syn16 interacts with Syn6 and Vti1a, but not Syn5, Syn7, Syn12, or Vti1b, and Syn6/Syn16/Vti1a interacts with VAMP3/cellubrevin and VAMP4, but not with VAMP7/TI-VAMP or VAMP8/endobrevin.
In agreement with the interaction studies, we found that EE/RE-to-TGN transport depends on two endosomal v-SNAREs. Indeed, anti-VAMP4 antibody and TeNT, which does not cleave VAMP4, both inhibited STxB and TGN38 transport to the TGN. The only TeNT-sensitive v-SNARE that we could detect in HeLa cells was VAMP3/cellubrevin, thus identifying this protein as a second candidate v-SNARE. Importantly, TeNT and anti-VAMP4 antibody had additive inhibitory effects, supporting the function of these v-SNAREs in separate molecular complexes. This conclusion is given further credence by the observation that VAMP3/cellubrevin did not coimmunoprecipitate with VAMP4, and vice versa.
The above-mentioned interaction data also suggests the formation of a specific t-SNARE complex between Syn6, Syn16, and Vti1a, and we indeed observed that Syn6, but not Syn5, stimulated binding of Syn16 to GST-VAMP4 (unpublished data). However, due to the low solubility of Syn16, binding was inefficient and final evidence for the existence of a stable and functional Syn6/Syn16/Vti1a t-SNARE complex will require in vitro fusion studies with VAMP3/cellubrevin and VAMP4 containing liposomes, as described by McNew and colleagues (2000).
In this context it appears noteworthy that Syn6 is not only found on the TGN, but also on the EE (Bock et al., 1996, 1997), and the protein interacts with a number of early endosomal v-SNAREs, such as VAMP3/cellubrevin (Bock et al., 1997), VAMP4 (Steegmaier et al., 1999), and VAMP8/endobrevin (this study). Furthermore, Syn6 is the most distant member of the syntaxin family, and its sequence is similar to the SN2 domain of SNAP23/25/29, much as Vti1a is similar to the SN1 domain (Bock et al., 2001). Therefore, it appears likely that Syn6 and Vti1a may serve as light chains of t-SNARE complexes with a more general function at the EE/RE-TGN interface (Bock et al., 2001). In contrast, Syn16 localization on the TGN appears to be very restrictive (this study), suggesting that this molecule is of primary importance for the definition of specificity in EE/RE-to-TGN transport.
The yeast SNARE proteins Tlg1p and Tlg2p show sequence similarity with Syn6 and Syn16, respectively. Conflicting results have been obtained on the function(s) of these proteins. They have been implicated in several pathways, such as cytosol-to-vacuole (Tlg2p) (Abeliovich et al., 1999), EE-to-TGN (Tlg1p and Tlg2p) (Lewis et al., 2000), endocytosis (Tlg2p) (Seron et al., 1998), and intra-Golgi transport (Tlg1p) (Coe et al., 1999). Assuming that Tlg1p and Tlg2p are in fact functional homologues of Syn6 and Syn16, respectively, our results establish their role in EE/RE-to-TGN transport. Indeed, in addition to the approach of expression of dominant negative mutants in whole cells, we have also used a permeabilized cell approach that allows the reconstitution of the key retrograde transport steps within the complex array of post-Golgi traffic. Similarly, the role of Vti1 proteins in yeast (Lupashin et al., 1997; Fischer von Mollard and Stevens, 1999; Ungermann et al., 1999; Sato et al., 2000), plants (Zheng et al., 1999), and mammals (Xu et al., 1998) has remained a matter of debate, particularly in the light of their multiple interactions with other SNAREs. Using in vitro assays, the mammalian Vti1b was found to function in homotypic fusion of late endosomes, and Vti1a in that of the early endosome (Antonin et al., 2000). In conjunction with our data, the latter observation indicates a more generalized role for Vti1a in membrane fusion on the EE.
A Rab6 isoform regulates EE/RE-to-TGN transport
In addition to the previously suggested role of Rab11 in EE/RE-to-TGN transport (Wilcke et al., 2000), we have now provided evidence that another Rab protein, Rab6, is involved in this transport step. Lower eukaryotes, such as the yeast Saccharomyces cerevisiae and the parasite Plasmodium falciparum express only one Rab6 protein (Echard et al., 2000). In mammalian cells, an exon duplication within the Rab6a gene has given rise to two different isoforms, Rab6a and Rab6a′, of which the latter appears to represent the ancestral form of Rab6 (Echard et al., 2000). In agreement with the hypothesis that Ypt6p and Rab6a′ are evolutionarily related, we found here that the Rab6a′ isoform specifically regulates retrograde transport to the TGN, as suggested for Ypt6p in yeast (Tsukada and Gallwitz, 1996; Bensen et al., 2001; Siniossoglou and Pelham, 2001). Because Ypt6p/Rab6a′ are located on TGN membranes, their function could be to regulate targeting/docking of EE/RE-derived vesicles with the TGN.
Without ruling out that also Rab6a may play a role in docking of EE/RE-derived vesicles at the TGN, Rab6a was previously shown to function in Golgi-to-ER retrograde transport (Martinez et al., 1997; Girod et al., 1999; White et al., 1999). It appears therefore that in mammalian cells, two isoforms of the same Rab protein control successive steps in retrograde transport. In addition, through an interaction with Rabkinesin-6 (Echard et al., 2000), Rab6a may participate in the coordination between retrograde membrane transport and progression through the cell cycle (Hill et al., 2000; Fontijn et al., 2001).
The complexity of the EE/RE-to-Golgi retrograde transport route in mammalian cells
The fact that EE/RE-to-TGN transport is regulated by specific SNAREs and Rab proteins suggests that it is a physiological, preexisting cellular pathway. The question arises as to whether transport from the EE, which is accessible during low temperature incubations used as a starting point in this study, to the TGN necessarily transits via the RE. That such a passage exists appears likely considering the accumulation of TGN38 (Ghosh et al., 1998) and STxB (Mallard et al., 1998) in the RE. Furthermore, modulating the activity of Rab11, a marker of the RE (Sheff et al., 1999), affects post-Golgi retrograde transport to the TGN (this study and Wilcke et al., 2000). However, both in permeabilized and intact cells, the Rab11 effect is weak as compared with the modulation of Rab6a′ activity. One interpretation of this data is that both GTPases act on two sequential steps of retrograde transport to the TGN, a hypothesis that is in agreement with the observation that modulating the activities of Rab6 and Rab11 in the same system does not result in additive inhibition of retrograde transport. Rab6a′ would also control an additional, Rab11-independent transport reaction to the TGN. VAMP3/cellubrevin- and VAMP4-dependent transport inhibition is additive, but not complete. Whether this reflects technical limitations or a high degree of versatility in membrane exchange between the early endocytic pathway and the TGN, as reported by Lippincott-Schwartz and colleagues (Nichols et al., 2001), remains to be determined.
In this study, we have provided the first molecular dissection of the EE/RE-to-TGN retrograde transport step in mammalian cells. The observed transport may correspond to the one required in S. cerevisiae for proper localization and/or recycling of TGN proteins. Indeed, the same molecules appear to be involved: Rab6a′ may be the functional homologue of Ypt6p, and Tlg1p and Tlg2p, recently found to interact with Ypt6p via the Vps52/53/54p complex (Siniossoglou and Pelham, 2001), show sequence similarity with Syn6 and Syn16, respectively. However, the fact that in the mammalian system two v-SNAREs appear to functionally interact with the same t-SNAREs suggests that EE/RE-to-TGN transport has gained in complexity during evolution, with the appearance of the RE and the involvement of Rab11 in higher eucaryotes.
Materials and methods
Recombinant proteins, antibodies, other reagents and media
TeNT and TeNT(E234Q) light chains (Galli et al., 1994), (His)6-α-SNAP, (His)6-α-SNAP(L294A) (Barnard et al., 1997), and STxB-Sulf2 (Mallard et al., 1998), were obtained as described. Polyclonal antibodies against the cytosolic domains of Syn6, Syn7, Syn10, Syn16, and Vti1a, or the first 15 amino acids of human VAMP4 were produced in rabbits. Control rabbit IgG (Sigma-Aldrich), monoclonal anti-Syn6, anti-Vti1a, anti-Vti1b antibodies (Transduction Laboratories), SLO, a gift of Dr. S. Bhakdi (Johannes-Gutenberg-Universität, Mainz, Germany), and radioactive sulfate (Amersham Pharmacia Biotech) were purchased. HBSS and MEM without sulfate (Johannes et al., 1997) and radiolabeled [35S]-PAPS (Itin et al., 1997) were prepared as described. ATP regenerating system was made of 1 mM ATP, 0.2 mM GTP (omitted where indicated), 50 μM UTP (Boehringer), 5 mM creatine phosphate and 15 μg/ml creatine phosphokinase (Sigma-Aldrich) in 10 mM Hepes, pH 7.2.
HeLa S3 cytosol
6 × 109 HeLa S3 cells were resuspended in 20 ml (final volume) ice-cold HK buffer (90 mM KCl, 50 mM Hepes, pH 7.2) supplemented with protease inhibitor cocktail. Cells were then homogenized in a potter. The supernatant of low-speed (10 min, 6,000 g, 4°C) and high-speed centrifugation (45 min, 105 g, 4°C) was dialyzed against ICT/DTT buffer (78 mM KCl, 4 mM MgCl2, 8.4 mM CaCl2, 10 mM EGTA, 1 mM DTT, 50 mM Hepes, pH 7.2). Protein concentration was around 10 mg/ml.
Immunofluorescence, immunoelectron microscopy, and immunoprecipitation.
Immunofluorescence and immuno-EM were performed essentially as described (Johannes et al., 1997). Coimmunoprecipitations: NRK or HeLa cells were lysed in 50 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, 0.1 mM PMSF and a protease inhibitor cocktail, and lysates were incubated with affinity-purified antibodies and protein A-Sepharose beads or IgG-coupled Dynabeads (Dynal). Immunoprecipitates were tested by Western blotting for the indicated proteins. In some cases, cells on ice were treated with either 1 mM NEM for 15 min followed by 15 min with 2 mM DTT, or for 30 min with 1 mM NEM quenched with 2 mM DTT, followed by incubated for 30 min at 37°C before lysis and immunoprecipitation.
STxB transport assay in SLO-permeabilized HeLa cells.
105 HeLa cells were incubated for 90 min in MEM without sulfate at 37°C and for 45 min at 19.5°C in the presence of 1 μM STxB-Sulf2. Cells were then placed on ice and incubated for 10 min in the presence of 1 μg/ml of SLO in ICT/DTT. Unbound SLO was removed by washes and cells were transferred to 37°C for 10 min in ICT/DTT. Transport of STxB-Sulf2 to the TGN was assayed by incubating the permeabilized cells for 30 min at 37°C in the presence of cytosol mix (3 mg/ml cytosol and ATP regenerating system in ICT/DTT, except when otherwise stated), supplemented with 1 μCi/ml [35S]sulfate (or 10 μCi/ml [35S]-PAPS where indicated). After incubation, the cells were lysed in 1 ml RIPA buffer containing a protease inhibitor cocktail and subjected to quantitative immunoprecipitation with 13C4 antibody. Immunoprecipitated STxB-Sulf2 was separated from radioactive contaminants by migration on a Tris-Tricine gel, and the amount of sulfated STxB-Sulf2 for each condition was quantified using a PhosphorImager and the ImageQuant software (Molecular Dynamics). Sulfation of endogenous proteins and proteoglycans was quantified by scintillation counting of TCA-precipitable material. The corresponding results were used to normalize the amount of sulfated STxB-Sulf2 for each condition. Only experiments were included where endogenous sulfation was within a 10% range in all conditions.
Antibodies were added from the permeabilization step on, whereas recombinant (His)6-α-SNAP or (His)6-α-SNAP(L294A) were only added to the transport mix. For GDI or TeNT treatment, SLO-permeabilized cells were incubated at 37°C for 15 min in the presence of, respectively, 1 μM GDIβ, 100 nM TeNT or TeNT(E234Q).
STxB retrograde transport assay on intact cells
The indicated Rab proteins or cytosolic domains of syntaxins (residues 1–234 of Syn6, residues 1–237 of Syn7, and residues 1–284 of Syn16) were overexpressed for, respectively, 6 or 10 h in intact cells using the vaccinia virus expression system, as described (Martinez et al., 1997). Transfection efficiencies were routinely >70% in all conditions. Cy3-coupled STxB and STxB-Sulf2 transport to the TGN was assayed as described (Mallard et al., 1998). After STxB-Sulf2 binding, HeLa cells were incubated for 20 min in the presence of radioactive sulfate, followed by immunoprecipitation and gel electrophoresis.
Anti-Tac uptake experiments
CHO cells expressing a chimeric TGN38 protein with an extracellular Tac domain (Ghosh et al., 1998) were transfected with expression constructs for the cytoplasmic domains of Syn6, Syn7 or Syn16. 24 h after transfection, the cells were incubated for 1 h with culture supernatant containing Tac monoclonal IgGs, washed extensively, fixed and labeled with the indicated antibodies.
Acknowledgments
We would like to thank Suzanne Pfeffer (Stanford University, Stanford, CT) and Robert Burgoyne (Liverpool University, Liverpool, UK) for the NodQ2 and the α-SNAP and α-SNAP(L294A) expression plasmids, respectively; William Mallet and Frederick Maxfield (Cornell University, Ithaca, NY) for CHO cells expressing Tac-TGN38; Siew Heng Wong (Institute for Molecular and Cell Biology, Singapore, China) for polyclonal antibody against Syn6; Reinhard Jahn (Max-Planck-Institut für Biophysikalische Chemie, Göttingen, Germany) for the clone 10.1 antibody; Solange Monier for help in some experiments; and Franck Perez and Christophe Lamaze for critical reading of the manuscript.
This work was supported by grants from the Ligue Nationale contre le Cancer and the Association pour la Recherche sur le Cancer (grants 9028 and 9254) to L. Johannes, B. Goud, and C. Antony, and by Action concertée incitative – Jeune chercheurs (grants 5254 and 5233) to T. Galli and L. Johannes. W. Hong and B.L. Tang are supported by research grants from the Singapore National Science and Technology Board.
F. Mallard and B.L. Tang contributed equally to this work.
W. Hong, B. Goud, and L. Johannes were principal investigators.
Footnotes
Abbreviations used in this paper: EE, early endosome; NEM, N-ethylmaleimide; NSF, NEM-sensitive soluble factor; PAPS, 3′-phosphoadenosine 5′-phosphosulfate; RE, recycling endosome; SLO, streptolysin O; SNAP, soluble NSF attachment protein; SNARE, SNAP receptor; STxB, Shiga toxin; Syn, syntaxin; TeNT, tetanus neurotoxin; TGN, trans-Golgi network; VAMP, vesicle associated membrane protein.
References
- Abeliovich, H., T. Darsow, and S.D. Emr. 1999. Cytoplasm to vacuole trafficking of aminopeptidase I requires a t-SNARE-Sec1p complex composed of Tlg2p and Vps45p. EMBO J. 18:6005–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advani, R.J., H.R. Bae, J.B. Bock, D.S. Chao, Y.C. Doung, R. Prekeris, J.S. Yoo, and R.H. Scheller. 1998. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J. Biol. Chem. 273:10317–10324. [DOI] [PubMed] [Google Scholar]
- Antonin, W., C. Holroyd, D. Fasshauer, S. Pabst, G. Fischer Von Mollard, and R. Jahn. 2000. A SNARE complex mediating fusion of late endosomes defines conserved properties of SNARE structure and function. EMBO J. 19:6453–6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard, R.J., A. Morgan, and R.D. Burgoyne. 1997. Stimulation of NSF ATPase activity by alpha-SNAP is required for SNARE complex disassembly and exocytosis. J. Cell Biol. 139:875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensen, E.S., B.G. Yeung, and G.S. Payne. 2001. Ric1p and the Ypt6p GTPase function in a common pathway required for localization of trans-Golgi network membrane proteins. Mol. Biol. Cell. 12:13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock, J.B., R.C. Lin, and R.H. Scheller. 1996. A new syntaxin family member implicated in targeting of intracellular transport vesicles. J. Biol. Chem. 271:17961–17965. [DOI] [PubMed] [Google Scholar]
- Bock, J.B., J. Klumperman, S. Davanger, and R.H. Scheller. 1997. Syntaxin 6 functions in trans-Golgi network vesicle trafficking. Mol. Biol. Cell. 8:1261–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock, J.B., H.T. Matern, A.A. Peden, and R.H. Scheller. 2001. A genomic perspective on membrane compartment organization. Nature. 409:839–841. [DOI] [PubMed] [Google Scholar]
- Carroll, K.S., J. Hanna, I. Simon, J. Krise, P. Barbero, and S.R. Pfeffer. 2001. Role of Rab9 GTPase in facilitating receptor recruitment by TIP47. Science. 292:1373–1376. [DOI] [PubMed] [Google Scholar]
- Coe, J.G., A.C. Lim, J. Xu, and W. Hong. 1999. A role for Tlg1p in the transport of proteins within the Golgi apparatus of Saccharomyces cerevisiae. Mol. Biol. Cell. 10:2407–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump, C.M., Y. Xiang, L. Thomas, F. Gu, C. Austin, S.A. Tooze, and G. Thomas. 2001. PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic. EMBO J. 20:2191–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher, C., J. Matteson, and W.E. Balch. 1994. Syntaxin 5 regulates endoplasmic reticulum to Golgi transport. J. Biol. Chem. 269:29363–29366. [PubMed] [Google Scholar]
- Echard, A., F. Jollivet, O. Martinez, J.-J. Lacapère, A. Rousselet, I. Janoueix-Lerosey, and B. Goud. 1998. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 279:580–585. [DOI] [PubMed] [Google Scholar]
- Echard, A., F.J. Opdam, H.J. de Leeuw, F. Jollivet, P. Savelkoul, W. Hendriks, J. Voorberg, B. Goud, and J.A. Fransen. 2000. Alternative splicing of the human Rab6a gene generates two close but functionally different isoforms. Mol. Biol. Cell. 11:3819–3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard, G., and T.H. Stevens. 1999. The Saccharomyces cerevisiae v-SNARE Vti1p is required for multiple membrane transport pathways to the vacuole. Mol. Biol. Cell. 10:1719–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fölsch, H., M. Pypaert, P. Schu, and I. Mellman. 2001. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J. Cell Biol. 152:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontijn, R.D., B. Goud, A. Echard, F. Jollivet, J. van Marle, H. Pannekoek, and A.J. Horrevoets. 2001. The human kinesin-like protein RB6K is under tight cell cycle control and is essential for cytokinesis. Mol. Cell Biol. 21:2944–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli, T., T. Chilcote, O. Mundigl, T. Binz, H. Niemann, and P. De Camilli. 1994. Tetanus toxin-mediated cleavage of cellubrevin impairs exocytosis of transferrin receptor-containing vesicles in CHO cells. J. Cell Biol. 125:1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli, T., A. Zahraoui, V.V. Vaidyanathan, G. Raposo, J.M. Tian, M. Karin, H. Niemann, and D. Louvard. 1998. A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol. Biol. Cell. 9:1437–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, R.N., W.G. Mallet, T.T. Soe, T.E. McGraw, and F.R. Maxfield. 1998. An endocytosed TGN38 chimeric protein is delivered to the TGN after trafficking through the endocytic recycling compartment in CHO cells. J. Cell Biol. 142:923–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girod, A., B. Storrie, J.C. Simpson, L. Johannes, B. Goud, L.M. Roberts, J.M. Lord, T. Nilsson, and R. Pepperkok. 1999. Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat. Cell Biol. 1:423–430. [DOI] [PubMed] [Google Scholar]
- Hay, J.C., and R.H. Scheller. 1997. SNAREs and NSF in targeted membrane fusion. Curr. Opin. Cell Biol. 9:505–512. [DOI] [PubMed] [Google Scholar]
- Hill, E., M. Clarke, and F.A. Barr. 2000. The Rab6-binding kinesin, Rab6-KIFL, is required for cytokinesis. EMBO J. 19:5711–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itin, C., C. Rancano, Y. Nakajima, and S.R. Pfeffer. 1997. A novel assay reveals a role for soluble N-ethylmaleimide-sensitive fusion attachment protein in mannose 6-phosphate receptor transport from endosomes to the trans Golgi network. J. Biol. Chem. 272:27737–27744. [DOI] [PubMed] [Google Scholar]
- Johannes, L., and T. Galli. 1998. Exocytosis: SNAREs drum up! Eur. J. Neurosci. 10:415–422. [DOI] [PubMed] [Google Scholar]
- Johannes, L., D. Tenza, C. Antony, and B. Goud. 1997. Retrograde transport of KDEL-bearing B-fragment of Shiga toxin. J. Biol. Chem. 272:19554–19561. [DOI] [PubMed] [Google Scholar]
- Khine, A.A., and C.A. Lingwood. 2000. Functional significance of globotriaosyl ceramide in interferon-alpha(2)/type 1 interferon receptor-mediated antiviral activity. J. Cell Physiol. 182:97–108. [DOI] [PubMed] [Google Scholar]
- Khine, A.A., M. Firtel, and C.A. Lingwood. 1998. CD77-dependent retrograde transport of CD19 to the nuclear membrane: functional relationship between CD77 and CD19 during germinal center B-cell apoptosis. J. Cell Physiol. 176:281–292. [DOI] [PubMed] [Google Scholar]
- Lewis, M.J., B.J. Nichols, C. Prescianotto-Baschong, H. Riezman, and H.R. Pelham. 2000. Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol. Biol. Cell. 11:23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi, D., T. Soldati, M.A. Riederer, Y. Goda, M. Zerial, and S.R. Pfeffer. 1993. Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO J. 12:677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupashin, V.V., I.D. Pokrovskaya, J.A. McNew, and M.G. Waters. 1997. Characterization of a novel yeast SNARE protein implicated in Golgi retrograde traffic. Mol. Biol. Cell. 8:2659–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard, F., D. Tenza, C. Antony, J. Salamero, B. Goud, and L. Johannes. 1998. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of Shiga toxin B-fragment transport. J. Cell Biol. 143:973–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez, O., C. Antony, G. Pehau-Arnaudet, E.G. Berger, J. Salamero, and B. Goud. 1997. GTP-bound forms of rab6 induce the redistribution of Golgi proteins into the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 94:1828–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, A. 1999. Intracellular membrane fusion: SNAREs only? Curr. Opin. Cell Biol. 11:447–452. [DOI] [PubMed] [Google Scholar]
- Meyer, C., D. Zizioli, S. Lausmann, E.L. Eskelinen, J. Hamann, P. Saftig, K. von Figura, and P. Schu. 2000. μ1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 19:2193–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon, H.T., Y.A. Ushkaryov, L. Edelmann, E. Link, T. Binz, H. Niemann, R. Jahn, and T.C. Sudhof. 1993. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature. 364:346–349. [DOI] [PubMed] [Google Scholar]
- McNew, J.A., F. Parlati, R. Fukuda, R.J. Johnston, K. Paz, F. Paumet, T.H. Söllner, and J.E. Rothman. 2000. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature. 407:153–159. [DOI] [PubMed] [Google Scholar]
- Nakamura, N., A. Yamamoto, Y. Wada, and M. Futai. 2000. Syntaxin 7 mediates endocytic trafficking to late endosomes. J. Biol. Chem. 275:6523–6529. [DOI] [PubMed] [Google Scholar]
- Nichols, B.J., A.K. Kenworthy, R.S. Polishchuk, R. Lodge, T.H. Roberts, K. Hirschberg, R.D. Phair, and J. Lippincott-Schwartz. 2001. Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153:529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohn, W.M., Y. Rouille, S. Waguri, and B. Hoflack. 2000. Bi-directional trafficking between the trans-Golgi network and the endosomal/lysosomal system. J. Cell Sci. 113:2093–2101. [DOI] [PubMed] [Google Scholar]
- Rothman, J.E., and F.T. Wieland. 1996. Protein sorting by transport vesicles. Science. 272:227–234. [DOI] [PubMed] [Google Scholar]
- Rowe, T., C. Dascher, S. Bannykh, H. Plutner, and W.E. Balch. 1998. Role of vesicle-associated syntaxin 5 in the assembly of pre-Golgi intermediates. Science. 279:696–700. [DOI] [PubMed] [Google Scholar]
- Sato, T.K., P. Rehling, M.R. Peterson, and S.D. Emr. 2000. Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol. Cell. 6:661–671. [DOI] [PubMed] [Google Scholar]
- Scales, S.J., Y.A. Chen, B.Y. Yoo, S.M. Patel, Y.C. Doung, and R.H. Scheller. 2000. SNAREs contribute to the specificity of membrane fusion. Neuron. 26:457–464. [DOI] [PubMed] [Google Scholar]
- Seron, K., V. Tieaho, C. Prescianotto-Baschong, T. Aust, M.O. Blondel, P. Guillaud, G. Devilliers, O.W. Rossanese, B.S. Glick, H. Riezman, S. Keranen, and R. Haguenauer-Tsapis. 1998. A yeast t-SNARE involved in endocytosis. Mol. Biol. Cell. 9:2873–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheff, D.R., E.A. Daro, M. Hull, and I. Mellman. 1999. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 145:123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen, A., B. Bremnes, E. Ronning, R. Aasland, and H. Stenmark. 1998. Syntaxin-16, a putative Golgi t-SNARE. Eur. J. Cell Biol. 75:223–231. [DOI] [PubMed] [Google Scholar]
- Siniossoglou, S., and H.R. Pelham. 2001. An effector of Ypt6p binds the SNARE Tlg1p and mediates selective fusion of vesicles with late Golgi membranes. EMBO J. 20:5991–5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner, T., S.W. Whiteheart, M. Brunner, H. Erdjument-Bromage, S. Geromanos, P. Tempst, and J.E. Rothman. 1993. SNAP receptors implicated in vesicle targeting and fusion. Nature. 362:318–324. [DOI] [PubMed] [Google Scholar]
- Steegmaier, M., J. Klumperman, D.L. Foletti, J.S. Yoo, and R.H. Scheller. 1999. Vesicle-associated membrane protein 4 is implicated in trans-Golgi network vesicle trafficking. Mol. Biol. Cell. 10:1957–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, B.L., D.Y. Low, S.S. Lee, A.E. Tan, and W. Hong. 1998. a. Molecular cloning and localization of human syntaxin 16, a member of the syntaxin family of SNARE proteins. Biochem. Biophys. Res. Commun. 242:673–679. [DOI] [PubMed] [Google Scholar]
- Tang, B.L., D.Y. Low, A.E. Tan, and W. Hong. 1998. b. Syntaxin 10: a member of the syntaxin family localized to the trans-Golgi network. Biochem. Biophys. Res. Commun. 242:345–350. [DOI] [PubMed] [Google Scholar]
- Tang, B.L., A.E. Tan, L.K. Lim, S.S. Lee, D.Y. Low, and W. Hong. 1998. c. Syntaxin 12, a member of the syntaxin family localized to the endosome. J. Biol. Chem. 273:6944–6950. [DOI] [PubMed] [Google Scholar]
- Thieblemont, N., and S.D. Wright. 1999. Transport of bacterial lipopolysaccharide to the Golgi apparatus. J. Exp. Med. 190:523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada, M., and D. Gallwitz. 1996. Isolation and characterization of SYS genes from yeast, multicopy suppressors of the functional loss of the transport GTPase Ypt6p. J. Cell Sci. 109:2471–2481. [DOI] [PubMed] [Google Scholar]
- Ungermann, C., G.F. von Mollard, O.N. Jensen, N. Margolis, T.H. Stevens, and W. Wickner. 1999. Three v-SNAREs and two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic fusion. J. Cell Biol. 145:1435–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, T., B.V. Zemelman, J.A. McNew, B. Westermann, M. Gmachl, F. Parlati, T.H. Sollner, and J.E. Rothman. 1998. SNAREpins: minimal machinery for membrane fusion. Cell. 92:759–772. [DOI] [PubMed] [Google Scholar]
- White, J., L. Johannes, F. Mallard, A. Girod, S. Grill, S. Reinsch, P. Keller, A. Echard, B. Goud, and E.H.K. Stelzer. 1999. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J. Cell Biol. 147:743–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcke, M., L. Johannes, T. Galli, V. Mayau, B. Goud, and J. Salamero. 2000. Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-Golgi network. J. Cell Biol. 151:1207–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, S.H., T. Zhang, Y. Xu, V.N. Subramaniam, G. Griffiths, and W. Hong. 1998. Endobrevin, a novel synaptobrevin/VAMP-like protein preferentially associated with the early endosome. Mol. Biol. Cell. 9:1549–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y., S.H. Wong, B.L. Tang, V.N. Subramaniam, T. Zhang, and W. Hong. 1998. A 29-kilodalton Golgi soluble N-ethylmaleimide-sensitive factor attachment protein receptor (Vti1-rp2) implicated in protein trafficking in the secretory pathway. J. Biol. Chem. 273:21783–21789. [DOI] [PubMed] [Google Scholar]
- Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107–117. [DOI] [PubMed] [Google Scholar]
- Zheng, H., G.F. von Mollard, V. Kovaleva, T.H. Stevens, and N.V. Raikhel. 1999. The plant vesicle-associated SNARE AtVTI1a likely mediates vesicle transport from the trans-Golgi network to the prevacuolar compartment. Mol. Biol. Cell. 10:2251–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]