Abstract
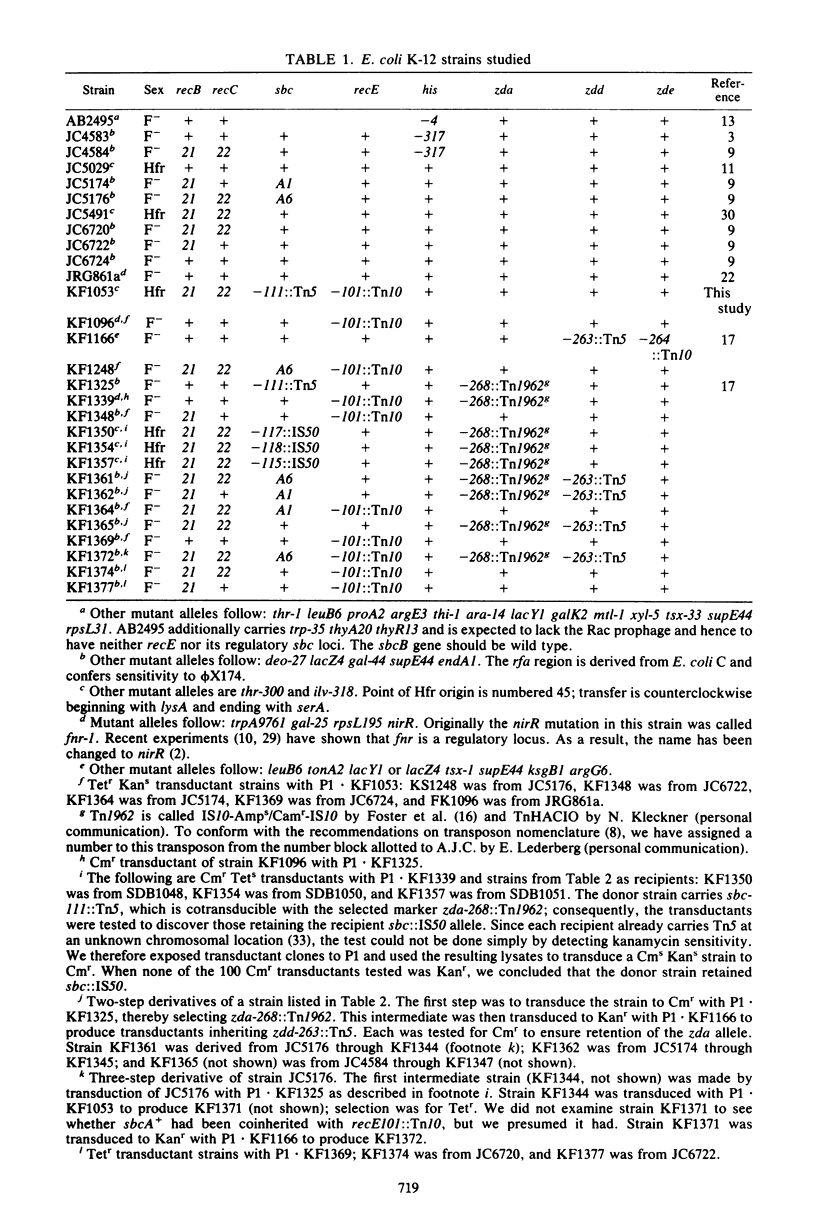
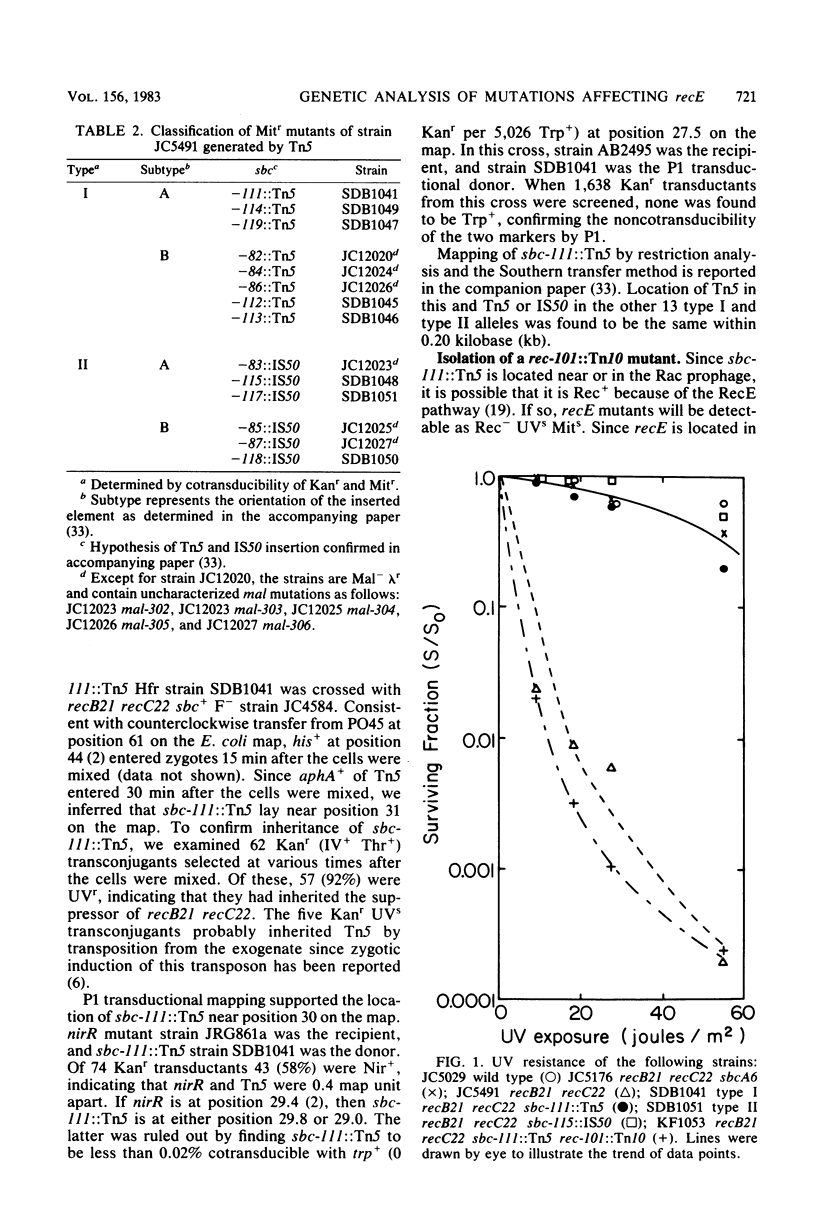
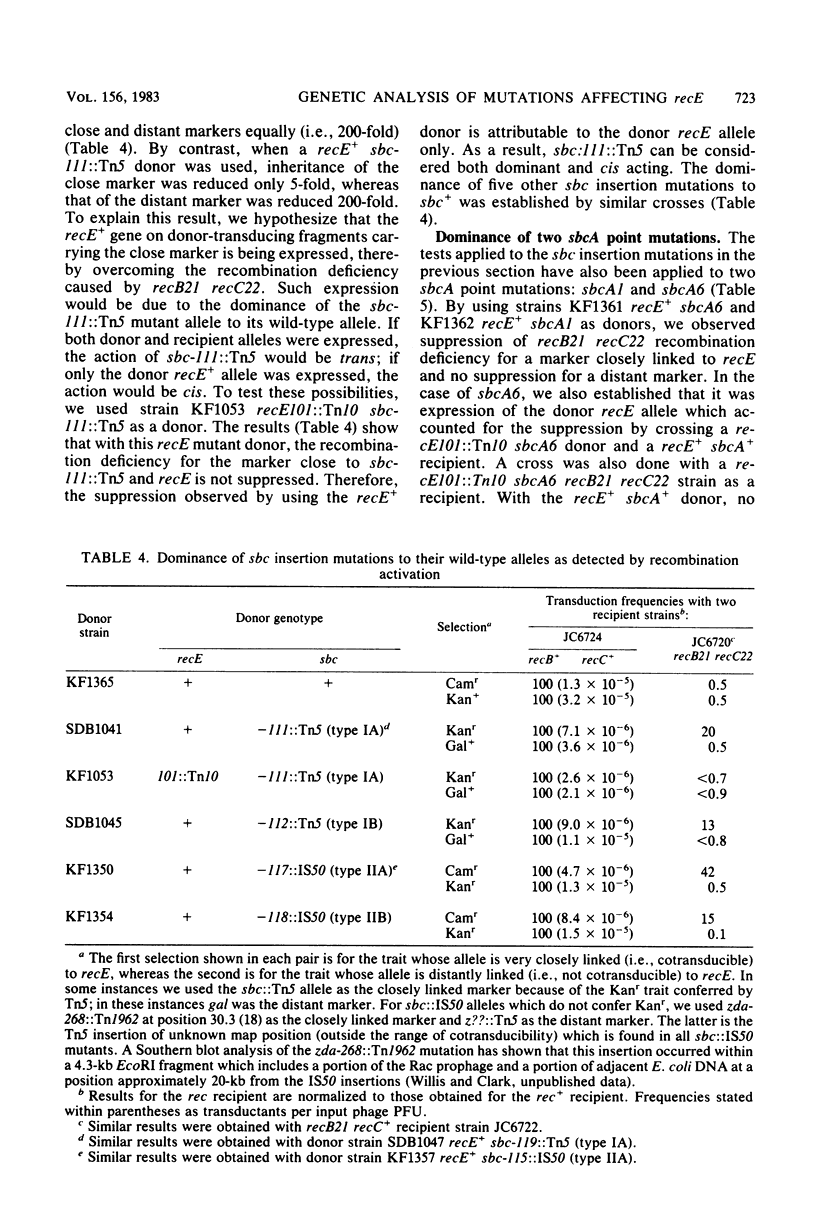
Fourteen mitomycin-resistant revertants of a recB21 recC22 strain were isolated after Tn5 mutagenesis. Eight of the mutations (type I) were essentially inseparable from aphA+ (Kanr) of Tn5; six (type II) were not. We hypothesize that the former are Tn5 and that the latter are IS50 insertions. Because of their phenotypic similarity to sbcA and sbcB mutations, which also suppress recB21 recC22, we have called them sbc mutations. sbc-lll::Tn5 was cotransducible with nirR and has thereby been located at position 29.8 on the Escherichia coli map in the vicinity of the Rac prophage and sbcA mutations. A recB21 recC22 sbc-lll::Tn5 strain was subjected to Tn10 mutagenesis, and a mitomycin- and UV-sensitive mutant was isolated. tet+ of Tn10 was 85% cotransducible with aphA+ of Tn5, locating these two transposons 0.1 map unit apart. A three-point cross located the Tn10 mutation at position 29.7. We hypothesize that the Tn10 insertion is located in recE and that the Tn5 and IS50 insertions activate expression of this gene. sbc-lll::Tn5 was found to be cis acting and dominant to its wild-type allele as were two sbcA mutations (sbcA1 and sbcA6). Five other type I and type II insertion mutations were dominant to their wild-type alleles. We hypothesize that the sbc insertion and sbcA mutations affect transcription regulation of recE and discuss the possibility that they do so differently.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour S. D., Nagaishi H., Templin A., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. II. Rec+ revertants caused by indirect suppression of rec- mutations. Proc Natl Acad Sci U S A. 1970 Sep;67(1):128–135. doi: 10.1073/pnas.67.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biek D., Roth J. R. Regulation of Tn5 transposition in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6047–6051. doi: 10.1073/pnas.77.10.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttin G., Wright M. Enzymatic DNA degradation in E. coli: its relationship to synthetic processes at the chromosome level. Cold Spring Harb Symp Quant Biol. 1968;33:259–269. doi: 10.1101/sqb.1968.033.01.030. [DOI] [PubMed] [Google Scholar]
- Capaldo-Kimball F., Barbour S. D. Involvement of recombination genes in growth and viability of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):204–212. doi: 10.1128/jb.106.1.204-212.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux M., Bonnefoy-Orth V., Ratouchniak J., Pascal M. C. Operon fusions in the nitrate reductase operon and study of the control gene nir R in Escherichia coli. Mol Gen Genet. 1981;182(3):477–479. doi: 10.1007/BF00293938. [DOI] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J. The beginning of a genetic analysis of recombination proficiency. J Cell Physiol. 1967 Oct;70(2 Suppl):165–180. doi: 10.1002/jcp.1040700412. [DOI] [PubMed] [Google Scholar]
- Cole J. A., Ward F. B. Nitrite reductase-deficient mutants of Escherichia coli K12. J Gen Microbiol. 1973 May;76(1):21–29. doi: 10.1099/00221287-76-1-21. [DOI] [PubMed] [Google Scholar]
- Emmerson P. T. Recombination deficient mutants of Escherichia coli K12 that map between thy A and argA. Genetics. 1968 Sep;60(1):19–30. doi: 10.1093/genetics/60.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R., Seeley N. R., Kuempel P. L. Loss of rac locus DNA in merozygotes of Escherichia coli K12. Mol Gen Genet. 1979 Oct 1;175(3):245–250. doi: 10.1007/BF00397223. [DOI] [PubMed] [Google Scholar]
- Foster T. J., Davis M. A., Roberts D. E., Takeshita K., Kleckner N. Genetic organization of transposon Tn10. Cell. 1981 Jan;23(1):201–213. doi: 10.1016/0092-8674(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Fouts K. E., Barbour S. D. Insertion of transposons through the major cotransduction gap of Escherichia coli K-12. J Bacteriol. 1982 Jan;149(1):106–113. doi: 10.1128/jb.149.1.106-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouts K. E., Barbour S. D. Transductional mapping of ksgB and a new Tn5-induced kasugamycin resistance gene, ksgD, in Escherichia coli K-12. J Bacteriol. 1981 Feb;145(2):914–919. doi: 10.1128/jb.145.2.914-919.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillen J. R., Willis D. K., Clark A. J. Genetic analysis of the RecE pathway of genetic recombination in Escherichia coli K-12. J Bacteriol. 1981 Jan;145(1):521–532. doi: 10.1128/jb.145.1.521-532.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser K., Murray N. E. On the nature of sbcA mutations in E. coli K 12. Mol Gen Genet. 1980;179(3):555–563. doi: 10.1007/BF00271745. [DOI] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Clark A. J. Isolation of exonuclease VIII: the enzyme associated with sbcA indirect suppressor. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3593–3597. doi: 10.1073/pnas.71.9.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Guest J. R. Mutants of Escherichia coli K12 unable to use fumarate as an anaerobic electron acceptor. J Gen Microbiol. 1976 Dec;97(2):145–160. doi: 10.1099/00221287-97-2-145. [DOI] [PubMed] [Google Scholar]
- Lloyd R. G., Barbour S. D. The genetic location of the sbcA gene of Escherichia coli. Mol Gen Genet. 1974;134(2):157–171. doi: 10.1007/BF00268417. [DOI] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Restoration by the rac locus of recombinant forming ability in recB - and recC - merozygotes of Escherichia coli K-12. Mol Gen Genet. 1973 Apr 12;122(2):119–130. doi: 10.1007/BF00435185. [DOI] [PubMed] [Google Scholar]
- Oishi M. An ATP-dependent deoxyribonuclease from Escherichia coli with a possible role in genetic recombination. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1292–1299. doi: 10.1073/pnas.64.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. J., Guest J. R. Molecular cloning of the fnr gene of Escherichia coli K12. Mol Gen Genet. 1981;181(1):95–100. doi: 10.1007/BF00339011. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J Bacteriol. 1969 Oct;100(1):231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J., Low B. Genetic location of certain mutations conferring recombination deficiency in Escherichia coli. J Bacteriol. 1969 Jan;97(1):244–249. doi: 10.1128/jb.97.1.244-249.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. K., Fouts K. E., Barbour S. D., Clark A. J. Restriction nuclease and enzymatic analysis of transposon-induced mutations of the Rac prophage which affect expression and function of recE in Escherichia coli K-12. J Bacteriol. 1983 Nov;156(2):727–736. doi: 10.1128/jb.156.2.727-736.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]


