Abstract
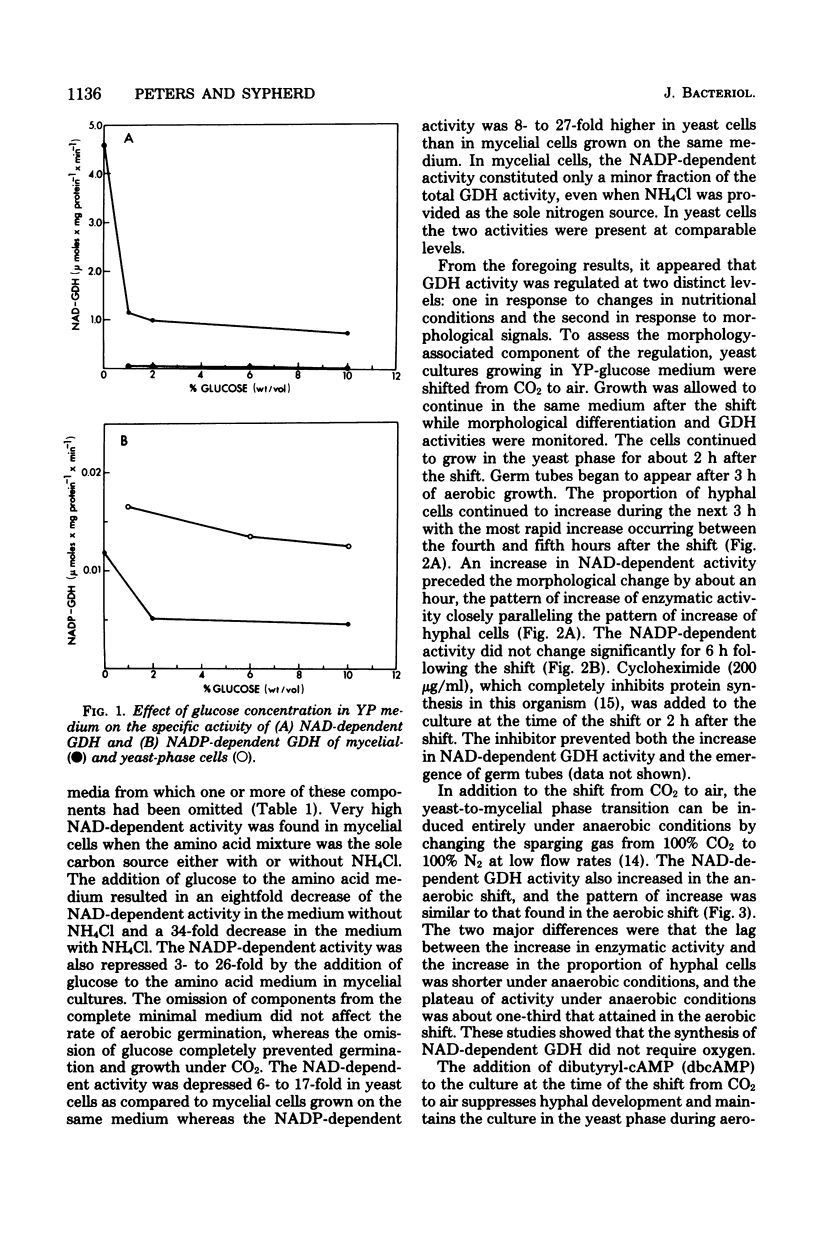
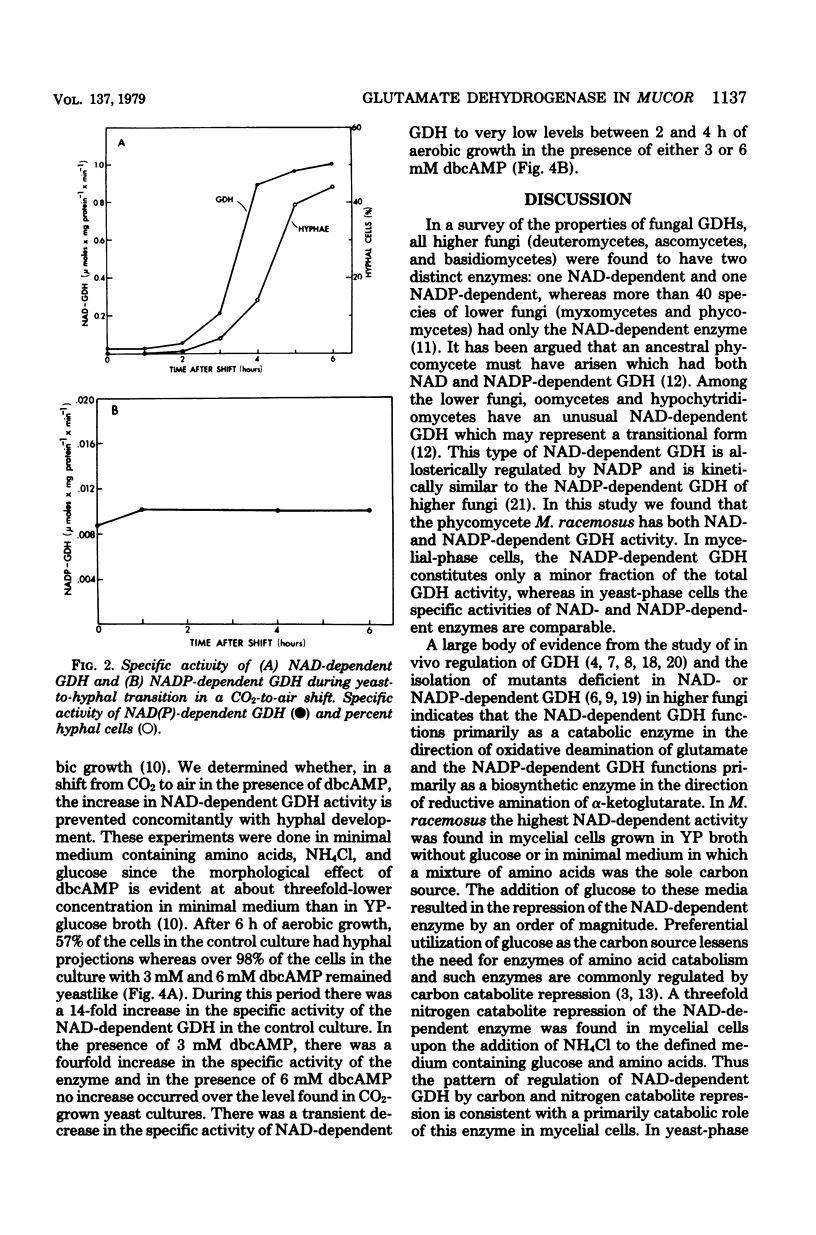
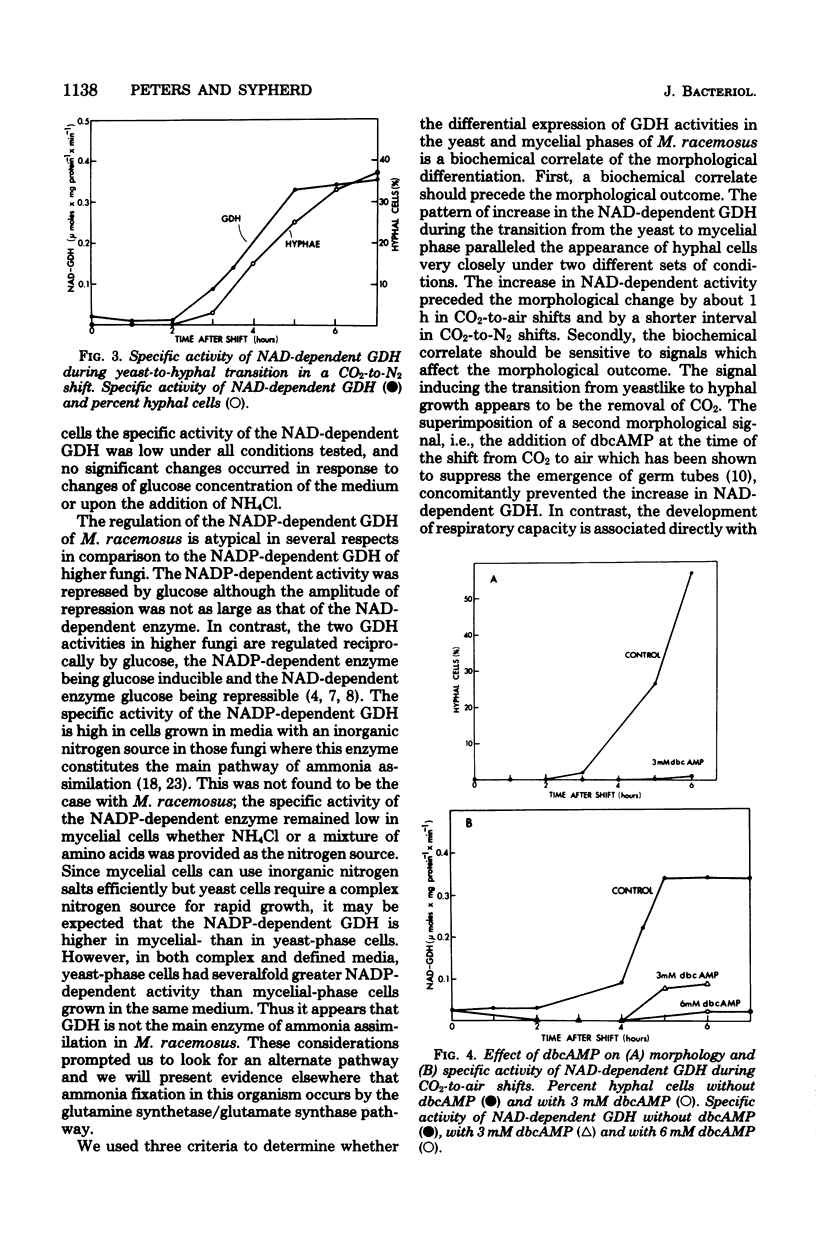
The in vivo regulation of glutamate dehydrogenase (GDH) was studied in Mucor racemosus as a function of nutritional conditions and morphological state. Both nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP)-dependent GDH activities were found. The effect of carbon and nitrogen source on the specific activity of the NAD-dependent GDH suggests that its role is primarily catabolic. The NAD-dependent activity was generally an order of magnitude greater in mycelial cells than in yeast-phase cells grown on the same medium. During yeast-to-hyphal morphogenesis the increase in NAD-dependent activity preceded the appearance of hyphal cells both under aerobic and anaerobic conditions. Exogenous dibutyryl-cyclic AMP prevented the increase in NAD-dependent GDH concomitantly with the suppression of morphological differentiation. The NADP-dependent activity did not change appreciably during morphogenesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTNICKI-GARCIA S., NICKERSON W. J. Induction of yeast-like development in Mucor by carbon dioxide. J Bacteriol. 1962 Oct;84:829–840. doi: 10.1128/jb.84.4.829-840.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Nutrition, growth, and morphogenesis of Mucor rouxii. J Bacteriol. 1962 Oct;84:841–858. doi: 10.1128/jb.84.4.841-858.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer G. W., Nickerson W. J. Nutritional requirements for growth and yeastlike development of Mucor rouxii under carbon dioxide. J Bacteriol. 1970 Feb;101(2):595–602. doi: 10.1128/jb.101.2.595-602.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M., Dubois E., Piotrowska M., Drillien R., Aigle M. Ammonia assimilation in Saccharomyces cerevisiae as mediated by the two glutamate dehydrogenases. Evidence for the gdhA locus being a structural gene for the NADP-dependent glutamate dehydrogenase. Mol Gen Genet. 1974;128(1):73–85. doi: 10.1007/BF00267295. [DOI] [PubMed] [Google Scholar]
- Hynes M. J. The effects of carbon source on glutamate dehydrogenase activities in Aspergillus nidulans. J Gen Microbiol. 1974 Mar;81(1):165–170. doi: 10.1099/00221287-81-1-165. [DOI] [PubMed] [Google Scholar]
- Kapoor M., Grover A. K. Catabolite-controlled regulation of glutamate dehydrogenases of Neurospora crassa. Can J Microbiol. 1970 Jan;16(1):33–40. doi: 10.1139/m70-006. [DOI] [PubMed] [Google Scholar]
- Kinghorn J. R., Pateman J. A. NAD and NADP l-glutamate dehydrogenase activity and ammonium regulation in Aspergillus nidulans. J Gen Microbiol. 1973 Sep;78(1):39–46. doi: 10.1099/00221287-78-1-39. [DOI] [PubMed] [Google Scholar]
- Larsen A. D., Sypherd P. S. Cyclic adenosine 3',5'-monophosphate and morphogenesis in Mucor racemosus. J Bacteriol. 1974 Feb;117(2):432–438. doi: 10.1128/jb.117.2.432-438.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- Mooney D. T., Sypherd P. S. Volatile factor involved in the dimorphism of Mucor racemosus. J Bacteriol. 1976 Jun;126(3):1266–1270. doi: 10.1128/jb.126.3.1266-1270.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez de Castro I., Ugarte M., Cano A., Mayor F. Effect of glucose, galactose, and different nitrogen-sources on the activity of yeast glutamate dehydrogenase (NAD and NADP-linked) from normal strain and impaired respiration mutant. Eur J Biochem. 1970 Nov;16(3):567–570. doi: 10.1111/j.1432-1033.1970.tb01118.x. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Sypherd P. S. Protein synthesis during morphogenesis of Mucor racemosus. J Bacteriol. 1977 Oct;132(1):209–218. doi: 10.1128/jb.132.1.209-218.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paznokas J. L., Sypherd P. S. Respiratory capacity, cyclic adenosine 3',5'-monophosphate, and morphogenesis of Mucor racemosus. J Bacteriol. 1975 Oct;124(1):134–139. doi: 10.1128/jb.124.1.134-139.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roon R. J., Even H. L. Regulation of the nicotinamide adenine dinucleotide- and nicotinamide adenine dinucleotide phosphate-dependent glutamate dehydrogenases of Saccharomyces cerevisiae. J Bacteriol. 1973 Oct;116(1):367–372. doi: 10.1128/jb.116.1.367-372.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANWAL B. D., LATA M. Effect of glutamic acid on the formation of two glutamic acid dehydrogenases of Neurospora. Biochem Biophys Res Commun. 1962 Jan 24;6:404–409. doi: 10.1016/0006-291x(62)90364-9. [DOI] [PubMed] [Google Scholar]
- SANWAL B. D., LATA M. Glutamic dehydrogenase in single-gene mutants of Neurospora deficient in amination. Nature. 1961 Apr 15;190:286–287. doi: 10.1038/190286a0. [DOI] [PubMed] [Google Scholar]
- Stevenson R. M., LéJohn H. B. Glutamic dehydrogenases of Oomycetes. Kinetic mechanism and possible vvolutionary history. J Biol Chem. 1971 Apr 10;246(7):2127–2135. [PubMed] [Google Scholar]
- Storck R., Morrill R. C. Respiratory-deficient, yeastlike mutant of Mucor. Biochem Genet. 1971 Oct;5(5):467–479. doi: 10.1007/BF00487136. [DOI] [PubMed] [Google Scholar]
- Thomulka K. W., Moat A. G. Inorganic nitrogen assimilation in yeasts: alteration in enzyme activities associated with changes in cultural conditions and growth phase. J Bacteriol. 1972 Jan;109(1):25–33. doi: 10.1128/jb.109.1.25-33.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


