Abstract
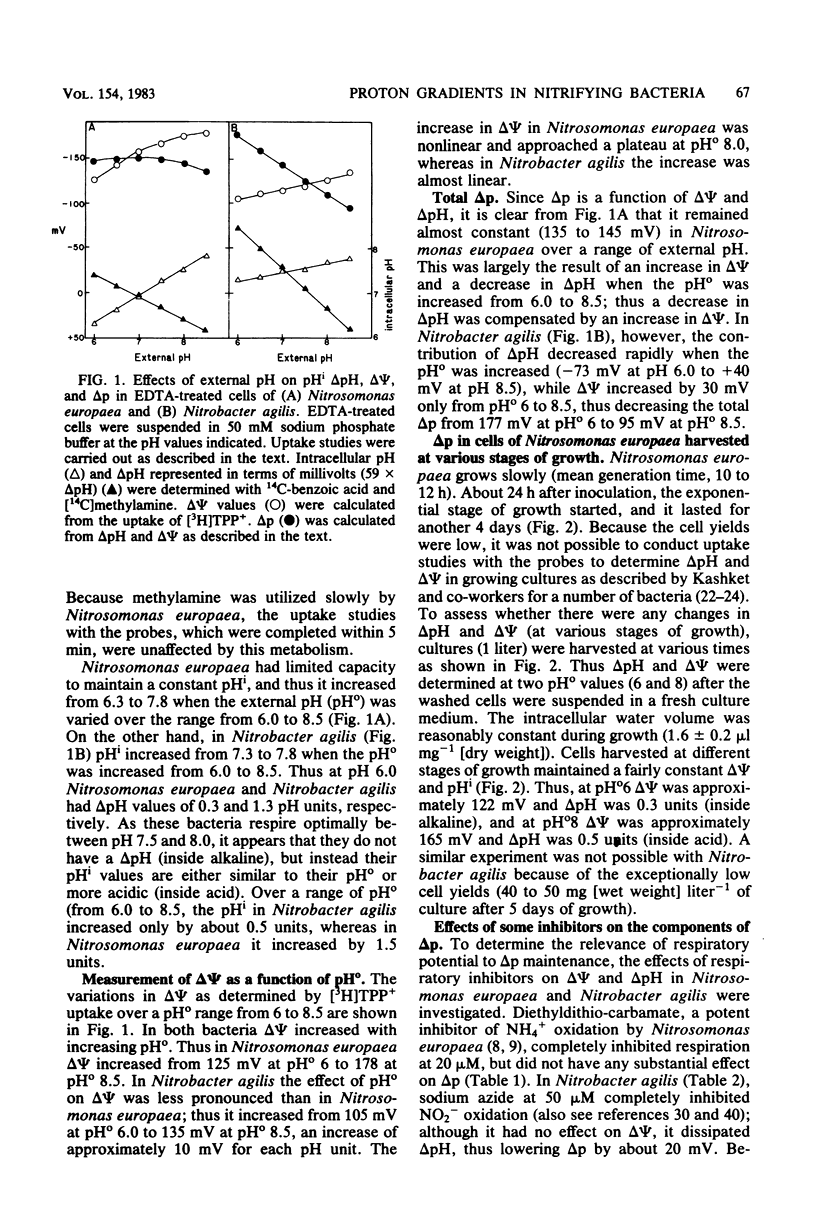
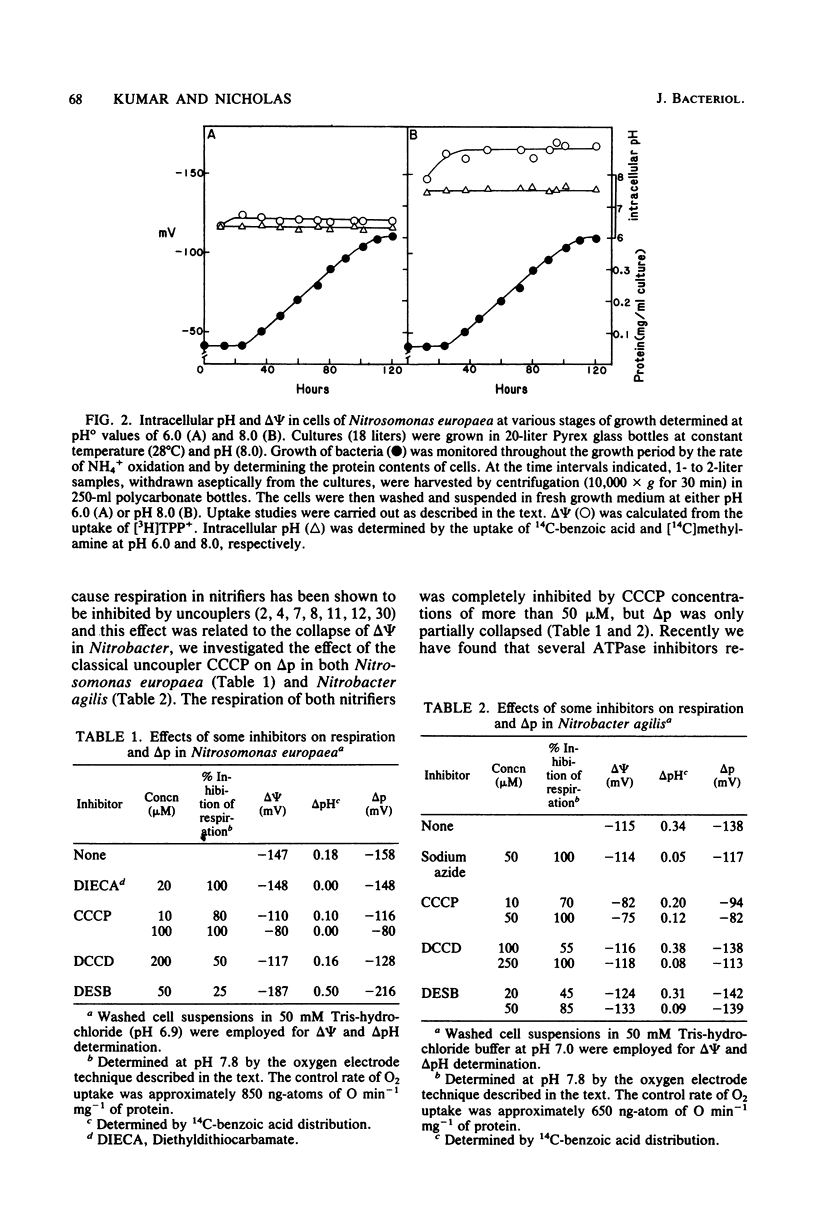
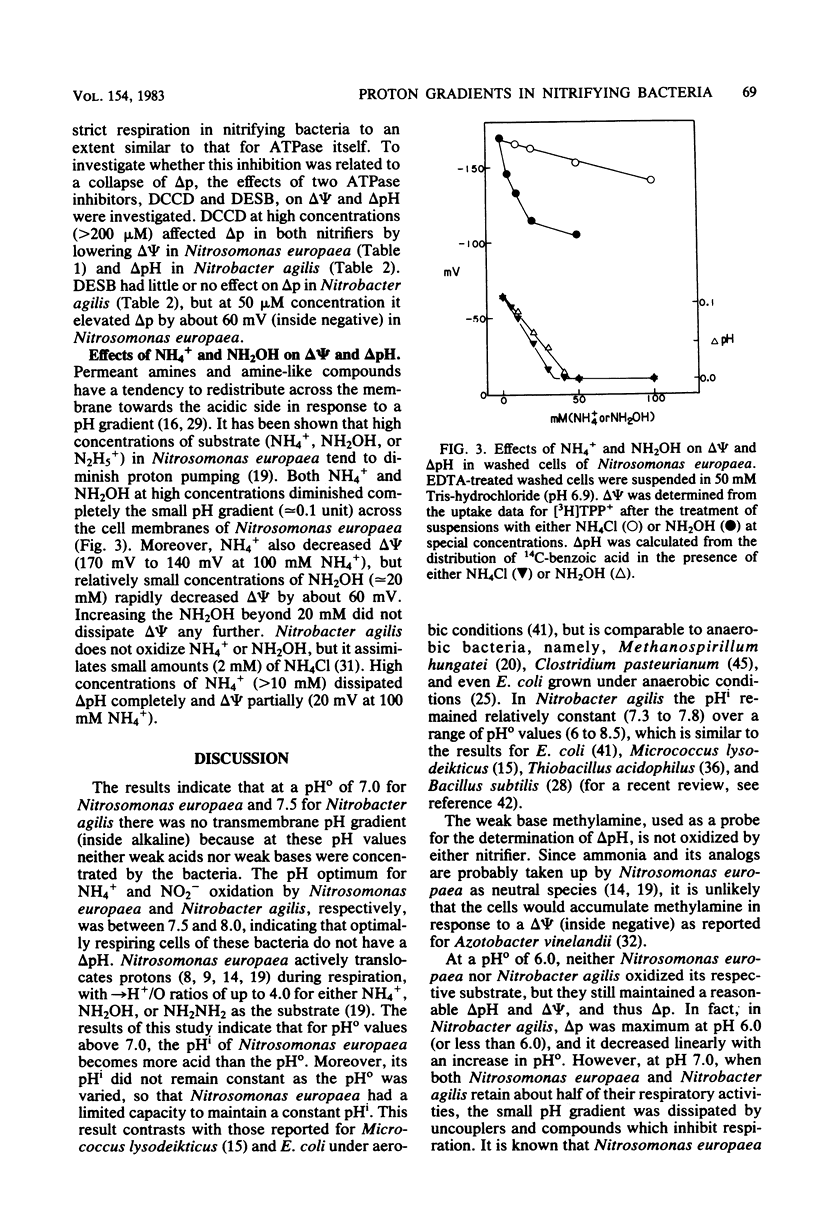
The components of the proton motive force (Δp), namely, membrane potential (Δψ) and transmembrane pH gradient (ΔpH), were determined in the nitrifying bacteria Nitrosomonas europaea and Nitrobacter agilis. In these bacteria both Δψ and ΔpH were dependent on external pH. Thus at pH 8.0, Nitrosomonas europaea and Nitrobacter agilis had Δψ values of 173 mV and 125 mV (inside negative), respectively, as determined by the distribution of the lipophilic cation [3H]tetraphenyl phosphonium. Intracellular pH was determined by the distribution of two weak acids, 14C-benzoic and 14C-acetyl salicylic, and the weak base [14C]methylamine. Nitrosomonas europaea accumulated 14C-benzoic acid and 14C-acetyl salicylic acid when the external pH was below 7.0 and [14C]methylamine at alkaline pH. Similarly, Nitrobacter agilis accumulated the two weak acids below an external pH of about 7.5 and [14C]methylamine above this pH. As these bacteria grow best between pH 7.5 and 8.0, they do not appear to have a ΔpH (inside alkaline). Thus, above pH 7.0 for Nitrosomonas europaea and pH 7.5 for Nitrobacter agilis, Δψ only contributed to Δp. In Nitrosomonas europaea the total Δp remained almost constant (145 to 135 mV) when the external pH was varied from 6 to 8.5. In Nitrobacter agilis, Δp decreased from 178 mV (inside negative) at pH 6.0 to 95 mV at pH 8.5. Intracellular pH in Nitrosomonas europaea varied from 6.3 at an external pH of 6.0 to 7.8 at external pH 8.5. In Nitrobacter agilis, however, intracellular pH was relatively constant (7.3 to 7.8) over an external pH range of 6 to 8.5. In Nitrosomonas europaea, Δp and its components (Δψ and ΔpH) remained constant in cells at various stages of growth, so that the metabolic state of cells did not affect Δp. Such an experiment was not possible with Nitrobacter agilis because of low cell yields. The effects of protonophores and ATPase inhibitors on ΔpH and Δψ in the two nitrifying bacteria are considered.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEEM M. I., LEES H. ADENOSINE TRIPHOSPHATE-DEPENDENT REDUCTION OF NICOTINAMIDE ADENINE DINUCLEOTIDE BY FERRO-CYTOCHROME C IN CHEMOAUTOTROPHIC BACTERIA. Nature. 1963 Nov 23;200:759–761. doi: 10.1038/200759a0. [DOI] [PubMed] [Google Scholar]
- ALEEM M. I., LEES H. Autotrophic enzyme systems. I. Electron transport systems concerned with hydroxylamine oxidation in Nitrosomonas. Can J Biochem Physiol. 1963 Mar;41:763–778. [PubMed] [Google Scholar]
- Aleem M. I., Nason A. PHOSPHORYLATION COUPLED TO NITRITE OXIDATION BY PARTICLES FROM THE CHEMOAUTOTROPH, NITROBACTER AGILIS. Proc Natl Acad Sci U S A. 1960 Jun;46(6):763–769. doi: 10.1073/pnas.46.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Mainzer S. E., Allen K. E., Slayman C. W. Effects of inhibitors on the plasma membrane and mitochondrial adenosine triphosphatases of Neurospora crassa. Biochim Biophys Acta. 1978 Sep 11;512(1):13–28. doi: 10.1016/0005-2736(78)90214-6. [DOI] [PubMed] [Google Scholar]
- Cobley J. G. Energy-conserving reactions in phosphorylating electron-transport particles from Nitrobacter winogradskyi. Activation of nitrite oxidation by the electrical component of the protonmotive force. Biochem J. 1976 Jun 15;156(3):481–491. doi: 10.1042/bj1560481c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobley J. G. Reduction of cytochromes by nitrite in electron-transport particles from Nitrobacter winogradskyi: proposal of a mechanism for H+ translocation. Biochem J. 1976 Jun 15;156(3):493–498. doi: 10.1042/bj1560493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch C. J., Kula T. Transmembrane electrical and pH gradients of Paracoccus denitrificans and their relationship to oxidative phosphorylation. FEBS Lett. 1978 Mar 1;87(1):145–151. doi: 10.1016/0014-5793(78)80154-9. [DOI] [PubMed] [Google Scholar]
- Drozd J. W. Energy coupling and respiration in Nitrosomonas europaea. Arch Microbiol. 1976 Nov 2;110(23):257–262. doi: 10.1007/BF00690236. [DOI] [PubMed] [Google Scholar]
- Friedberg I., Kaback H. R. Electrochemical proton gradient in Micrococcus lysodeikticus cells and membrane vesicles. J Bacteriol. 1980 May;142(2):651–658. doi: 10.1128/jb.142.2.651-658.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOOD N. E. Activation of the Hill reaction by amines. Biochim Biophys Acta. 1960 Jun 3;40:502–517. doi: 10.1016/0006-3002(60)91391-3. [DOI] [PubMed] [Google Scholar]
- Guffanti A. A., Susman P., Blanco R., Krulwich T. A. The protonmotive force and alpha-aminoisobutyric acid transport in an obligately alkalophilic bacterium. J Biol Chem. 1978 Feb 10;253(3):708–715. [PubMed] [Google Scholar]
- Hollocher T. C., Kumar S., Nicholas D. J. Respiration-dependent proton translocation in Nitrosomonas europaea and its apparent absence in Nitrobacter agilis during inorganic oxidations. J Bacteriol. 1982 Mar;149(3):1013–1020. doi: 10.1128/jb.149.3.1013-1020.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell K. F., Sprott G. D. The transmembrane electrical potential and intracellular pH in methanogenic bacteria. Can J Microbiol. 1981 Jul;27(7):720–728. doi: 10.1139/m81-110. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Molecular biology and energetics of membrane transport. J Cell Physiol. 1976 Dec;89(4):575–593. doi: 10.1002/jcp.1040890414. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Blanchard A. G., Metzger W. C. Proton motive force during growth of Streptococcus lactis cells. J Bacteriol. 1980 Jul;143(1):128–134. doi: 10.1128/jb.143.1.128-134.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. Effects of aerobiosis and nitrogen source on the proton motive force in growing Escherichia coli and Klebsiella pneumoniae cells. J Bacteriol. 1981 Apr;146(1):377–384. doi: 10.1128/jb.146.1.377-384.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. Proton motive force in growing Streptococcus lactis and Staphylococcus aureus cells under aerobic and anaerobic conditions. J Bacteriol. 1981 Apr;146(1):369–376. doi: 10.1128/jb.146.1.369-376.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Wong P. T. The intracellular pH of Escherichia coli. Biochim Biophys Acta. 1969 Oct 14;193(1):212–214. doi: 10.1016/0005-2736(69)90074-1. [DOI] [PubMed] [Google Scholar]
- Kell D. B., John P., Ferguson S. J. The protonmotive force in phosphorylating membrane vesicles from Paracoccus denitrificans. Magnitude, sites of generation and comparison with the phosphorylation potential. Biochem J. 1978 Jul 15;174(1):257–266. doi: 10.1042/bj1740257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. P. Autotrophy: concepts of lithotrophic bacteria and their organic metabolism. Annu Rev Microbiol. 1971;25:177–210. doi: 10.1146/annurev.mi.25.100171.001141. [DOI] [PubMed] [Google Scholar]
- Khan S., Macnab R. M. Proton chemical potential, proton electrical potential and bacterial motility. J Mol Biol. 1980 Apr 15;138(3):599–614. doi: 10.1016/s0022-2836(80)80019-2. [DOI] [PubMed] [Google Scholar]
- Krogmann D. W., Jagendorf A. T., Avron M. Uncouplers of Spinach Chloroplast Photosynthetic Phosphorylation. Plant Physiol. 1959 May;34(3):272–277. doi: 10.1104/pp.34.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laane C., Krone W., Konings W., Haaker H., Veeger C. Short-term effect of ammonium chloride on nitrogen fixation by Azotobacter vinelandii and by bacteroids of Rhizobium leguminosarum. Eur J Biochem. 1980 Jan;103(1):39–46. doi: 10.1111/j.1432-1033.1980.tb04286.x. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., WATSON S. W. STRUCTURE OF NITROSOCYSTIS OCEANUS AND COMPARISON WITH NITROSOMONAS AND NITROBACTER. J Bacteriol. 1965 Jun;89:1594–1609. doi: 10.1128/jb.89.6.1594-1609.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney P. C., Kashket E. R., Wilson T. H. A protonmotive force drives ATP synthesis in bacteria. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3896–3900. doi: 10.1073/pnas.71.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel K. G., Guffanti A. A., Krulwich T. A. Monovalent cation/proton antiporters in membrane vesicles from Bacillus alcalophilus. J Biol Chem. 1980 Aug 10;255(15):7391–7396. [PubMed] [Google Scholar]
- Matin A., Wilson B., Zychlinsky E., Matin M. Proton motive force and the physiological basis of delta pH maintenance in thiobacillus acidophilus. J Bacteriol. 1982 May;150(2):582–591. doi: 10.1128/jb.150.2.582-591.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- NICHOLAS D. J. THE METABOLISM OF INORGANIC NITROGEN AND ITS COMPOUNDS IN MICRO-ORGANISMS. Biol Rev Camb Philos Soc. 1963 Nov;38:530–568. doi: 10.1111/j.1469-185x.1963.tb00792.x. [DOI] [PubMed] [Google Scholar]
- O'Kelley J. C., Becker G. E., Nason A. Characterization of the particulate nitrite oxidase and its component activities from the chemoautotroph Nitrobacter agilis. Biochim Biophys Acta. 1970 Jun 30;205(3):409–425. doi: 10.1016/0005-2728(70)90107-6. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981 Dec;650(2-3):151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Peck H. D., Jr Energy-coupling mechanisms in chemolithotrophic bacteria. Annu Rev Microbiol. 1968;22:489–518. doi: 10.1146/annurev.mi.22.100168.002421. [DOI] [PubMed] [Google Scholar]
- Prochaska L. J., Bisson R., Capaldi R. A., Steffens G. C., Buse G. Inhibition of cytochrome c oxidase function by dicyclohexylcarbodiimide. Biochim Biophys Acta. 1981 Sep 14;637(2):360–373. doi: 10.1016/0005-2728(81)90175-4. [DOI] [PubMed] [Google Scholar]
- Riebeling V., Thauer R. K., Jungermann K. The internal-alkaline pH gradient, sensitive to uncoupler and ATPase inhibitor, in growing Clostridium pasteurianum. Eur J Biochem. 1975 Jul 1;55(2):445–453. doi: 10.1111/j.1432-1033.1975.tb02181.x. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Suzuki I. Mechanisms of inorganic oxidation and energy coupling. Annu Rev Microbiol. 1974;28(0):85–101. doi: 10.1146/annurev.mi.28.100174.000505. [DOI] [PubMed] [Google Scholar]
- Wallace W., Knowles S. E., Nicholas D. J. Intermediary metabolism of carbon compounds by nitrifying bacteria. Arch Mikrobiol. 1970;70(1):26–42. doi: 10.1007/BF00691058. [DOI] [PubMed] [Google Scholar]
- Wallace W., Nicholas D. J. The biochemistry of nitrifying microorganisms. Biol Rev Camb Philos Soc. 1969 Jul;44(3):359–391. doi: 10.1111/j.1469-185x.1969.tb01216.x. [DOI] [PubMed] [Google Scholar]
- Walter B., Sidransky E., Kristjansson J. K., Hollocher T. C. Inhibition of denitrification by uncouplers of oxidative phosphorylation. Biochemistry. 1978 Jul 25;17(15):3039–3045. doi: 10.1021/bi00608a015. [DOI] [PubMed] [Google Scholar]
- Wilson D. M., Alderette J. F., Maloney P. C., Wilson T. H. Protonmotive force as the source of energy for adenosine 5'-triphosphate synthesis in Escherichia coli. J Bacteriol. 1976 Apr;126(1):327–337. doi: 10.1128/jb.126.1.327-337.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


