Abstract
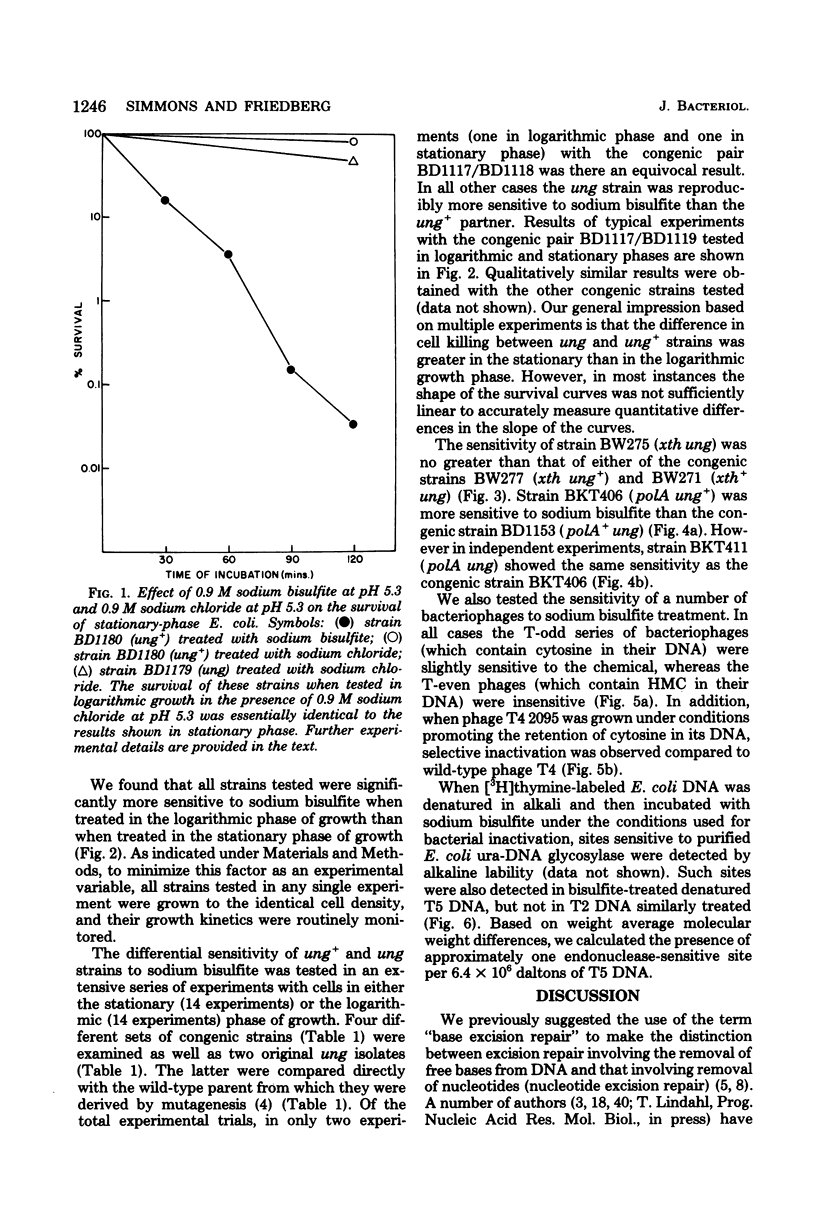
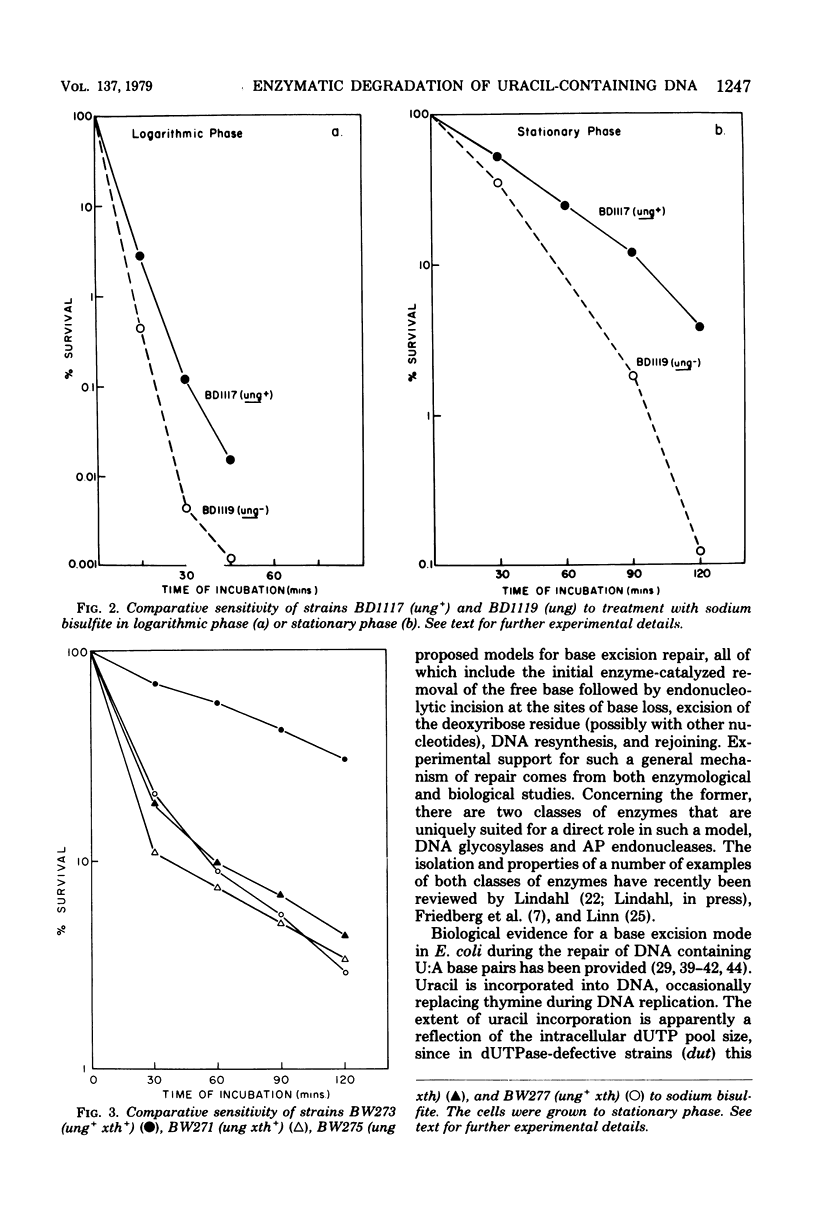
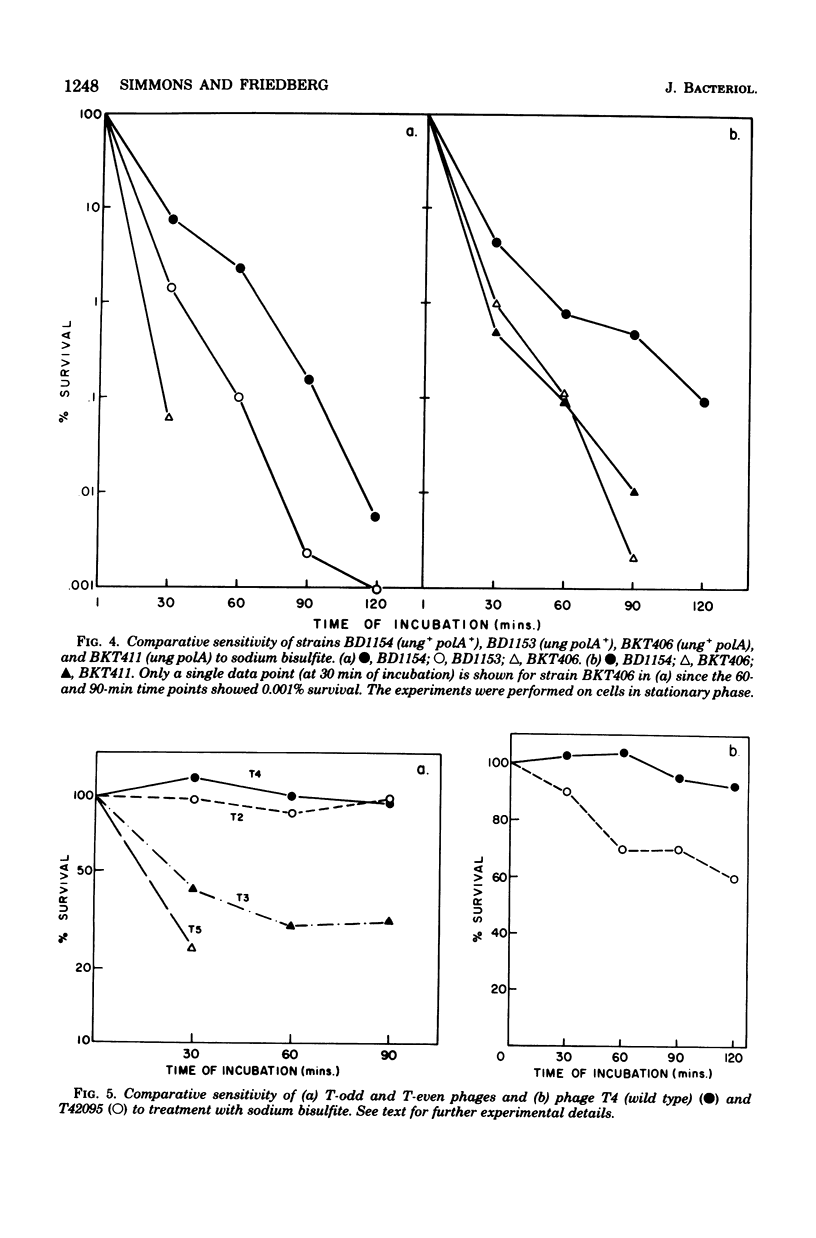
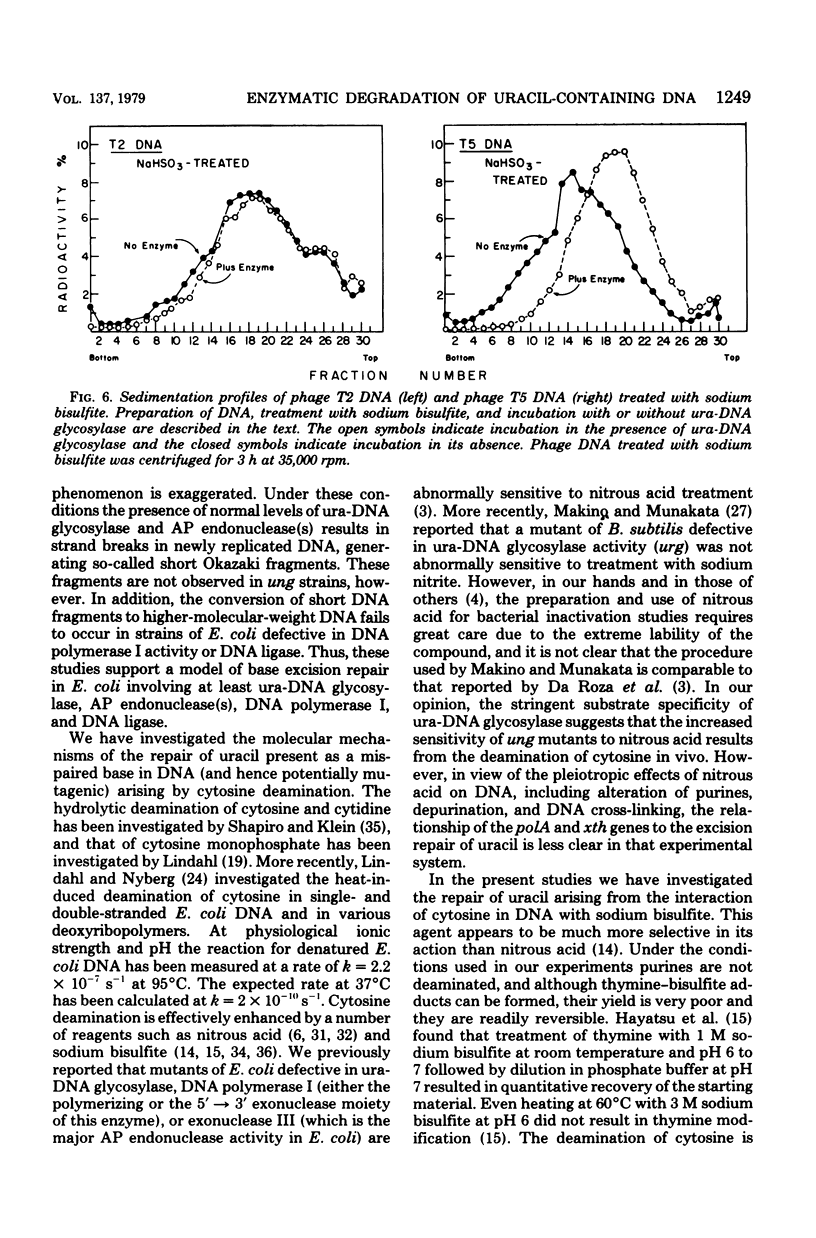
A number of mutants of Escherichia coli defective in the ung gene (structural gene for uracil-deoxyribonucleic acid [ura-DNA] glycosylase) are shown to be abnormally sensitive to treatment with sodium bisulfite when compared with congenic ung+ strains. These results provide further evidence that sodium bisulfite causes the deamination of cytosine to uracil in DNA and that ura-DNA glycosylase is required for the repair of U-G mispairs. The effect of the chemical is apparently selective with respect to base damage; coliphages containing cytosine in their DNA are inactivated by treatment with sodium bisulfite, whereas those containing hydroxymethylcytosine are not. ura-DNA glycosylase and the major apurinic-apyrimidinic endonuclease of E. coli may function in the same repair pathway, since the extent of inactivation of a congenic set of strains which are ung xth (structural gene for the major apurinic-apyrimidinic endonuclease of E. coli) or ung xth+ is the same.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brynolf K., Eliasson R., Reichard P. Formation of Okazaki fragments in polyoma DNA synthesis caused by misincorporation of uracil. Cell. 1978 Mar;13(3):573–580. doi: 10.1016/0092-8674(78)90330-6. [DOI] [PubMed] [Google Scholar]
- Cone R., Duncan J., Hamilton L., Friedberg E. C. Partial purification and characterization of a uracil DNA N-glycosidase from Bacillus subtilis. Biochemistry. 1977 Jul 12;16(14):3194–3201. doi: 10.1021/bi00633a024. [DOI] [PubMed] [Google Scholar]
- Da Roza R., Friedberg E. C., Duncan B. K., Warner H. R. Repair of nitrous acid damage to DNA in Escherichia coli. Biochemistry. 1977 Nov 1;16(22):4934–4939. doi: 10.1021/bi00641a030. [DOI] [PubMed] [Google Scholar]
- Duncan B. K., Rockstroh P. A., Warner H. R. Escherichia coli K-12 mutants deficient in uracil-DNA glycosylase. J Bacteriol. 1978 Jun;134(3):1039–1045. doi: 10.1128/jb.134.3.1039-1045.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J., Hamilton L., Friedberg E. C. Enzymatic degradation of uracil-containing DNA. II. Evidence for N-glycosidase and nuclease activities in unfractionated extracts of Bacillus subtilis. J Virol. 1976 Aug;19(2):338–345. doi: 10.1128/jvi.19.2.338-345.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C., Ganesan A. K., Minton K. N-Glycosidase activity in extracts of Bacillus subtilis and its inhibition after infection with bacteriophage PBS2. J Virol. 1975 Aug;16(2):315–321. doi: 10.1128/jvi.16.2.315-321.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C., King J. J. Dark repair of ultraviolet-irradiated deoxyribonucleic acid by bacteriophage T4: purification and characterization of a dimer-specific phage-induced endonuclease. J Bacteriol. 1971 May;106(2):500–507. doi: 10.1128/jb.106.2.500-507.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates F. T., 3rd, Linn S. Endonuclease V of Escherichia coli. J Biol Chem. 1977 Mar 10;252(5):1647–1653. [PubMed] [Google Scholar]
- Gates F. T., Linn S. Endonuclease from Escherichia coli that acts specifically upon duplex DNA damaged by ultraviolet light, osmium tetroxide, acid, or x-rays. J Biol Chem. 1977 May 10;252(9):2802–2807. [PubMed] [Google Scholar]
- Hayatsu H. Bisulfite modification of nucleic acids and their constituents. Prog Nucleic Acid Res Mol Biol. 1976;16:75–124. doi: 10.1016/s0079-6603(08)60756-4. [DOI] [PubMed] [Google Scholar]
- Hayatsu H., Wataya Y., Kai K., Iida S. Reaction of sodium bisulfite with uracil, cytosine, and their derivatives. Biochemistry. 1970 Jul 7;9(14):2858–2865. doi: 10.1021/bi00816a016. [DOI] [PubMed] [Google Scholar]
- Hochhauser S. J., Weiss B. Escherichia coli mutants deficient in deoxyuridine triphosphatase. J Bacteriol. 1978 Apr;134(1):157–166. doi: 10.1128/jb.134.1.157-166.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isolation and characterization of a Bacillus subtilis mutant with a defective N-glycosidase activity for uracil-containing deoxyribonucleic acid. J Bacteriol. 1977 Aug;131(2):438–445. doi: 10.1128/jb.131.2.438-445.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnlein U., Lee B., Linn S. Human uracil DNA N-glycosidase: studies in normal and repair defective cultured fibroblasts. Nucleic Acids Res. 1978 Jan;5(1):117–125. doi: 10.1093/nar/5.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laval J. Two enzymes are required from strand incision in repair of alkylated DNA. Nature. 1977 Oct 27;269(5631):829–832. doi: 10.1038/269829a0. [DOI] [PubMed] [Google Scholar]
- Lindahl T. An N-glycosidase from Escherichia coli that releases free uracil from DNA containing deaminated cytosine residues. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3649–3653. doi: 10.1073/pnas.71.9.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. Irreversible heat inactivation of transfer ribonucleic acids. J Biol Chem. 1967 Apr 25;242(8):1970–1973. [PubMed] [Google Scholar]
- Lindahl T., Ljungquist S., Siegert W., Nyberg B., Sperens B. DNA N-glycosidases: properties of uracil-DNA glycosidase from Escherichia coli. J Biol Chem. 1977 May 25;252(10):3286–3294. [PubMed] [Google Scholar]
- Lindahl T. New class of enzymes acting on damaged DNA. Nature. 1976 Jan 1;259(5538):64–66. doi: 10.1038/259064a0. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Heat-induced deamination of cytosine residues in deoxyribonucleic acid. Biochemistry. 1974 Jul 30;13(16):3405–3410. doi: 10.1021/bi00713a035. [DOI] [PubMed] [Google Scholar]
- Ljungquist S. A new endonuclease from Escherichia coli acting at apurinic sites in DNA. J Biol Chem. 1977 May 10;252(9):2808–2814. [PubMed] [Google Scholar]
- Makino F., Munakata N. Deoxyuridine residues in DNA of thymine-requiring Bacillus subtilis strains with defective N-glycosidase activity for uracil-containing DNA. J Bacteriol. 1978 Apr;134(1):24–29. doi: 10.1128/jb.134.1.24-29.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M. DNA intermediates at the Escherichia coli replication fork: effect of dUTP. Proc Natl Acad Sci U S A. 1978 Jan;75(1):238–242. doi: 10.1073/pnas.75.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera R. M., Bonhoeffer E. Replication of Escherichia coli requires DNA polymerase I. Nature. 1974 Aug 9;250(5466):513–514. doi: 10.1038/250513a0. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M., Hayakawa H., Makino F., Tanaka K., Okada Y. A human enzyme that liberates uracil from DNA. Biochem Biophys Res Commun. 1976 Nov 22;73(2):293–299. doi: 10.1016/0006-291x(76)90706-3. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Braverman B., Louis J. B., Servis R. E. Nucleic acid reactivity and conformation. II. Reaction of cytosine and uracil with sodium bisulfite. J Biol Chem. 1973 Jun 10;248(11):4060–4064. [PubMed] [Google Scholar]
- Shapiro R., Klein R. S. The deamination of cytidine and cytosine by acidic buffer solutions. Mutagenic implications. Biochemistry. 1966 Jul;5(7):2358–2362. doi: 10.1021/bi00871a026. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Yamaguchi H. Nucleic acid reactivity and conformation. I. Deamination of cytosine by nitrous acid. Biochim Biophys Acta. 1972 Nov 9;281(4):501–506. doi: 10.1016/0005-2787(72)90150-5. [DOI] [PubMed] [Google Scholar]
- Tamanoi F., Okazaki T. Uracil incorporation into nascent DNA of thymine-requiring mutant of Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1978 May;75(5):2195–2199. doi: 10.1073/pnas.75.5.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye B. K., Chien J., Lehman I. R., Duncan B. K., Warner H. R. Uracil incorporation: a source of pulse-labeled DNA fragments in the replication of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1978 Jan;75(1):233–237. doi: 10.1073/pnas.75.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye B. K., Lehman I. R. Excision repair of uracil incorporated in DNA as a result of a defect in dUTPase. J Mol Biol. 1977 Dec 5;117(2):293–306. doi: 10.1016/0022-2836(77)90128-0. [DOI] [PubMed] [Google Scholar]
- Tye B. K., Nyman P. O., Lehman I. R. Excision repair of uracil during replication of phiX174 DNA in vitro. Biochem Biophys Res Commun. 1978 May 30;82(2):434–441. doi: 10.1016/0006-291x(78)90894-x. [DOI] [PubMed] [Google Scholar]
- Tye B. K., Nyman P. O., Lehman I. R., Hochhauser S., Weiss B. Transient accumulation of Okazaki fragments as a result of uracil incorporation into nascent DNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):154–157. doi: 10.1073/pnas.74.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman J. S., McCarthy B. J. Alkali deamination of cytosine residues in DNA. Biochim Biophys Acta. 1973 Feb 4;294(1):396–404. doi: 10.1016/0005-2787(73)90094-4. [DOI] [PubMed] [Google Scholar]


