Abstract
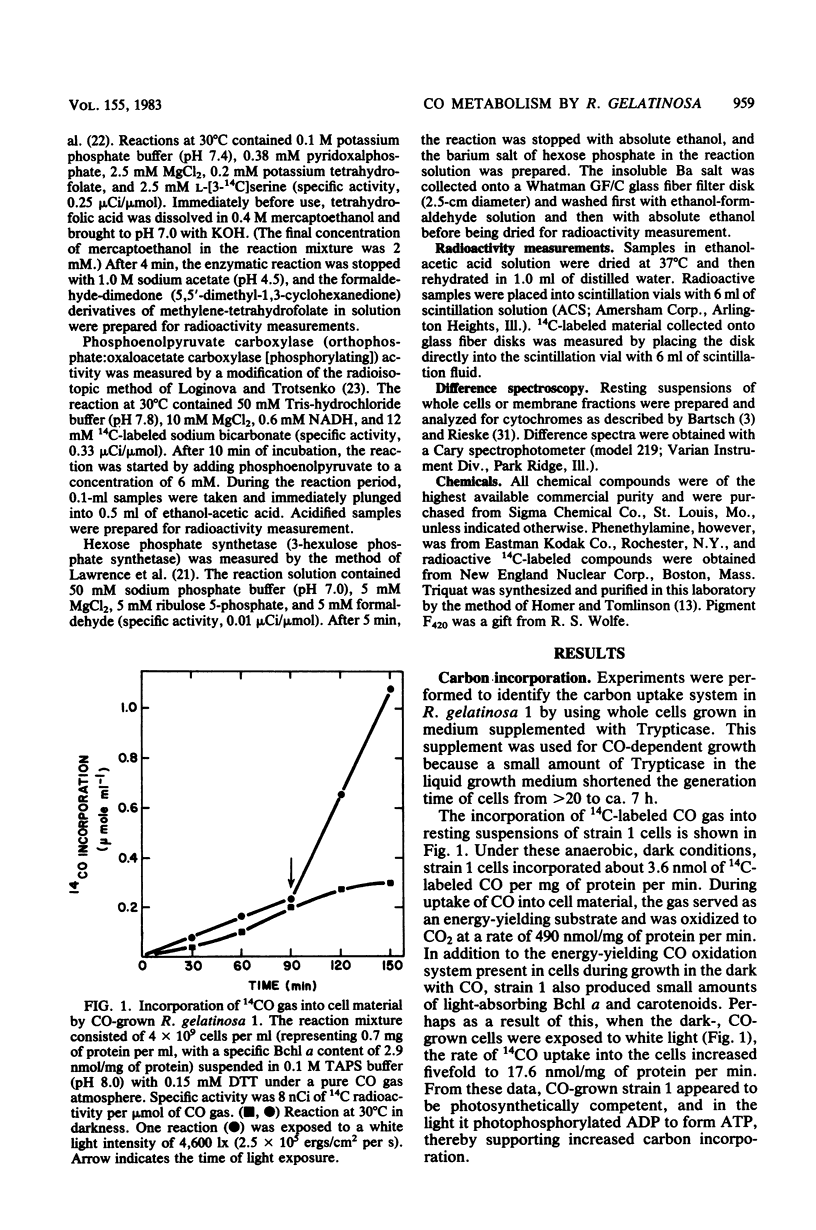
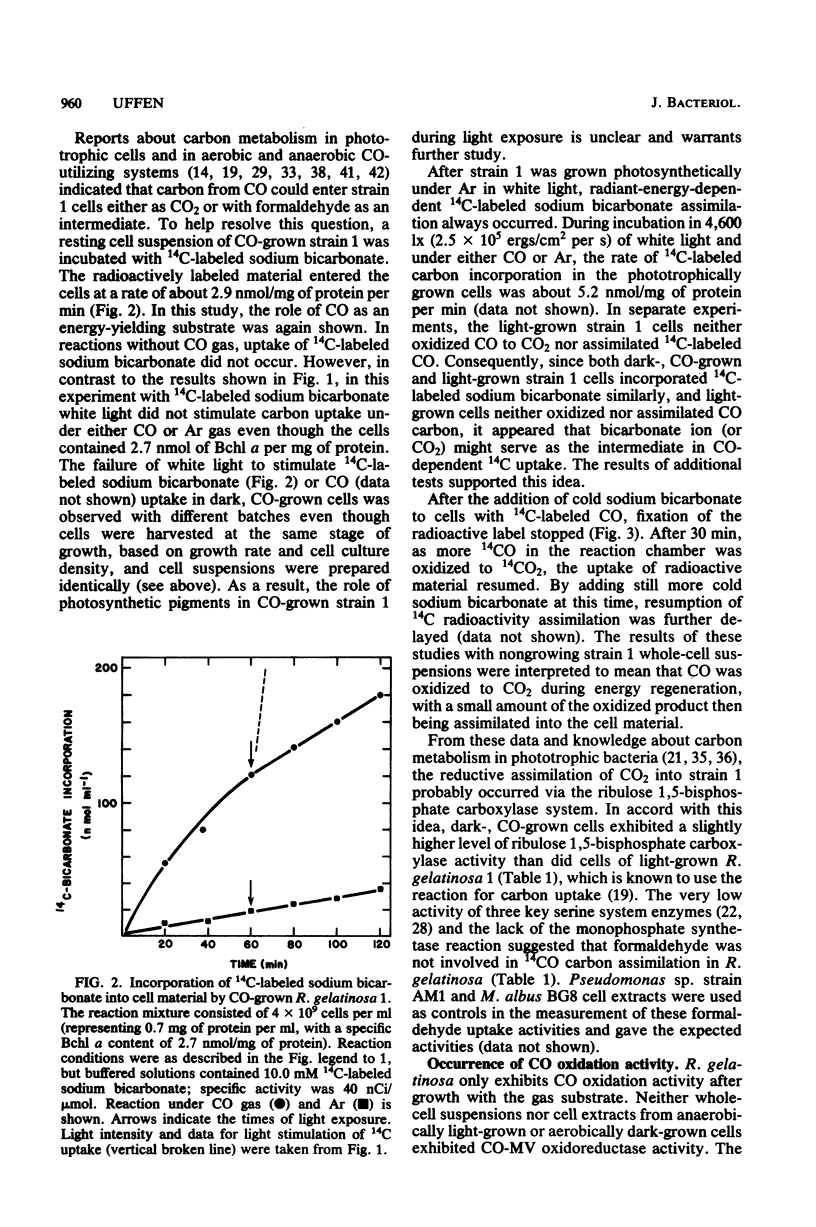
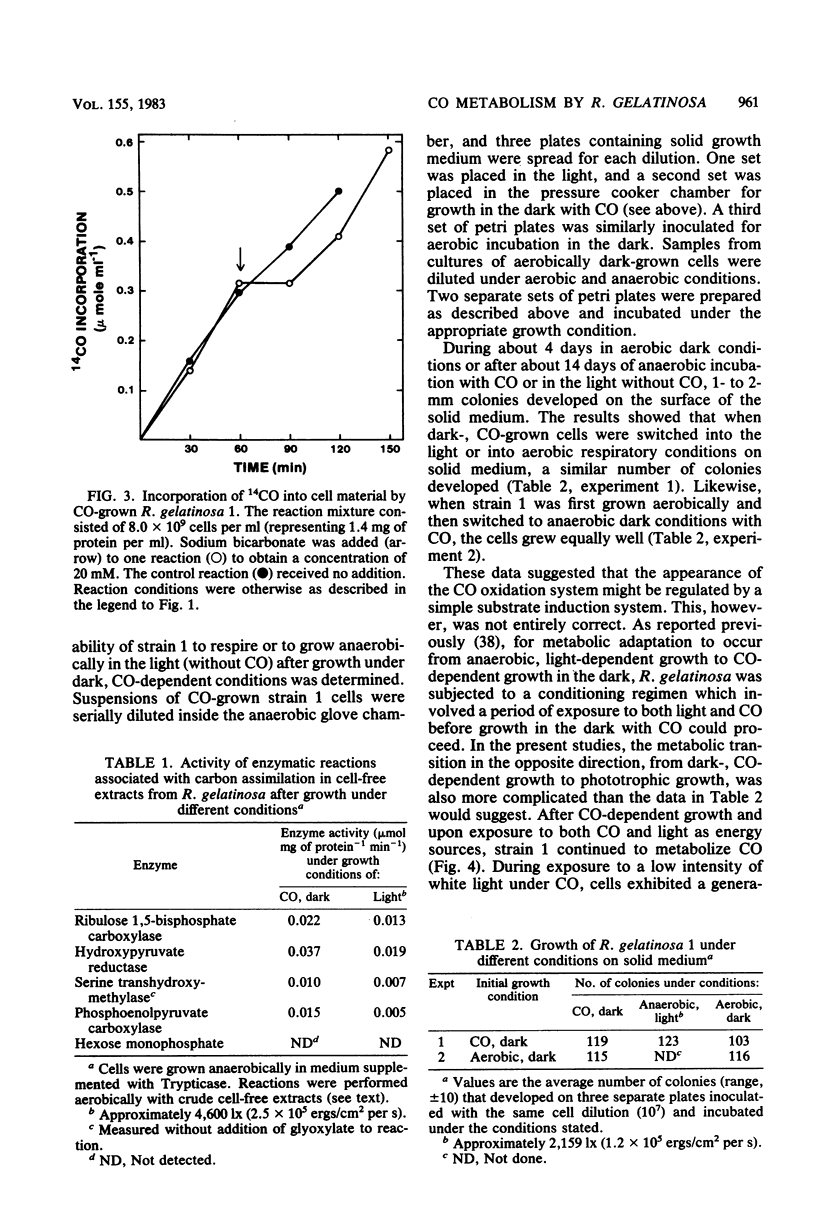
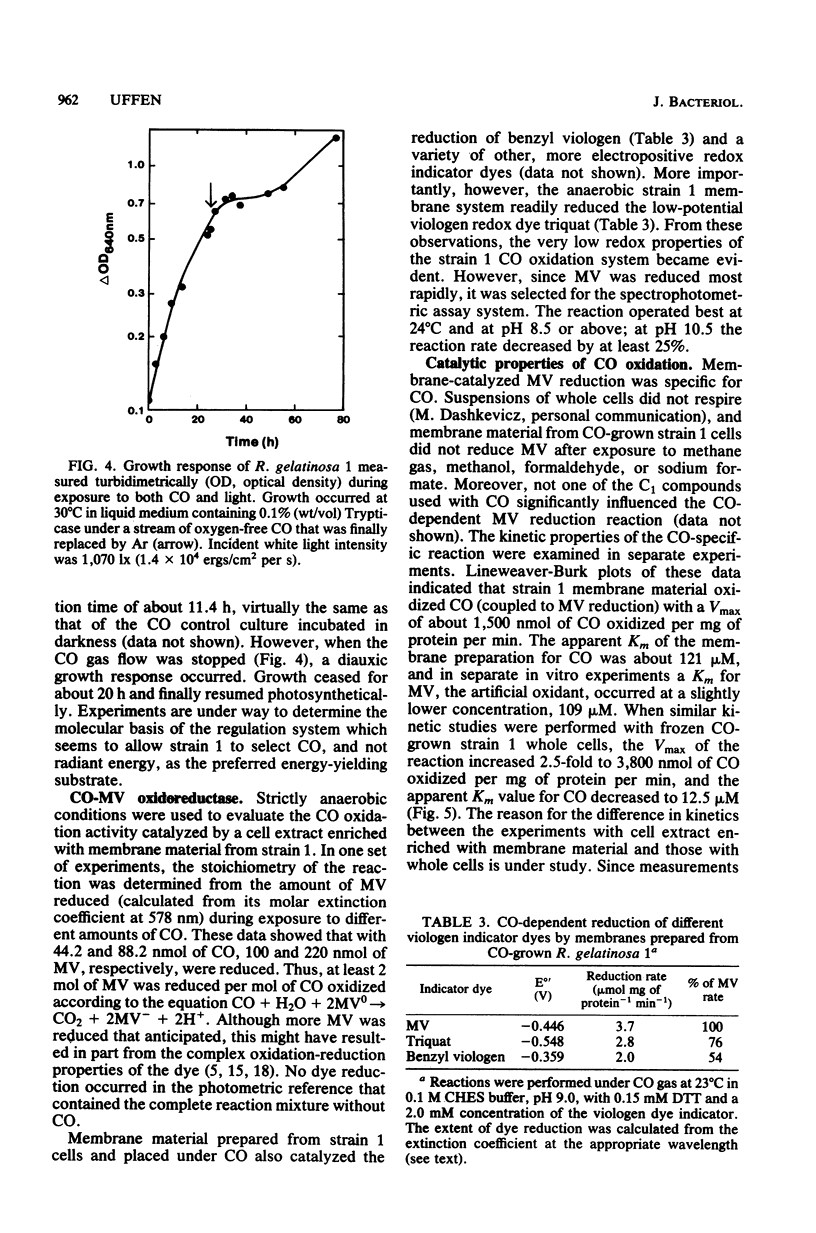
Rhodopseudomonas gelatinosa 1 grew as an anaerobic facultative methylotroph with carbon monoxide as the sole carbon and energy source. Carbon from CO was assimilated into cell material via the ribulose 1,5-bisphosphate carboxylase cycle. The CO oxidation system in R. gelatinosa was induced during growth with the gas substrate. Light-grown cells did not oxidize CO. Surprisingly, when strain 1 cells grown in the dark with CO were transferred to growth with both CO and light, they continued to use CO and then photometabolized after the CO gas flow was stopped. This change in the energy-yielding substrate resulted in a diauxic growth response. The use of CO in preference to light energy forms the basis of a system in the cells that controls photosynthetic differentiation. CO oxidation was assayed as CO-methyl viologen oxidoreductase. Methyl viologen reduction only occurred with CO; the dye was not reduced with other C1 compounds. In vitro methyl viologen was reduced best at 24 degrees C and at pH values above 8.5. Whole cells exhibited a Km of 12.5 microM for CO and a Vmax of 3,800 nmol of CO oxidized per mg of protein per min. This was a low-potential oxidation reaction that readily reduced the viologen dye triquat (1,1'-trimethylene-2,2'-dipyridilium dibromide) (E degrees' = -548 mV).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aranki A., Freter R. Use of anaerobic glove boxes for the cultivation of strictly anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1329–1334. doi: 10.1093/ajcn/25.12.1329. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAYTON R. K. TOWARD THE ISOLATION OF A PHOTOCHEMICAL REACTION CENTER IN RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1963 Nov 29;75:312–323. doi: 10.1016/0006-3002(63)90618-8. [DOI] [PubMed] [Google Scholar]
- Corwin A. H., Arellano R. R., Chivvis A. B. Anomalies of viologens in bases and water. Biochim Biophys Acta. 1968 Nov 26;162(4):533–538. doi: 10.1016/0005-2728(68)90060-1. [DOI] [PubMed] [Google Scholar]
- Daniels L., Zeikus J. G. One-carbon metabolism in methanogenic bacteria: analysis of short-term fixation products of 14CO2 and 14CH3OH incorporated into whole cells. J Bacteriol. 1978 Oct;136(1):75–84. doi: 10.1128/jb.136.1.75-84.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner B. R., Bryant M. P. Growth of Eubacterium limosum with Carbon Monoxide as the Energy Source. Appl Environ Microbiol. 1982 Jan;43(1):70–74. doi: 10.1128/aem.43.1.70-74.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrell T. E., Uffen R. L. Fermentative metabolism of pyruvate by Rhodospirillum rubrum after anaerobic growth in darkness. J Bacteriol. 1977 Aug;131(2):533–543. doi: 10.1128/jb.131.2.533-543.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. I., Drake H. L., Wood H. G. Synthesis of acetyl coenzyme A from carbon monoxide, methyltetrahydrofolate, and coenzyme A by enzymes from Clostridium thermoaceticum. J Bacteriol. 1982 Feb;149(2):440–448. doi: 10.1128/jb.149.2.440-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. W., Garland P. B. Sites and specificity of the reaction of bipyridylium compounds with anaerobic respiratory enzymes of Escherichia coli. Effects of permeability barriers imposed by the cytoplasmic membrane. Biochem J. 1977 Apr 15;164(1):199–211. doi: 10.1042/bj1640199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok B., Rurainski H. J., Owens O. V. The reducing power generated in photoact I of photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):347–356. doi: 10.1016/0926-6585(65)90162-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Large P. J., Quayle J. R. Microbial growth on C(1) compounds. 5. Enzyme activities in extracts of Pseudomonas AM1. Biochem J. 1963 May;87(2):386–396. doi: 10.1042/bj0870386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence A. J., Kemp M. B., Quayle J. R. Synthesis of cell constituents by methane-grown Methylococcus capsulatus and Methanomonas methanooxidans. Biochem J. 1970 Feb;116(4):631–639. doi: 10.1042/bj1160631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd L., Kerby R., Zeikus J. G. Carbon monoxide metabolism of the methylotrophic acidogen Butyribacterium methylotrophicum. J Bacteriol. 1982 Jan;149(1):255–263. doi: 10.1128/jb.149.1.255-263.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- PEEL D., QUAYLE J. R. Microbial growth on C1 compounds. I. Isolation and characterization of Pseudomonas AM 1. Biochem J. 1961 Dec;81:465–469. doi: 10.1042/bj0810465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiser G. D., Lizada M. C., Yang S. F. Dark metabolism of carbon monoxide in lettuce leaf discs. Plant Physiol. 1982 Aug;70(2):397–400. doi: 10.1104/pp.70.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater J. H., Morris I. Photosynthetic carbon dioxide assimilation by Rhodospirillum rubrum. Arch Mikrobiol. 1973;88(3):213–223. doi: 10.1007/BF00421847. [DOI] [PubMed] [Google Scholar]
- Smith A. J., Hoare D. S. Specialist phototrophs, lithotrophs, and methylotrophs: a unity among a diversity of procaryotes? Bacteriol Rev. 1977 Jun;41(2):419–448. doi: 10.1128/br.41.2.419-448.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaiman D., Uffen R. L. Pyruvate-dependent diauxic growth of Rhodospirillum rubrum in light. J Bacteriol. 1982 Dec;152(3):1175–1187. doi: 10.1128/jb.152.3.1175-1187.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L. Anaerobic growth of a Rhodopseudomonas species in the dark with carbon monoxide as sole carbon and energy substrate. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3298–3302. doi: 10.1073/pnas.73.9.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L. Growth properties of Rhodospirillum rubrum mutants and fermentation of pyruvate in anaerobic, dart conditions. J Bacteriol. 1973 Nov;116(2):874–884. doi: 10.1128/jb.116.2.874-884.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim B. T., Uffen R. L. Membrane association of the carbon monoxide oxidation system in Rhodopseudomonas gelatinosa. J Bacteriol. 1983 Jan;153(1):571–573. doi: 10.1128/jb.153.1.571-573.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Fuchs G., Kenealy W., Thauer R. K. Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J Bacteriol. 1977 Nov;132(2):604–613. doi: 10.1128/jb.132.2.604-613.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


