Abstract
In the current study, we show that macrophages adaptively resist anthrax lethal toxin (LT) through a toxin-activated process termed toxin-induced resistance (TIR). TIR was triggered by pretreatment of RAW 264.7 or J774A.1 macrophages with a low dose of LT for at least 6 h, which resulted in resistance to high doses of LT for 96 h. Activation of TIR required functional toxin, because LT subunits, mutants, and heat-inactivated toxin were unable to trigger resistance. TIR macrophages were not altered in toxin receptor levels or cell cycle profiles. Treatment of TIR macrophages with high doses of LT resulted in a sustained decline in full-length mitogen-activated protein kinase kinase 2, a known target of lethal factor, and a marked reduction in diphosphorylated extracellular response kinases 1,2 for 24 h. However, despite the sustained loss of full-length mitogen-activated protein kinase kinase 2, by 48 h, TIR macrophages regained diphosphorylated extracellular response kinases 1,2, suggesting an adaptation led to recovery of this signaling pathway. TIR macrophages were also able to maintain normal levels of ubiquitinylated proteins, whereas sensitive cells show a rapid reduction in ubiquitin-modified proteins before cell death, indicating a possible alteration in proteasome activity contributed to resistance. These results provide a paradigm for toxin-cell interactions and suggest macrophages are capable of adapting to and tolerating toxic doses of LT.
Atripartite toxin composed of protective antigen (PA), lethal factor (LF), and edema factor (EF) is produced by Bacillus anthracis. During cellular intoxication, PA works in combination with LF or EF to yield lethal toxin (LT) or edema toxin (ET), respectively (1). After PA-mediated cell entry, EF acts as a Ca2+ and calmodulin-dependent adenylate cyclase, whereas LF functions as a Zn2+-dependent metalloprotease and cleaves mitogen-activated protein kinase kinases (MAPKKs). LF has been shown to cleave six different MAPKKs, including MEK1, MEK2, MKK3, MKK4, MKK6, and MKK7 (2), leading to potential disruption of three signaling pathways that encompass extracellular signal-regulated kinases 1 and 2 [extracellular response kinase 1,2 (ERK1,2)], as well as c-jun N-terminal kinase and p38.
Macrophages, putative targets of LT in mouse models (3) and key elements of innate immunity, present a hostile environment leading to the destruction of bacterial pathogens and initiation of inflammatory responses. Furthermore, macrophages are among the first cells to encounter B. anthracis spores at the onset of infection. The dichotomy of B. anthracis-macrophage interaction is such that early events require macrophage survival, because germination occurs within these cells. At later time points in disease, the vegetative form of B. anthracis must avoid destruction by the macrophage. In these secondary stages of disease, both anthrax toxin and the organism's poly(D)-glutamic acid capsule may prevent macrophage-mediated destruction of B. anthracis. Yet, these scenarios of anthrax disease do not take into account possible adaptations on the part of the macrophage, which could change the dynamics of host-pathogen interaction in favor of the host. There is no reason why this should be so, because macrophages demonstrate elegant versatility during the disease process. Indeed, adaptive responses are a hallmark of macrophage activity, because these cells successfully recognize pathogens, destroy the organism, and attenuate responses potentially detrimental to the host.
As an example of macrophage adaptation, it is well established that macrophages mount a robust response to inflammatory stimuli such as lipopolysaccharide (LPS), yet after initial exposure to LPS, macrophages become tolerant to this molecule (4). Secondary treatment with LPS yields a blunted macrophage response lacking detectable amounts of tumor necrosis factor-α, IL-1β, and IL-6 (5, 6). Other inflammatory stimuli, including lipoteichoic acid, lipoaribinomannan, and muramyl dipeptide, have been found to induce macrophage tolerance (7-9). To date, induced macrophage tolerance or resistance to other bacterial factors, such as exotoxins, has not been reported.
During recent studies, we observed a small population (≈2.0%) of RAW 264.7 macrophages that survived treatment with high doses of LT. When expanded in vitro, these cells maintained resistance to LT for ≈5 d. Herein, we report on a putative mechanism for this process, which we term toxin-induced resistance (TIR). Analysis of TIR provides a paradigm for toxin-cell interactions, which suggests macrophages adapt to and resist LT.
Materials and Methods
Cell Lines and Media. Murine macrophage-like cells, RAW 264.7, J774A.1, and IC-21, were obtained from the American Type Cell Culture Collection (ATCC TIB-71, -67, and -186, respectively) and cultured in RPMI medium 1640 supplemented with 10% FCS. CHO-R1.1 cells (deficient in anthrax toxin receptor ATR) were kindly provided by Kenneth Bradley (University of California, Los Angeles) (10). Assays were performed on cells between passages 5 and 30. Media and reagents were confirmed LPS-free by the limulus amebocyte lysate assay (BioWhittaker; sensitivity ≈0.03 units/ml).
Protein Purification. PA, LF, and EF were purified from Escherichia coli/BL-21 (DE3) by using His-tagged affinity chromatography according to the manufacturer's protocol (Novagen). LPS was removed from purified PA and LF by polymyxin B chromatography (Sigma). Proteins were tested for LPS before each assay, as described above.
Cytotoxicity Assays and TIR. Target macrophages were grown in 96-well plates (1 × 104 cells per well) in 100 μl of medium as described above. To analyze TIR, cells were pretreated for 24 h with LT, PA/LFn (N-terminal region of LF), PA/LFH690C, boiled LT, and PA or LF at the appropriate concentrations. After the initial treatment, cells were exposed to a high dose of LT consisting of 1 μg/ml each PA and LF at the indicated time points. Control cells were incubated with an equal volume of LPS-free 20 mM Tris, pH 8.0, during pretreatment or secondary challenge. Cell viability was determined colorimetrically by using WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt (Dojindo Molecular Technologies, Gaithersburg, MD)]. Values are expressed relative to viability of cells (nonpretreated and pretreated) incubated in medium with LPS-free 20 mM Tris, pH 8.0, and are an average of at least four measurements (±SD) from two separate experiments.
Quantification of Receptor Number, cAMP, and Cell Cycle Profile of TIR Macrophages. Receptor number in TIR and control macrophages was determined by using a protocol similar to Bradley et al. (10). Briefly, a cysteine reactive mutant of PA (PAK563C) was labeled with Alexa Fluor 488 maleimide (Molecular Probes). TIR and control macrophages were washed once in cold PBS, centrifuged, and washed once with RP10. Cells were then resuspended in 1 ml of medium containing 100 nM Alexa Fluor 488-labeled PA (AM-PA) and incubated at 4°C for 1 h. To determine specificity, macrophages were incubated in the presence of labeled PA with a 200-fold molar excess of unlabeled PA. Alternatively, CHO-R1.1 cells were treated as above and incubated with 100 nM AM-PA. Cells were subsequently washed in RP10 medium and receptor-staining intensity was measured by fluorescence on a FACScan flow cytometer (Becton Dickinson). Gating of live cells by propidium iodide (PI) and data analysis were performed by using CELLQUEST software (Becton Dickinson). The presented data are representative of three independent experiments.
To determine TIR sensitivity to ET, RAW 264.7 macrophages were pretreated for 24 h with a low dose of LT, as described above before treatment with ET (1 μg/ml PA + 2 μg/ml EF). Levels of cAMP were assayed after 24 h by using the cAMP enzyme immunoassay system (Amersham Biosciences).
Cell-cycle profiles were analyzed in TIR and control macrophages (1 × 104 cells) on a FACScan flow cytometer (Becton Dickinson) by using MODFIT LT ver. 2.0 software. Briefly, cells were washed once in PBS and fixed in 70% ethanol. After washing with 5 ml of PBS, cells were incubated in propidium iodide (PI) staining buffer (0.1% Triton-X-100, 0.2 mg/ml DNAse free RNase, 20 μg/ml PI) for 30 min at room temperature in the dark. Results are representative of two independent experiments.
Immunoblot Analysis of Mitogen-Activated Protein Kinases, Mitogen-Activated Protein Kinase Kinases, and Ubiquitin. Lysates from experimental and control macrophages were collected by TRIzol extraction (Life Technologies, Grand Island, NY) and examined by Western blot by using enhanced chemiluminescence detection (Amersham Biosciences). When indicated, RAW 264.7 cells were preincubated with 10 μM lactacystin (Calbiochem) for 1 h before treatment with a high dose of LT. Samples (10 μg) were resolved on 10% polyacrylamide gel, transferred to poly(vinylidene difluoride) membrane, and probed with one of the following primary antibodies: MEK2 (N-terminal; 1:500; catalogue no. sc-524, Santa Cruz Biotechnology), p-ERK1,2-Y (1:1,000; catalogue no. sc-7383, Santa Cruz Biotechnology), p-ERK1,2-YT (1:1,000; catalogue no. 9101, Cell Signaling Technology, Beverly, MA), ERK1,2 (1:1,000; catalogue no. 9102, Cell Signaling Technology), or antiubiquitin (1:1,000; catalogue no. 662099, Calbiochem). The primary antibody was revealed with horse-radish peroxidase-conjugated secondary antibodies (catalogue no. NXA 931; anti-mouse IgG or catalogue no. NA934, anti-rabbit IgG, Amersham Biosciences).
Proteasome Activity and Characterization. Fluorescence-based determination of proteasome activity was performed as follows: RAW 264.7 cells were pretreated with a low dose of LT as described above or pretreated with the corresponding volume of LPS-free 20 mM Tris, pH 8.0, for 24 h before exposure to a high-dose treatment of LT (1 μg/ml each LF and PA). After 60 min of treatment, cells were washed with PBS, and the protein extracts were prepared in 100 mM Hepes, 10% sucrose, and 0.1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate. Suc-LLVY-7-amino-4-methylcoumarin (AMC) (catalogue no. I-1395, Bachem), Z-LLE-AMC (catalogue no. 539141, Calbiochem), and Z-VVR-AMC (catalogue no. I-1540, Bachem) were resuspended in DMSO and used at 50 μM in the assay. Substrates were incubated with protein extracts (50 μg) in 5 mM MgCl2/50 mM Tris·HCl, pH 7.8/20 mM KCl/5 mM Mg(C2H3O2)2 in a total volume of 200 μl. Degradation of fluoropeptides was determined by measuring the fluorescence of AMC at 460 nm (excitation at 355 nm) by using a FLUOstar (Durham, NC) OPTIMA fluorimeter over 30 min at 37°C.
Results
TIR After Exposure to Subcytotoxic Amounts of LT. During initial experiments, we observed a small population of RAW 264.7 macrophages (≈2%) that retained viability during treatment with a high dose of LT (1 μg/ml LF, 1 μg/ml PA). This observation was made under multiple growth conditions in both tissue-culture flasks and 96-well plates, with varying cell densities and at different cell passages. Interestingly, further treatment of these cells with higher doses of LT (5 μg LF/ml, 1 μg PA/ml) did not result in loss of cell viability (data not shown). Yet, when grown for 2 wk, these cells regained sensitivity to LT, indicating the resistance was transient and not the result of a selected spontaneous mutation (data not shown).
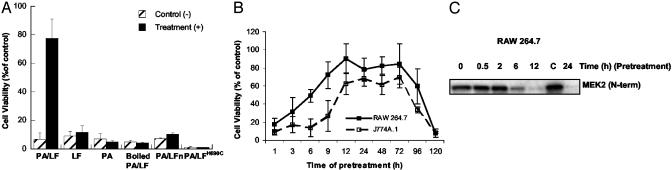
A plausible hypothesis for TIR was that resistant cells arose as a result of exposure to lower doses of the toxin during initial treatment, which then led to the adaptive resistance. In testing this hypothesis, RAW 264.7 and J774A.1 macrophages were pretreated with a range of doses of LT and assayed for viability and subsequent resistance to treatment with cytotoxic amounts of LT. A dose curve is shown in Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org, and represents doses ranging in amounts causing >95% cytotoxicity to <5% cytotoxicity during pretreatment. One dose (5 ng/ml LF plus 1 μg/ml PA) was capable of triggering resistance to subsequent high-dose treatment, without causing a high level of cytotoxicity in RAW 264.7 macrophages (Fig. 1A). Cells that survived original treatments at higher doses (>5ng/ml LF) were also resistant to subsequent treatment but made up only ≈5% of the starting number of cells, because most cells were killed at this dose of the toxin. Similar effects were also observed in J774A.1 macrophages (data not shown). Doses of LF below 5 ng/ml neither caused detectable cytotoxicity nor activated TIR. To determine whether functional toxin is required to activate resistance, RAW 264.7 macrophages were pretreated with PA alone, LF alone, PA plus LFn (a truncated form of LF capable of PA interaction and cell entry), heat-inactivated LT, or PA plus an inactive mutant of LF (LFH690C). As shown in Fig. 1 A, TIR required both functional PA and LF and could not be conferred by noncytotoxic derivatives of the toxin.
Fig. 1.
Activation of TIR by pretreatment with subcytotoxic doses of anthrax LT. (A) Comparison of TIR activation in RAW 264.7 cells treated with functional or inactive LT. Cells were preincubated 24 h with LF/PA, LF, PA, heat-inactivated LF/PA, PA/LFn (N-terminal region of LF), and PA/LFH690C (filled bars), or pretreated with LPS-free 20 mM Tris, pH 8.0 (hatched bars). PA was used at 1 μg/ml and LF, LFn, or LFH690C was used at 5 ng/ml. Cells were subsequently challenged with 1 μg/ml of each PA and LF and assayed for viability 24 h posttreatment. (B) Time course of TIR activation and retention. RAW 264.7 and J774A.1 cells were pretreated with 5 ng/ml LF plus 1 μg/ml PA for the indicated times before challenge with 1 μg/ml PA and LF. Cells were assayed for viability 24 h after high-dose treatment. (C) LF-mediated cleavage of MEK2 during activation of TIR. RAW 264.7 cells were pretreated for the indicated times with the TIR-inducing dose of LT. Cell extracts were collected and analyzed for substrate cleavage by using antibody reactive to the N terminus of MEK2.
To determine the time course for activation and retention of TIR, RAW 264.7 and J774A.1 macrophages were pretreated with a TIR-inducing dose of LT and subsequently treated with a high dose of LT at the indicated time points. As shown in Fig. 1B, treatment of cells with high doses of the toxin resulted in a loss of cell viability when the pretreatment was <6 h or >96 h. This time of 6 h also correlated with the cleavage of MEK2, a known target of LF (see Fig. 1C).
TIR is similar to another process reported for inflammatory stimuli such as LPS, lipoteichoic acid, and mycolic acid (4). In this hyposensitivity, macrophages pretreated with one inflammatory stimulus are refractory to treatment with other stimuli. Because LT has been reported to activate proinflammatory cytokine production (11), the ability of LPS to confer resistance to LT was tested, and no detectable impact on LT cytotoxicity was found (data not shown). This result suggested that, although similar in that resistance can be conferred, the mechanism of transient resistance triggered by LT may be different from that of other inflammatory stimuli.
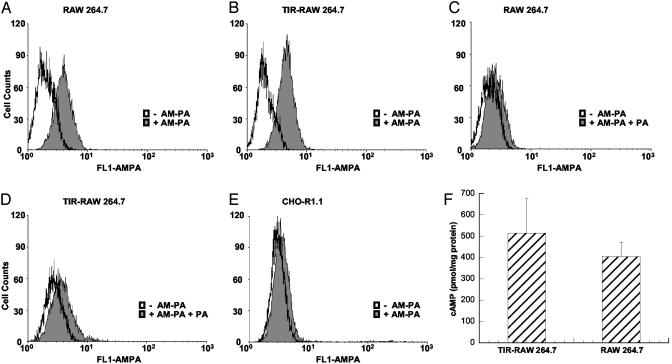
Receptor Levels, Sensitivity to ET, and Cell-Cycle Profiles of TIR Cells. To address the possibility that TIR was reducing accessible PA receptors or blocking toxin entry, the receptor number was examined after low-dose treatment with LT. In this experiment, a functional cysteine mutant of PA (PAK563C) was fluorescently labeled by using Alexa Fluor 488 Maleimide as described by Bradley et al. (10). Labeled PA was then used to quantify receptor number in TIR and control cells (including CHO-R1.1 ATR-deficient cells) by flow cytometric analysis. As shown in Fig. 2 A-D, there was no discernible difference in receptor levels for TIR and control macrophages. Furthermore, TIR and control cells showed similar sensitivity to ET (Fig. 2F). These results suggested that pretreatment did not inhibit receptor binding or prevent subsequent PA-mediated entry.
Fig. 2.
PA-receptor levels on TIR and non-TIR macrophages and TIR macrophage susceptibility to ET. (A and C) RAW 264.7 cells pretreated with LPS-free 20 mM Tris, pH 8.0. (B and D) RAW 264.7 cells pretreated with 1 μg/ml PA plus 5 ng/ml LF for 24 h. After treatment, cells were incubated for 1 h on ice with 100 nM AM-PA (A and B) or with 100 nM AM-PA plus 200-fold molar excess of cold PA (C and D), washed once, and analyzed by flow cytometry. (E) CHO-R1.1 cells were incubated with AM-PA as above. (F) Control and TIR-RAW 264.7 were treated for 24 h with ET (1 μg/ml PA plus 2 μg/ml EF) and assayed for intracellular cAMP levels.
TIR cells were also examined for changes in cell-cycle profiles, because this could explain the ability of some cells in the population to survive LT treatment. As shown in Table 1, which is published as supporting information on the PNAS web site, although TIR and control cells had a slightly different distribution of cells at various stages of the cell cycle, this could not explain TIR. Regardless of the cell cycle stage (G0/G1, S, or G2/M), TIR macrophages were resistant to high doses of LT.
Attenuation of MEK/ERK Signaling in TIR Cells. Initial observations indicated that TIR was dose-dependent, required functional toxin, and did not reduce receptor numbers, hinder toxin entry, or alter cell-cycle profiles. Collectively, these findings indicated that TIR could involve events downstream of cell entry. For this reason, effects of low-dose treatment on signaling pathways targeted by LT were investigated. Phosphorylated p38 and c-jun N-terminal kinase were not detected in untreated cells or after low- or high-dose treatments of LT, suggesting these pathways were quiescent under our experimental conditions (data not shown).
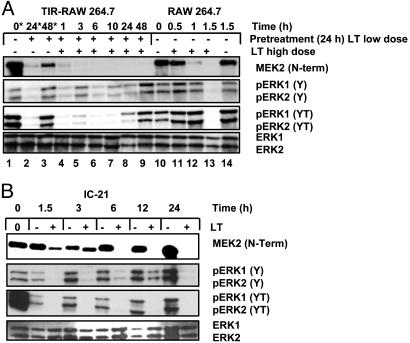
For the above reasons, we examined, by immunoblot analysis, relative levels of MEK2, ERK1,2, and phosphorylated ERK1,2 in TIR cells and TIR cells treated with a high dose of LT. As shown in Fig. 3A, when RAW 264.7 macrophages were treated with the TIR-inducing dose of LT, there was a sustained reduction in full-length MEK2 for at least 24 h, with detectable levels returning by ≈48 h. As shown in Fig. 3A, lane 2, concomitant with this decline in full-length MEK2 is a reduction in monophosphorylated ERK1,2 (phosphorylated at Y204) and diphosphorylated ERK1,2 (phosphorylated at Y204 and T202). By 48 h after low-dose treatment, levels of both monophosphorylated and diphosphorylated ERK1,2 return to near-normal amounts (see lane 3). When cells were pretreated with low-dose LT to induce TIR for 24 h and subsequently treated with a high dose of LT, there was a temporal shift in the overall MEK/ERK profile (see lanes 4-9). The amount of full-length MEK2 remained below the level of detection for at least 48 h after high-dose treatment.
Fig. 3.
MEK2 cleavage and ERK1,2 phosphorylation in LT-sensitive and -resistant macrophages. (A) MEK/ERK profile in TIR and non-TIR macrophages. Lane 1, mock-treated control; lanes 2 and 3, pretreated low dose; lanes 4-9, TIR macrophages treated with high-dose LT (1 μg/ml PA and LF); lane 10, mock-treated control; lanes 11-13, non-TIR macrophages treated with high dose of LT; lane 14, mock-treated control. Time corresponds to LT high-dose treatment unless indicated by *, where time is relative to initial treatment. (B) MEK/ERK profile in IC-21 macrophages. LT-resistant macrophage cells (IC-21) were treated with a high dose of the toxin (1 μg/ml PA and LF) for the indicated times. Lysates were analyzed for MEK2 cleavage and ERK1,2 phosphorylation by immunoblotting.
Interestingly, after high-dose treatment of TIR cells with LT, there was a detectable increase in the amount of monophosphorylated ERK1,2, although there was no corresponding change in the level of full-length MEK2 or diphosphorylated ERK1,2. Both remained below levels of detection for at least 24 h. As shown in lane 9, incongruent with the predicted mechanism of MEK/ERK signaling, levels of both monophosphorylated and diphosphorylated ERK1,2 approached similar to control by 48 h after high-dose treatment of TIR macrophages, despite the fact that full-length MEK2 remained undetectable. High-dose treatment of non-TIR RAW 264.7 macrophages resulted in cleavage of MEK2 by 1 h, and full-length MEK2 fell below the levels of detection by 90 min. Levels of monophosphorylated and diphosphorylated ERK were unchanged up to 1 h after LT treatment, although MEK2 was cleaved by that time point and was undetectable 90 min after treatment.
Past studies have shown that macrophages from particular strains of inbred mice are resistant to LT. Whether this mechanism of stable resistance was similar to TIR observed in RAW 264.7 and J774A.1 macrophages was unclear. For this reason, IC-21 macrophages, known to be resistant to LT, were examined for changes in ERK1,2 phosphorylation after high-dose treatment with the toxin. As shown in Fig. 3B, similar to the profile identified in TIR macrophages, IC-21 macrophages demonstrated a rapid decrease in monophosphorylated and diphosphorylated ERK1,2, although the decrease was more dramatic for the latter.
A reasonable explanation for TIR was that reduction in levels of phosphorylated ERK1,2, conditioned the cell to survive the subsequent high-dose treatment. To address the possibility that TIR occurred due to inactivation of MEK/ERK signaling, macrophages were pretreated with inhibitors (PD98059 and U0126), which block activation of ERK1,2. These inhibitor-treated cells were then subjected to high-dose treatment with LT. Under these experimental conditions, pretreatment with PD98059 or U0126 had no impact on cell survival when macrophages were exposed to high doses of LT (see Fig. 7, which is published as supporting information on the PNAS web site). The absence of protection from LT by PD98059 and U0126 suggested that, whereas abrogation of ERK1,2 signaling may be an important component of TIR, it is not the sole contributor to this protective effect.
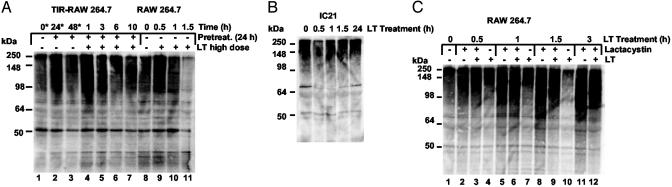
Putative Role of Ubiquitin/Proteasome Activity in TIR. Previous studies by Tang and Leppla (12) found that proteasome activity was necessary for LT cytotoxicity. As reported, macrophages were protected from LT cytotoxicity when pretreated with lactacystin, an effective proteasome inhibitor. Because of this reported link between the proteasome and sensitivity to LT, TIR cells were examined for attenuation of proteasome activity. Both TIR cells and sensitive macrophages showed similar proteasome activity when assayed for the ability to hydrolyze small peptide fluorescent substrates (Fig. 8, which is published as supporting information on the PNAS web site). In contrast, unlike sensitive cells, TIR cells showed a sustained level of ubiquitinylated proteins, whereas non-TIR cells revealed a dramatic reduction in ubiquitin-conjugated proteins (see Fig. 4A). The decline in ubiquitin-modified proteins did not appear to be due to the loss of total cell protein, because control and LT-treated cells showed similar profiles by SDS/PAGE analysis (Fig. 9, which is published as supporting information on the PNAS web site). When TIR macrophages were treated with a high dose of LT, there was no detectable reduction in the level of ubiquitin-conjugated proteins. In contrast to sensitive cells, TIR and IC-21 macrophages had steady levels of total ubiquitinylated proteins (Fig. 4 A and B) when treated with high doses of LT. Thus, although pretreatment did not detectably alter proteasome activity on small peptides, the levels of ubiquitin-conjugated proteins were distinctly different among the cell types. There was a direct correlation between normal levels of ubiquitinylated proteins and cell survival. In line with this, pretreatment with lactacystin also prevents a decline in the level of ubiquitinylated proteins as cells survive treatment with LT (see Fig. 4C). Again under these conditions, and similar to that reported by Tang and Leppla (12), lactacystin-pretreated cells survived high-dose LT treatment, whereas nonpretreated cells succumb to the toxin within <2 h.
Fig. 4.
Levels of ubiquitin-conjugated proteins in RAW 264.7, TIR-RAW 264.7, and RAW 264.7 cells treated with lactacystin. (A) Immunoblot analysis of polyubiquitinylated protein levels in TIR and non-TIR macrophages. (B) Immunoblot analysis of polyubiquitinylated protein levels in LT-resistant IC-21 macrophages. (C) Immunoblot analysis of polyubiquitinylated protein levels in LT-treated macrophages, preexposed to lactacystin.
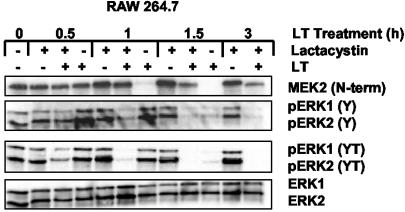
Because TIR macrophages and IC-21 macrophages show a noticeable decrease in the level of diphosphorylated ERK, the levels of this protein after treatment with lactacystin were analyzed. As shown in Fig. 5, treatment with lactacystin alone did not noticeably reduce the level of diphosphorylated ERK1,2; however, subsequent treatment with a high dose of LT for 1 h in lactacystin-treated cells resulted in the cleavage of MEK2. This event was concomitant with a reduction in diphosphorylated ERK1,2 below the levels of detection. At the same time, levels of diphosphorylated ERK1,2 were unchanged in LT-treated cells compared with untreated cells.
Fig. 5.
Correlation between inhibition of proteasome and loss of phosphorylated ERK1,2 leading to cell survival. RAW 264.7 cells were preincubated for 1 h with 10 μM lactacystin before treatment with LT (1 μg/ml PA and LF). Lysates from treated macrophages were analyzed by immunoblotting with antibodies against MEK2 and phosphorylated ERK1,2.
Discussion
In the current study, we present data indicating macrophages are capable of adaptive responses and resistance to LT. Given the important role of macrophages in anthrax disease, adaptive resistance to LT could provide a survival advantage to the host. In this regard, TIR resembles endotoxin tolerance, first reported >30 years ago, wherein it was found that preexposing macrophages to LPS reduces the inflammatory response to subsequent treatments with LPS (13). In fact, several inflammatory mediators, as well as surgical ischemia, have been shown to induce tolerance (14). The mechanism for LPS tolerance appears to be somewhat different from LT-induced tolerance, because we were not able to cross-confer resistance to LT by pretreatment with LPS.
Analysis of TIR led to the observation that macrophages are able to survive LT treatment despite the loss of ERK signaling. We present three examples of macrophage survival in the presence of MEK2 inactivation and loss of ERK1,2 phosphorylation. First, TIR macrophages show a sustained decrease in diphosphorylated ERK1,2 after high-dose treatment with LT, yet these cells survive. Second, ERK1,2 phosphorylation in LT-resistant IC-21 macrophages falls below the level of detection, and these cells survive despite the loss of ERK1,2 signaling for ≈24 h. Finally, treatment with lactacystin provides protection from LT despite the decline in phosphorylated ERK1,2. These observations suggest that, although a decline in ERK1,2 signaling may be important to LT-induced cell death, such a decrease might only synergize with other LT activities. Additionally, the increase in phosphorylated ERK1,2 48 h after LT treatment, despite the undetectable level of full-length MEK2, may indicate a cross-talk mechanism in this pathway. In the sustained absence of MEK2 signaling, cells may invoke a yet-undefined alternative pathway for activation of ERK1,2. It is also worth noting that, after toxin treatment, there are detectable levels of monophosphorylated ERK1,2, whereas levels of diphosphorylated ERK1,2 and full-length MEK2 remain undetectable. Reportedly, a critical threshold of monophosphorylated ERK1,2 must be reached before diphosphorylation of this signaling kinase (15). Thus, it is possible that the amount of functional MEK is such that phosphorylation of ERK1,2 occurs at a much slower rate but accumulates to a level (by 48 h) sufficient to trigger the diphosphorylation and completed activation of ERK1,2.
The detectable decrease in the level of ubiquitinylated proteins during normal intoxication and the previously reported protective effects of lactacystin (12), as well as results from TIR macrophages, propose an important role for proteasome activity after exposure to LT. The decline in ubiquitin-conjugated proteins may be the result of increased proteasome activity, which contributes to cell death. Results from the lactacystin protection studies presented here and first described by Tang and Leppla (12) indicate that the proteasome may degrade factors important to the survival of the intoxicated cell. It is reasonable to suspect TIR occurs due to modulation of events in a fashion similar to lactacystin. Yet the alteration in proteasome activity seems to be limited to ubiquitin-conjugated proteins, because we were unable to detect changes in the rate of cleavage of small peptides.
The rate of intoxication may also be a governing event leading to TIR. At the cytotoxic dose of LT, MEK2 cleavage is >90% complete by 1 h, whereas cleavage of MEK2 by low-dose LT takes at least 6 h and is not complete until ≈12 h after initial treatment. The window of time from 0 to 12 h posttreatment may be sufficient to allow the macrophage to invoke adaptive responses, such as protein relocalization, changes in cell signaling, or altered proteasome activity before completion of cytotoxicity. In line with this observation, cleavage of MEK2 within IC-21 macrophages also occurs at a slower rate, between 6 and 12 h, than that found in RAW 264.7 macrophages (see Fig. 3B). Further supporting this explanation for TIR is the observation that pretreatment with lactacystin slows the rate of MEK2 cleavage (see Fig. 5).
Our results are not the first to identify an approach to protect macrophages from LT, yet this is the only example of a method that uses functional toxin to provide resistance. Previous studies have found that cells can be protected from LT by pretreatment with inhibitors of the proteasome (12), protein translation (16), or vacuolar ATPases (17), indicating these processes are necessary for cytotoxicity. Furthermore, Singh et al. (18) reported that a PA mutant, deficient in LF/EF binding, could provide protection from LT by competing with wild-type PA for receptor binding. In turn, Bradley et al. (10) along with Scobie et al. (19) have shown that soluble forms of PA's two known receptors, tumor endothelial marker 8 and capillary morphogenesis protein 2, protect cells from LT cytotoxicity. Finally, Sellman et al. (20) have described PA mutants, which act as dominant negative inhibitors of wild-type PA and hold promise as novel therapeutics.
In contrast to the above approaches, TIR represents a mechanism of instilling toxin resistance by using functional toxin and could reflect a process that occurs in a real disease setting. Of particular interest to the current studies are the findings of Molnar and Altenbern (21) ≈40 years ago, which showed that Fisher rats were able to survive exposure to LT if pretreated with PA for at least 4 h. Similar to our findings on TIR in macrophages, Molnar and Altenbern reported this effect was transient, and rats regained sensitivity to LT within 24 h after pretreatment with PA. It is worth noting here that the assays of Molnar and Altenbern (21) were performed by using partially purified proteins of anthrax toxin, and it is reasonable to suspect PA contained contaminating amounts of LF. In fact, these investigators point out a second crossreacting band in their preparation of PA, which may very well have been trace amounts of LF.
The exponential growth of bacterial pathogens during the disease process is likely to be linked to incremental increases in specific virulence factors. Thus, although most experimental approaches expose cells or animals to a bolus of toxin, in the natural setting, cells will more likely experience an increasing gradient of these virulence factors. Initial exposure to the lower nontoxic amounts of the toxin may allow the cell to adapt for the impending higher dose. Such a process would represent an elegant, but perhaps more primitive, mechanism of host resistance to pathogen-derived virulence factors and may explain, in part, why several pathogens have evolved elaborate quorumsensing mechanisms or growth state regulation of toxin expression. In doing so, the organism may ensure that host cells are exposed to the toxin only when there is an amount adequate to overcome resistance. Continued consideration should be given to the possibility that mammalian cells (even those not involved with immunity) are not passive targets of bacterial virulence factors and invoke adaptive responses to promote survival of the host.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health National Center for Research Resources Grant RR15564.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PA, protective antigen; EF, edema factor; LT, lethal toxin; LF, lethal factor; TIR, toxin-induced resistance; ERK, extracellular response kinase; LPS, lipopolysaccharide; AM-PA, Alexa Fluor 488-labeled PA; ET, edema toxin.
References
- 1.Brossier, F. & Mock, M. (2001) Toxicon 39, 1747-1755. [DOI] [PubMed] [Google Scholar]
- 2.Vitale, G., Bernardi, L., Napolitani, G., Mock, M. & Montecucco, C. (2000) Biochem. J. 352, 739-745. [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon, T. C., Meselson, M., Guillemin, J. & Hanna, P. C. (1999) N. Engl. J. Med. 341, 815-826. [DOI] [PubMed] [Google Scholar]
- 4.West, M. A. & Heagy, W. (2002) Crit. Care Med. 30, S64-S73. [PubMed] [Google Scholar]
- 5.Leon, P., Redmond, H. P., Shou, J. & Daly, J. M. (1992) Arch. Surg. 127, 146-151. [DOI] [PubMed] [Google Scholar]
- 6.Zuckerman, S. H., Evans, G. F. & Butler, L. D. (1991) Infect. Immun. 59, 2774-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreira, M. E., Coelho, M. M. & Pela, I. R. (2001) Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R162-R169. [DOI] [PubMed] [Google Scholar]
- 8.Riedel, D. D. & Kaufmann, S. H. (2000) Microb. Infect. 2, 463-471. [DOI] [PubMed] [Google Scholar]
- 9.Lehner, M. D., Morath, S., Michelsen, K. S., Schumann, R. R. & Hartung, T. (2001) J. Immunol. 166, 5161-5167. [DOI] [PubMed] [Google Scholar]
- 10.Bradley, K. A., Mogridge, J., Mourez, M., Collier, R. J. & Young, J. A. (2001) Nature 414, 225-229. [DOI] [PubMed] [Google Scholar]
- 11.Hanna, P. C., Acosta, D. & Collier, R. J. (1993) Proc. Natl. Acad. Sci. USA 90, 10198-10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang, G. & Leppla, S. H. (1999) Infect. Immun. 67, 3055-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooke, M. S. (1965) Nature 206, 635-636. [DOI] [PubMed] [Google Scholar]
- 14.Park, K. M., Chen, A. & Bonventre, J. V. (2001) J. Biol. Chem. 276, 11870-11876. [DOI] [PubMed] [Google Scholar]
- 15.Pearson, G., Robinson, F., Beers Gibson, T., Xu, B. E., Karandikar, M., Berman, K. & Cobb, M. H. (2001) Endocr. Rev. 22, 153-183. [DOI] [PubMed] [Google Scholar]
- 16.Bhatnagar, R. & Friedlander, A. M. (1994) Infect. Immun. 62, 2958-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menard, A., Altendorf, K., Breves, D., Mock, M. & Montecucco, C. (1996) FEBS Lett. 386, 161-164. [DOI] [PubMed] [Google Scholar]
- 18.Singh, Y., Chaudhary, V. K. & Leppla, S. H. (1989) J. Biol. Chem. 264, 19103-19107. [PubMed] [Google Scholar]
- 19.Scobie, H. M., Rainey, G. J., Bradley, K. A. & Young, J. A. (2003) Proc. Natl. Acad. Sci. USA 100, 5170-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sellman, B. R., Mourez, M. & Collier, R. J. (2001) Science 292, 695-697. [DOI] [PubMed] [Google Scholar]
- 21.Molnar, D. M. & Altenbern, R. A. (1963) Proc. Soc. Exp. Biol. Med. 114, 294-297. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.