Abstract
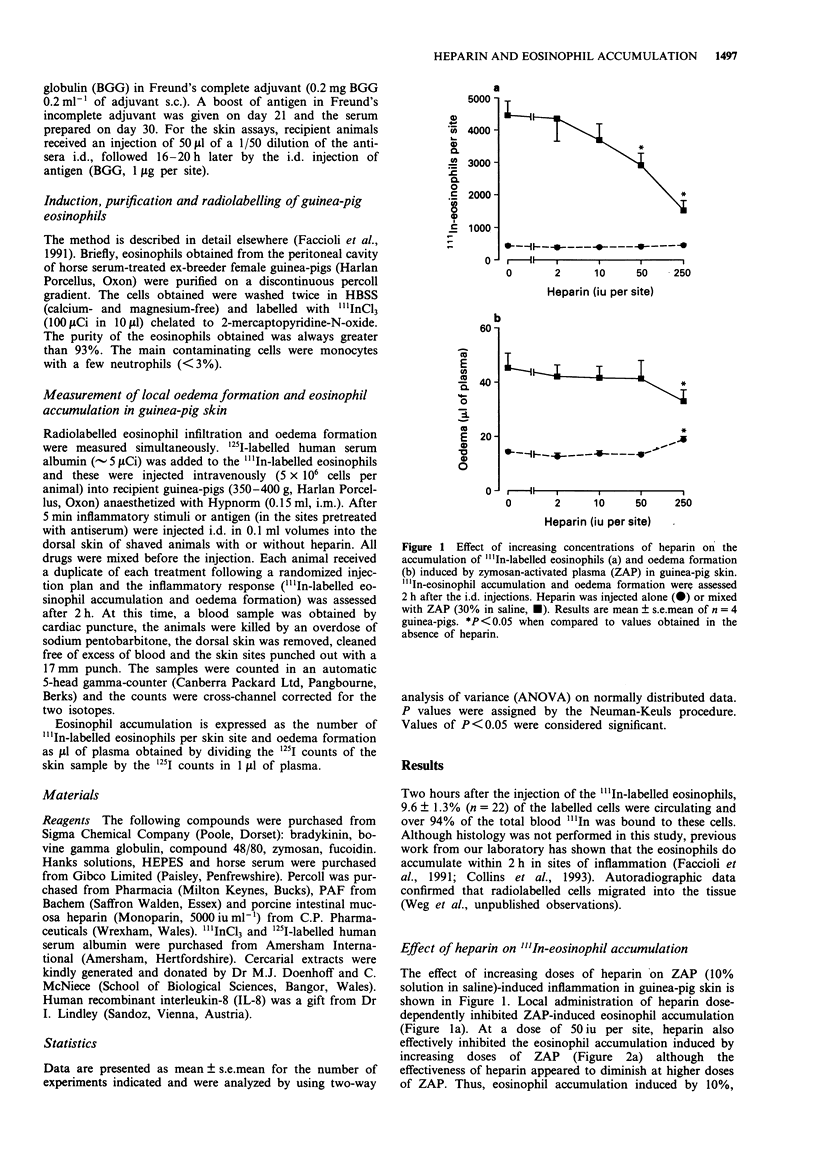
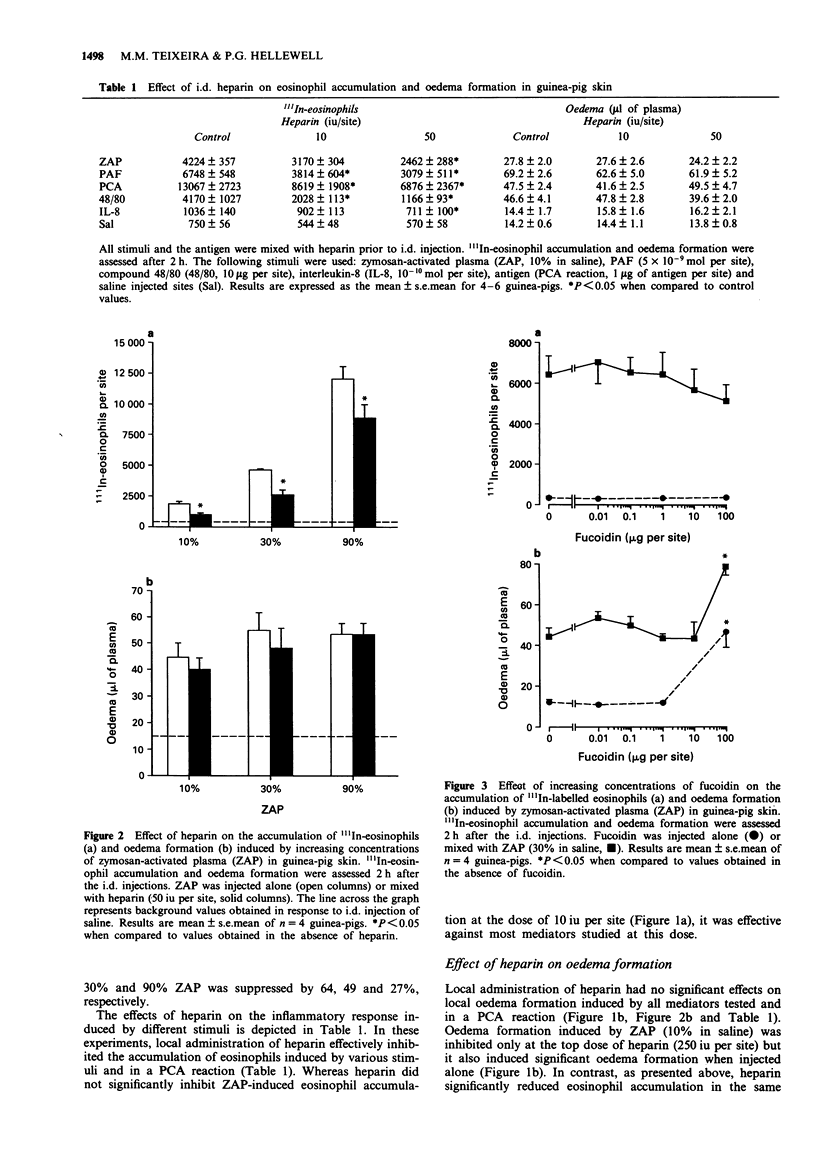
1. Heparin is widely used in the treatment of thrombotic disorders and as an aid in surgery. Anti-inflammatory effects of heparin have also been described. In this study, we have investigated the effects of locally-injected heparin on the oedema formation and eosinophil accumulation induced by various inflammatory stimuli in guinea-pig skin. 2. Heparin dose-dependently suppressed the accumulation of 111In-labelled eosinophils induced by i.d. injection of zymosan-activated plasma (ZAP). The 111In-eosinophil accumulation induced by other inflammatory stimuli (compound 48/80, platelet activating factor, interleukin-8 and in a passive cutaneous anaphylaxis reaction) was also suppressed by locally-injected heparin. 3. Oedema formation in response to these same stimuli was not altered by the local injection of heparin. 4. Fucoidin, a negatively-charged sulphated algal polymer, had no effect on the 111In-eosinophil accumulation or oedema formation induced by ZAP. Nevertheless, fucoidin significantly suppressed the oedema formation induced by i.d. injection of cationic protein-containing extracts of Schistosoma mansoni larvae. Heparin also inhibited oedema induced by the extracts, suggesting that both fucoidin and heparin were effectively neutralizing the cationic protein of the extracts to inhibit their oedema-inducing activity. 5. Thus, heparin significantly inhibited the accumulation of 111In-eosinophils, but not oedema formation, induced by various inflammatory stimuli. This, taken together with the lack of effect of fucoidin, suggests that heparin interferes with the process of eosinophil trafficking by a mechanism that does not depend on neutralisation of the charge of the stimulatory molecules.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed T., Abraham W. M., D'Brot J. Effects of inhaled heparin on immunologic and nonimmunologic bronchoconstrictor responses in sheep. Am Rev Respir Dis. 1992 Mar;145(3):566–570. doi: 10.1164/ajrccm/145.3.566. [DOI] [PubMed] [Google Scholar]
- Arfors K. E., Ley K. Sulfated polysaccharides in inflammation. J Lab Clin Med. 1993 Feb;121(2):201–202. [PubMed] [Google Scholar]
- Asch A. S., Tepler J., Silbiger S., Nachman R. L. Cellular attachment to thrombospondin. Cooperative interactions between receptor systems. J Biol Chem. 1991 Jan 25;266(3):1740–1745. [PubMed] [Google Scholar]
- BOYLE J. P., SMART R. H., SHIREY J. K. HEPARIN IN THE TREATMENT OF CHRONIC OBSTRUCTIVE BRONCHOPULMONARY DISEASE. Am J Cardiol. 1964 Jul;14:25–28. doi: 10.1016/0002-9149(64)90100-6. [DOI] [PubMed] [Google Scholar]
- Bazzoni G., Beltrán Nuñez A., Mascellani G., Bianchini P., Dejana E., Del Maschio A. Effect of heparin, dermatan sulfate, and related oligo-derivatives on human polymorphonuclear leukocyte functions. J Lab Clin Med. 1993 Feb;121(2):268–275. [PubMed] [Google Scholar]
- Bowler S. D., Smith S. M., Lavercombe P. S. Heparin inhibits the immediate response to antigen in the skin and lungs of allergic subjects. Am Rev Respir Dis. 1993 Jan;147(1):160–163. doi: 10.1164/ajrccm/147.1.160. [DOI] [PubMed] [Google Scholar]
- Bradding P., Feather I. H., Howarth P. H., Mueller R., Roberts J. A., Britten K., Bews J. P., Hunt T. C., Okayama Y., Heusser C. H. Interleukin 4 is localized to and released by human mast cells. J Exp Med. 1992 Nov 1;176(5):1381–1386. doi: 10.1084/jem.176.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J. The anti-inflammatory action of heparin: heparin as an antagonist to histamine, bradykinin and prostaglandin E1. Thromb Res. 1979;16(3-4):507–516. doi: 10.1016/0049-3848(79)90097-5. [DOI] [PubMed] [Google Scholar]
- Claman H. N. On scleroderma. Mast cells, endothelial cells, and fibroblasts. JAMA. 1989 Sep 1;262(9):1206–1209. doi: 10.1001/jama.262.9.1206. [DOI] [PubMed] [Google Scholar]
- Collins P. D., Weg V. B., Faccioli L. H., Watson M. L., Moqbel R., Williams T. J. Eosinophil accumulation induced by human interleukin-8 in the guinea-pig in vivo. Immunology. 1993 Jun;79(2):312–318. [PMC free article] [PubMed] [Google Scholar]
- Djukanović R., Roche W. R., Wilson J. W., Beasley C. R., Twentyman O. P., Howarth R. H., Holgate S. T. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990 Aug;142(2):434–457. doi: 10.1164/ajrccm/142.2.434. [DOI] [PubMed] [Google Scholar]
- Faccioli L. H., Nourshargh S., Moqbel R., Williams F. M., Sehmi R., Kay A. B., Williams T. J. The accumulation of 111In-eosinophils induced by inflammatory mediators, in vivo. Immunology. 1991 Jun;73(2):222–227. [PMC free article] [PubMed] [Google Scholar]
- Fine N. L., Shim C., Williams M. H., Jr Objective evaluation of heparin in the treatment of asthma. Am Rev Respir Dis. 1968 Nov;98(5):886–887. doi: 10.1164/arrd.1968.98.5.886. [DOI] [PubMed] [Google Scholar]
- Fredens K., Dahl R., Venge P. In vitro studies of the interaction between heparin and eosinophil cationic protein. Allergy. 1991 Jan;46(1):27–29. doi: 10.1111/j.1398-9995.1991.tb00538.x. [DOI] [PubMed] [Google Scholar]
- Freedman M. D. Pharmacodynamics, clinical indications, and adverse effects of heparin. J Clin Pharmacol. 1992 Jul;32(7):584–596. doi: 10.1002/j.1552-4604.1992.tb05765.x. [DOI] [PubMed] [Google Scholar]
- Hiebert L., Liu J. M. Protective action of polyelectrolytes on endothelium. Semin Thromb Hemost. 1991;17 (Suppl 1):42–46. [PubMed] [Google Scholar]
- Hirsh J. Heparin. N Engl J Med. 1991 May 30;324(22):1565–1574. doi: 10.1056/NEJM199105303242206. [DOI] [PubMed] [Google Scholar]
- Holgate S. T., Church M. K. Asthma. The mast cell. Br Med Bull. 1992 Jan;48(1):40–50. doi: 10.1093/oxfordjournals.bmb.a072540. [DOI] [PubMed] [Google Scholar]
- Jaques L. B. Heparins--anionic polyelectrolyte drugs. Pharmacol Rev. 1979 Jun;31(2):99–166. [PubMed] [Google Scholar]
- Kay A. B. Biological properties of eosinophils. Clin Exp Allergy. 1991 May;21 (Suppl 3):23–29. doi: 10.1111/j.1365-2222.1991.tb01760.x. [DOI] [PubMed] [Google Scholar]
- Kulmburg P. A., Huber N. E., Scheer B. J., Wrann M., Baumruker T. Immunoglobulin E plus antigen challenge induces a novel intercrine/chemokine in mouse mast cells. J Exp Med. 1992 Dec 1;176(6):1773–1778. doi: 10.1084/jem.176.6.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LECOMTE J., HUGUES J. Action inhibitrice de l'héparine sur le phénomène d'Arthus. Int Arch Allergy Appl Immunol. 1954;5(5):367–373. [PubMed] [Google Scholar]
- Ley K., Cerrito M., Arfors K. E. Sulfated polysaccharides inhibit leukocyte rolling in rabbit mesentery venules. Am J Physiol. 1991 May;260(5 Pt 2):H1667–H1673. doi: 10.1152/ajpheart.1991.260.5.H1667. [DOI] [PubMed] [Google Scholar]
- Ley K., Gaehtgens P., Fennie C., Singer M. S., Lasky L. A., Rosen S. D. Lectin-like cell adhesion molecule 1 mediates leukocyte rolling in mesenteric venules in vivo. Blood. 1991 Jun 15;77(12):2553–2555. [PubMed] [Google Scholar]
- Ley K., Linnemann G., Meinen M., Stoolman L. M., Gaehtgens P. Fucoidin, but not yeast polyphosphomannan PPME, inhibits leukocyte rolling in venules of the rat mesentery. Blood. 1993 Jan 1;81(1):177–185. [PubMed] [Google Scholar]
- Matzner Y., Marx G., Drexler R., Eldor A. The inhibitory effect of heparin and related glycosaminoglycans on neutrophil chemotaxis. Thromb Haemost. 1984 Oct 31;52(2):134–137. [PubMed] [Google Scholar]
- McCarthy J. B., Skubitz A. P., Qi Z., Yi X. Y., Mickelson D. J., Klein D. J., Furcht L. T. RGD-independent cell adhesion to the carboxy-terminal heparin-binding fragment of fibronectin involves heparin-dependent and -independent activities. J Cell Biol. 1990 Mar;110(3):777–787. doi: 10.1083/jcb.110.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Sporn M. Cytokines in context. J Cell Biol. 1991 Jun;113(5):981–986. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page C. P. One explanation of the asthma paradox: inhibition of natural anti-inflammatory mechanism by beta 2-agonists. Lancet. 1991 Mar 23;337(8743):717–720. doi: 10.1016/0140-6736(91)90289-2. [DOI] [PubMed] [Google Scholar]
- Plaut M., Pierce J. H., Watson C. J., Hanley-Hyde J., Nordan R. P., Paul W. E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989 May 4;339(6219):64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Rot A. Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration. Immunol Today. 1992 Aug;13(8):291–294. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- Saiki I., Makabe T., Yoneda J., Murata J., Ishizaki Y., Kimizuka F., Kato I., Azuma I. Inhibitory effect of fibronectin and its recombinant polypeptides on the adhesion of metastatic melanoma cells to laminin. Jpn J Cancer Res. 1991 Oct;82(10):1112–1119. doi: 10.1111/j.1349-7006.1991.tb01765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Adams D. H., Shaw S. Proteoglycans on endothelial cells present adhesion-inducing cytokines to leukocytes. Immunol Today. 1993 Mar;14(3):111–115. doi: 10.1016/0167-5699(93)90209-4. [DOI] [PubMed] [Google Scholar]
- Thorne K. J., Mazza G. Eosinophilia, activated eosinophils and human schistosomiasis. J Cell Sci. 1991 Mar;98(Pt 3):265–270. doi: 10.1242/jcs.98.3.265. [DOI] [PubMed] [Google Scholar]
- Völkl K. P., Konerding M. A., Kroll M. Wirkung von topischem Heparin auf Histamin-induzierte Quaddeln. Eine Doppelblindstudie. Fortschr Med. 1991 Aug 10;109(23):462–464. [PubMed] [Google Scholar]
- Wasserman S. I. The regulation of inflammatory mediator production by mast cell products. Am Rev Respir Dis. 1987 Jun;135(6 Pt 2):S46–S48. doi: 10.1164/arrd.1987.135.6P2.S46. [DOI] [PubMed] [Google Scholar]
- Weg V. B., Watson M. L., Cordeiro R. S., Williams T. J. Histamine, leukotriene D4 and platelet-activating factor in guinea pig passive cutaneous anaphylaxis. Eur J Pharmacol. 1991 Nov 5;204(2):157–163. doi: 10.1016/0014-2999(91)90700-z. [DOI] [PubMed] [Google Scholar]
- Williams T. J., Hellewell P. G. Endothelial cell biology. Adhesion molecules involved in the microvascular inflammatory response. Am Rev Respir Dis. 1992 Nov;146(5 Pt 2):S45–S50. doi: 10.1164/ajrccm/146.5_Pt_2.S45. [DOI] [PubMed] [Google Scholar]
- Yamashita Y., Nakagomi K., Takeda T., Hasegawa S., Mitsui Y. Effect of heparin on pulmonary fibroblasts and vascular cells. Thorax. 1992 Aug;47(8):634–639. doi: 10.1136/thx.47.8.634. [DOI] [PMC free article] [PubMed] [Google Scholar]


