Abstract
Induction of antigen-specific suppression elicited by environmental insults, such as ultraviolet (UV)-B radiation in sunlight, can inhibit an effective immune response in vivo and may contribute to the outgrowth of UV-induced skin cancer. Although UV-induced DNA damage is known to be an initiating event in the immune suppression of most antigen responses, the underlying mechanism(s) of such suppression remain undefined. In this report, we document that Fas ligand (FasL) is critical for UV-induced systemic immune suppression. Normal mice acutely exposed to UV exhibit a profound suppression of both contact hypersensitivity and delayed type hypersensitivity (DTH) reactions and the development of transferable antigen-specific suppressor cells. FasL-deficient mice exposed to UV lack both transferable suppressor cell activity and primary suppression to all antigens tested, with the exception of the DTH response to allogeneic spleen cells. Interestingly, suppression of this response is also known to occur independently of UV-induced DNA damage. Delivery of alloantigen as protein, rather than intact cells, restored the requirement for FasL in UV-induced immune suppression of this response. These results substantiate that FasL/Fas interactions are essential for systemic UV-induced suppression of immune responses that involve host antigen presentation and suggest an interrelationship between UV-induced DNA damage and FasL in this phenomenon. Collectively, our results suggest a model whereby UV-induced DNA damage disarms the immune system in a manner similar to that observed in immunologically privileged sites.
Keywords: Fas ligand, ultraviolet radiation, immunosuppression, CD95, T suppressor cells
Exposure to UV-B radiation from sunlight is a significant worldwide environmental hazard. UV exposure induces the most common malignancy in humans, skin cancer, with an estimated 1,000,000 cases per year in the United States (1). UV exposure also significantly downmodulates immune responses, exacerbating a number of infectious diseases and permitting the outgrowth of highly antigenic UV-induced skin tumors in mice (1, 2). The effects of UV on the immune system have been clinically exploited to attenuate pathophysiological responses associated with autoimmune disease in the skin (e.g., psoriasis), transfusion-associated graft-versus-host disease, and tumor growth (e.g., cutaneous T cell lymphoma) (3–5). Elucidating the mechanism(s) underlying UV-induced immunosuppression may permit significant advances in the therapeutic use of UV light and define a target for intervention in infectious diseases adversely affected by UV exposure. Moreover, the contribution of UV-induced immune suppression to skin carcinogenesis could be more accurately defined.
A large body of evidence supports the concept that exposure to UV radiation can suppress cellular immune responses in animals and humans to antigens applied both locally (at the site of irradiation) and distally (at a nonirradiated [NR]1 site) (6). Acute exposure to UV-B radiation alters the local site such that epicutaneous application of hapten fails to induce contact hypersensitivity (CHS) and instead induces tolerance in UV-B–susceptible mice (7, 8). Acute UV-B exposure can also result in diminished CHS and delayed type hypersensitivity (DTH) responses when antigens are applied epicutaneously or subcutaneously, respectively, at NR sites (6, 9, 10). Suppression of immune responses to antigens administered at distant, NR sites is known as systemic immune suppression and is characterized by the generation of antigen-specific splenic T suppressor cells (11– 13). Although the phenotype and mode of action of the T suppressor cell population remain largely uncharacterized, previous studies have documented that UV-induced immune suppression can be transferred to naive, NR animals by CD4+ cells from UV-irradiated (UVR), antigen-primed mice (14, 15). Suppressor cells cannot be elicited by either UVR or antigen alone but require the introduction of antigen at a critical time after irradiation (16, 17). Suppressor cell activity appears to be targeted to the Th population and requires IL-10 for both induction and function (18–20). Data from a number of laboratories suggest that one consequence of UV-B exposure is a shift from a Th1- to a Th2-type immune response, resulting in the suppression of CHS and DTH induction and the generation and maintenance of suppressor cells (19–24).
DNA damage (in the form of cyclobutane pyrimidine dimers) has been identified as the initial photobiological event triggering UV-induced systemic immune suppression to most antigens in the mouse (25, 26). Application of liposomes containing bacteriophage T4 endonuclease V (an excision repair enzyme specific for pyrimidine dimers) to UV-irradiated skin can repair the UV-induced DNA damage in the skin, diminish UV-induced Th2-type cytokine production, reverse UV-induced systemic suppression, and prevent the generation of UV-induced suppressor cells (27). Conversely, inducing DNA damage in the skin using liposomes containing HindIII can result in the production of Th2-type cytokines and the systemic suppression of CHS and DTH responses (28, 29). These observations point to DNA damage as a central mechanism underlying UV-induced immune suppression and suggest a sequence of events in which UV-initiated DNA damage triggers immunomodulatory cytokine production, the generation of CD4+ suppressor cells, and immune perturbations leading to diminished CHS and DTH responses. Considering that primary immunosuppression and the generation of suppressor cells are dissociable events and that immunomodulatory cytokine production is necessary, but not sufficient, to recapitulate the effects of UVR on the immune response, the existence of additional molecular linkages between DNA damage and immune suppression is likely.
Recent evidence has documented the interactions of Fas and Fas ligand (FasL) in the control of specific T cell–mediated immune responses (reviewed in 30). This complementary receptor–ligand pair initiates apoptosis in activated lymphocytes (31, 32) and is required for the maintenance of peripheral tolerance (33) and immune privilege (34, 35). Several lines of circumstantial evidence led us to query whether Fas/FasL interactions were involved in systemic UV-induced immune suppression. First, FasL is inducible by DNA damage (36) and is upregulated in normal skin following exposure to UV irradiation (3); second, Fas/FasL interactions have been shown to mediate antigen-specific immune suppression (35, 37); third, CD4+ T cells have been documented to upregulate FasL expression and induce autocrine, paracrine, or juxtacrine cell death (38–41); and finally, the preferential elimination of Th1 cells by FasL has been reported (42). To test the hypothesis that Fas and FasL are involved in the immunomodulatory effects of UVR, we investigated the biological consequences of Fas and FasL loss of function using lpr and gld mice, respectively, on UV-induced systemic immune suppression and the generation of transferable suppressor cells.
Materials and Methods
Mice.
Specific pathogen-free C3H/HeJ, Balb/c, and C57Bl/6 male mice were purchased from the National Cancer Institute– Frederick Cancer Research Facility Animal Production Area. C57Bl/6 gld/gld and C57Bl/6 lpr/lpr male mice were purchased from The Jackson Laboratory. C3H/HeJ gld/gld male mice were generated from a breeder colony maintained in our facility and used between 8 and 14 wk of age. Mice were housed in a pathogen-free barrier facility accredited by the American Association for Accreditation of Laboratory Animal Care, in accordance with current U.S. Department of Agriculture, Department of Health and Human Services, and National Institutes of Health regulations and standards. All animal procedures were approved by the Institutional Animal Care and Use Committee.
UV-B Radiation Source and Irradiation Procedure.
A bank of six Westinghouse FS40 sunlamps was used as a source of UV radiation as described (25).
DTH Responses to Candida albicans and Alloantigen.
DTH responses were assessed as previously described (9). In brief, mice were shaved and exposed to UV-B radiation (2–5 and 15 kJ/m2 for C. albicans and alloantigen, respectively). 3 d later, mice were sensitized by subcutaneous injection of antigen (107 formalin-fixed C. albicans or 5 × 107 Balb/c spleen cells or cell equivalents). 6–10 d after antigen sensitization, mice were challenged by injecting either purified C. albicans protein (Allercheck, Inc.) or 107 Balb/c spleen cells in the footpad. 24 h later, footpad swelling was quantitated using a spring loaded micrometer (Swiss Precision Instruments). Specific footpad swelling (Δswelling) was determined by subtracting the footpad swelling in mice that were challenged but not sensitized from that observed in mice that were sensitized and challenged. Percent suppression was calculated as: % suppression = 1 − (T − N/P − N) × 100, where N = negative control (response of unsensitized mice to challenge), P = positive control (response of sensitized mice to challenge), and T = test group (response of mice given UV irradiation before sensitization and challenge). Treatment groups consisted of 3–6 (typically 5) mice; both hind footpads were measured.
CHS Response to FITC.
CHS responses were determined as previously described (43). In brief, for FITC responses, the abdominal hair of mice was shaved, their ears protected with electrical tape, and the animals exposed to UV-B radiation (2 kJ/m2). 3 d later, the dorsal hair was shaved and the animals sensitized by epicutaneous application of 400 μl of 0.5% FITC (Isomer I, Aldrich Chemical Co.) in acetone–dibutylphthalate (1:1, vol/vol). 5–7 d later, the mice were challenged by applying either 10 μl 0.5% FITC to the ventral and dorsal surfaces of both ears. Ear swelling (Δswelling) was quantitated 24 h later using a spring loaded micrometer and specific ear swelling determined by subtracting the ear swelling in mice challenged but not sensitized from that observed in mice that had been sensitized and challenged; percent suppression was calculated as described for DTH responses.
Transfer of Spleen Cells.
For transfer of splenic suppressor cell populations, mice were killed, spleens harvested, and single cell suspensions prepared immediately following DTH or CHS analysis. Approximately 108 spleen cells were injected into the tail veins of NR, naive recipient mice and the animals immediately sensitized by subcutaneous injection (107 formalin-fixed C. albicans or 5 × 107 Balb/c spleen cells) or epicutaneous application (400 μl 0.5% FITC). 6–10 d later, mice were challenged as described above and DTH or CHS responses determined 24 h later.
Detection of FasL mRNA.
C3H/HeJ mice were shaved and exposed to 15 kJ/m2 UV radiation as described above. 3 d after UVR, mice were killed and inguinal, axillary, and brachial lymph nodes harvested. Lymph nodes were mechanically dissociated and washed, and RNA was extracted with Trizol (GIBCO BRL) per manufacturer's instructions. Reverse transcriptase (RT) and PCR reactions were performed with the GeneAmp PCR kit (Perkin-Elmer Corp.) using the following primer sequences: FasL, 5′-ATCCCTCTGGAATGGGAAGA-3′ (forward), 5′-CCATATCTGTCCAGTAGTGC-3′ (reverse); β actin, 5′-TCCTGTGGCATCCATGAAACT-3′ (forward), 5′-CTTCGTGAACGCCACGTGCTA-3′ (reverse). 35 cycles of PCR were performed: 30 s at 94°C, 45 s at 55°C, and 60 s at 72°C, using a Perkin-Elmer Gene Amp 9600.
Statistical Analysis.
For DTH and CHS analysis, the probability of no difference between treatment and controls was analyzed in a factorial ANOVA using Fisher's protected least significant difference test with a 5% significance level. Statistical analyses were performed with Statview software (Abacus Concepts; v4.5).
Results
UV-induced Systemic Immune Suppression Requires Fas/ FasL Interactions.
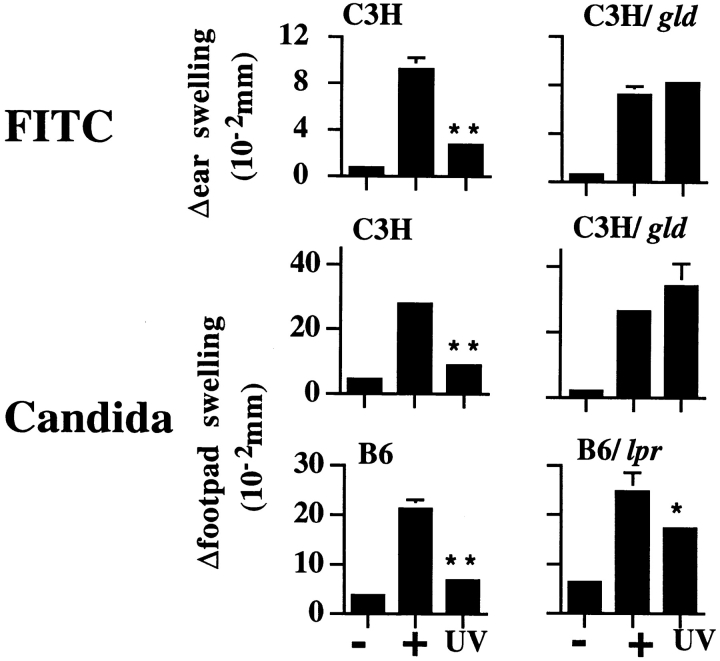
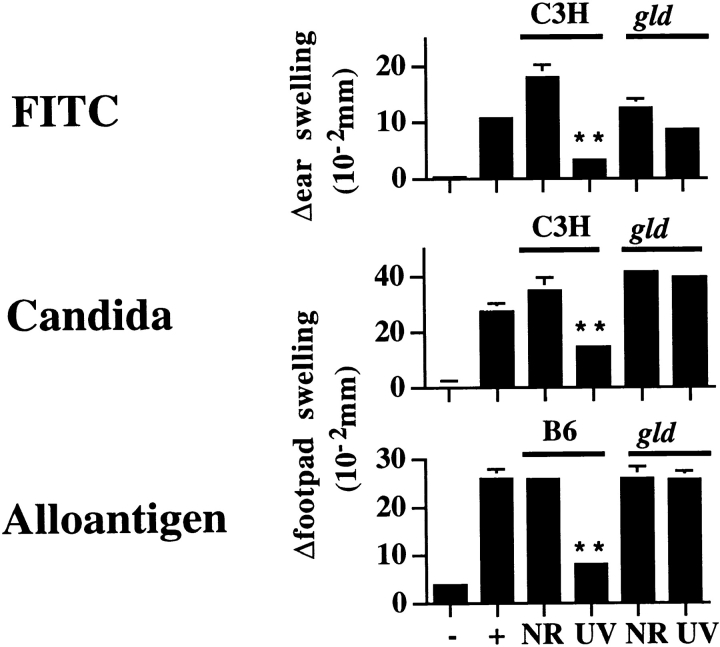
Normal mice exposed to a single dose of UVR before immunization at an NR site with formalin-fixed C. albicans or FITC exhibit a profound suppression of DTH or CHS response, respectively. To address the potential role of Fas/FasL interactions in UVR-induced systemic immune suppression, lpr or gld mice were evaluated for UV-induced immune suppression of CHS and DTH responses. The lpr mutation encodes an abnormal Fas gene containing an early retroviral transposon insertion that results in premature termination (44–47). Low levels of Fas expression (up to 50% of the level observed in wild-type mice) have been reported in lpr mice, demonstrating an incomplete Fas loss in these animals (46, 48). Mice harboring the gld mutation have a complete loss of biologically active FasL as a result of a point mutation in the FasL gene (49–51). The requirement for Fas/FasL interactions in UVR-induced immune suppression was first evaluated by comparing responses in wild-type C3H/HeJ (C3H) and FasL-deficient C3H/HeJ gld/gld (C3H/gld) mice. Both groups of animals were exposed to a single dose of UVR and immunized 3 d later at a distant NR site by epicutaneous application of FITC or subcutaneous injection of C. albicans (9). 6–10 d later, mice were challenged either on the pinnae or in the footpad with the sensitizing antigen to elicit CHS and DTH responses, respectively. Representative results from one such experiment are shown in Fig. 1. In wild-type C3H mice, UVR exposure potently suppressed both CHS and DTH responses compared with NR, positive control mice (76 and 82% suppression, respectively; P < 0.0001). In direct contrast, UVR-exposed C3H/gld mice exhibited no such immune suppression. Similar results were observed in C57Bl/6 (B6) and C57Bl/6 gld/gld (B6/gld) mice for DTH to C. albicans (data not shown). Thus, when FasL is nonfunctional, UVR-induced immune suppression is abrogated.
Figure 1.
UV-induced systemic suppression of antigen-specific responses to FITC and C. albicans. Mice were exposed to UVR, sensitized with antigen, and challenged, and Δswelling was determined 24 h later as described in Materials and Methods. Values shown represent mean ± SE for five mice per group using measurements from two footpads per mouse. −, negative (challenge only); +, positive (sensitized and challenged); and UV (UVR, sensitized, and challenged). **P < 0.0001 vs. positive control. *P < 0.05 vs. positive control.
To explore the effects of diminished Fas function on UVR-induced immune suppression, we next examined the DTH response of wild-type B6 and Fas-deficient C57Bl/6 lpr/lpr (B6/lpr) mice acutely exposed to a single dose of UVR and immunized with C. albicans. Results from a representative experiment are shown in Fig. 1. Unlike mice containing the gld mutation, DTH induction was significantly suppressed in both UVR-treated B6/lpr and wild-type B6 mice compared with NR control mice in each group. Notably, UV-induced suppression in lpr mice was only half that observed in wild-type B6 mice with intact Fas expression (40% suppression in lpr mice compared with 83% in wild-type mice). The observation that B6/lpr mice have intermediate sensitivity to UVR-induced immunosuppression is not likely the result of differences in genetic background between the two mouse strains, as B6/gld mice also exhibited a complete absence of UVR-induced immune suppression (data not shown). Instead, these findings likely reflect the leaky nature of the lpr mutation and, moreover, suggest that the induction of Fas after UV exposure may reestablish the capacity to induce systemic immune suppression. Indeed, Fas upregulation has been recently reported to occur in lpr mice exposed to gamma irradiation (52). Taken together, these results point toward a critical requirement for Fas/FasL interactions in primary, UVR-induced systemic suppression of CHS and DTH induction.
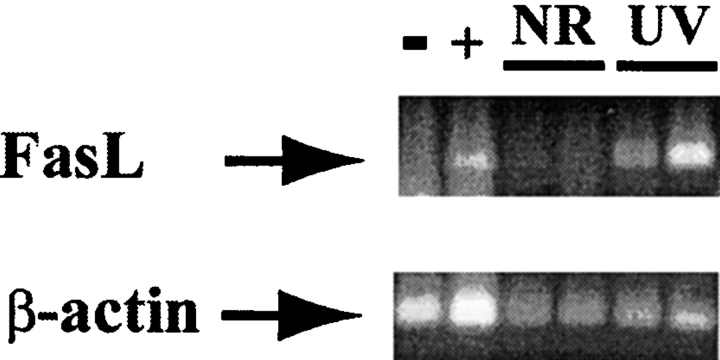
Earlier work in our laboratory demonstrated the presence of DNA damage (cyclobutane pyrimidine dimers) in skin-derived dendritic cells up to 1 wk after a single acute exposure of UVR (27). As DNA damage can induce FasL expression, a potential mechanism for the requirement for FasL in UV-mediated suppression might be the inappropriate expression of FasL in the draining lymph nodes after UVR. To explore this possibility, the skin-draining lymph nodes from wild-type mice were removed 3 d after UVR and FasL expression determined by RT-PCR. As shown in Fig. 2, FasL mRNA was markedly induced in the lymph nodes of mice that received 15 kJ/m2 UVR but was undetectable in untreated control animals. Specificity was demonstrated using L929 murine fibroblasts and unactivated lpr/ lpr splenocytes as negative and positive controls for FasL expression, respectively (53). FasL induction in the skin-draining lymph nodes after UVR points to an interrelationship with DNA damage and suggests a potential scenario in which inappropriate FasL expression eliminates antigen- responsive T cells (54) or serves to clonally expand a suppressor cell population (55). Experiments are currently underway to test these possibilities.
Figure 2.
FasL is induced in skin-draining lymph nodes after UVR. Skin-draining lymph nodes were harvested from mice that were NR and 3 d post-UVR. RNA was extracted and RT-PCR performed as described in Materials and Methods. −, negative control (L929 murine fibroblasts); +, positive control (lpr splenocytes); UV, UV-irradiated. Each lane represents a single mouse analyzed.
UV-induced Systemic Suppression of DTH to Alloantigen Does Not Require FasL.
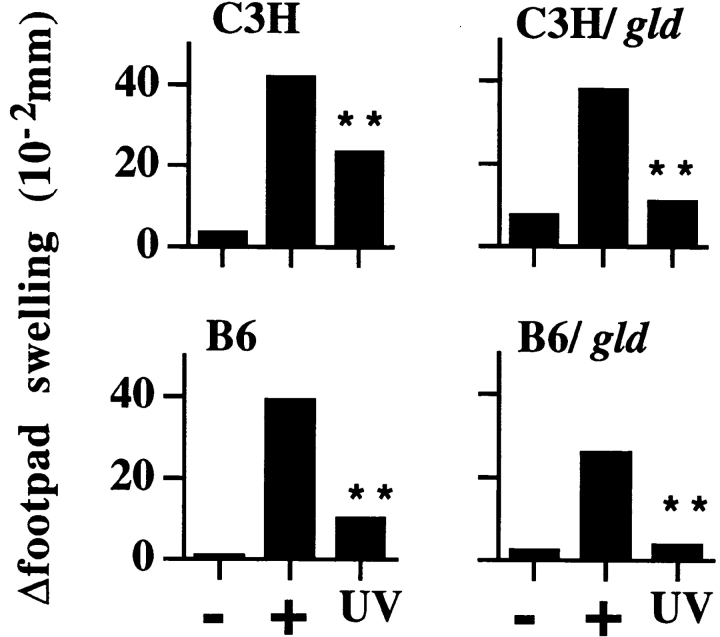
UVR-induced suppression of the DTH response to whole alloantigen differs from that of FITC or C. albicans in that it is mediated by photoisomerization of cis-urocanic acid and is UV-induced DNA damage–independent (Kripke, M.L., unpublished observations). Such findings suggest that UVR-induced suppression of the DTH response to alloantigen differs mechanistically from that of C. albicans. To evaluate the role of FasL in UVR-induced immune suppression of DTH responses to whole alloantigen, C3H, C3H/gld, B6, and B6/gld mice were exposed to a single dose of UVR and immunized with intact Balb/c spleen cells, and DTH responses were measured. In three independent experiments, UVR exposure potently suppressed DTH responses in both strains of FasL-deficient gld mice (Fig. 3) at levels comparable to those of matched, wild-type control mice. These results demonstrate that, in contrast to other antigen systems tested (FITC and C. albicans), primary UVR-induced suppression of DTH to whole alloantigen can proceed in a manner independent of host-derived FasL.
Figure 3.
Systemic suppression of alloantigen DTH responses in wild-type and FasL-deficient (gld) mice. Mice were exposed to UVR, sensitized with intact Balb/c spleen cells, and challenged, and Δswelling was determined 24 h later as described in Materials and Methods. Data are representative of two experiments for C3H mice and one for B6 mice. Values shown represent mean ± SE for five mice per group using measurements from two footpads per mouse. −, negative (challenge only); +, positive (sensitized and challenged); and UV (UVR, sensitized, and challenged). **P < 0.0001 vs. positive control.
Presentation of Alloantigen Determines the Requirement for Host-derived FasL in UVR-induced Immune Suppression.
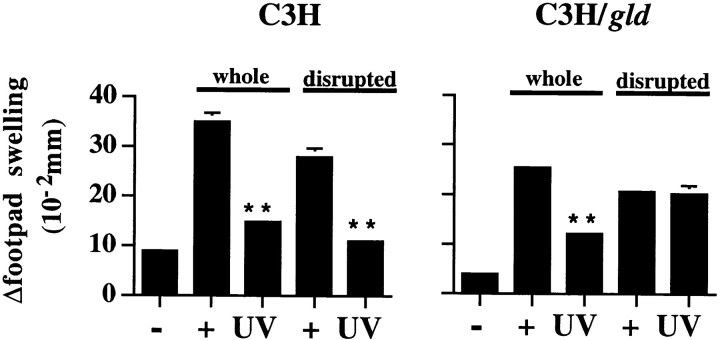
To resolve the differences in FITC, C. albicans, and alloantigen requirements for FasL in the mediation of UVR-induced immune suppression, a more detailed analysis of alloimmunization was undertaken. Whereas FITC and formalin-fixed C. albicans require processing and presentation by host APC, whole allogeneic splenocytes may bypass such a requirement through direct antigen presentation on the various cell populations in the sensitizing inoculum. To this end, we queried whether FasL was required for UVR- induced suppression when allogeneic splenocytes were disrupted before immunization. UVR-induced systemic suppression of responses to intact and disrupted allogeneic spleen cells was compared in C3H and C3H/gld mice by exposing mice to a single dose of UVR, immunizing 3 d later with either intact or disrupted allogeneic Balb/c spleen cells, and challenging 6–10 d later with intact spleen cells to elicit a DTH response. Disrupted allogeneic spleen cells were prepared by several freeze–thaw cycles and sonication to insure uniform dispersion. Results from one of two consistent experiments are shown in Fig. 4. DTH responses were suppressed in UVR-treated wild-type mice whether intact or disrupted alloantigen was used for sensitization (78 and 89% suppression, respectively, compared with positive control mice; P < 0.0001). In contrast to the potent suppression of DTH observed in UVR-treated C3H/gld mice immunized with intact allogeneic spleen cells (62% suppression; P < 0.0001), UVR-treated C3H/gld mice immunized with disrupted allogeneic spleen cells failed to demonstrate such suppression. The lack of primary, UV-induced immunosuppression in FasL-deficient mice immunized with disrupted alloantigen is in accordance with our findings using FITC and C. albicans (Fig. 1), suggesting that such suppression is dependent upon FasL and host-derived APC for antigen presentation.
Figure 4.
Effect of antigen presentation on FasL requirement for UV suppression. C3H and C3H/gld mice were exposed to UVR, sensitized with whole or disrupted Balb/c spleen cells, subsequently challenged with whole Balb/c spleen cells on day 10, and Δswelling was determined 24 h later as described in Materials and Methods. Values shown represent mean ± SE for five mice per group using measurements from two footpads per mouse. −, negative (challenge only); +, positive (sensitized and challenged); and UV (UVR, sensitized, and challenged). **P < 0.0001 vs. positive control.
Transfer of UV-induced Suppression Requires Donor-derived FasL.
Antigen-specific T suppressor cells capable of transferring antigen-specific suppression to a naive host exist in the spleens of mice exposed to UVR and sensitized to antigen (11–15). Although primary, UVR-mediated suppression was absent in FasL-deficient mice, it remained uncertain whether transferable suppressor cell function was also absent in such mice. To address this premise, we compared suppressor cell activity in adoptively transferred spleen cells from immunized, UVR-exposed wild-type and gld donors. These experiments were carried out by exposing C3H and C3H/gld mice to a single dose of UVR followed by immunization and challenge with FITC, C. albicans, or alloantigen. On the day that CHS and DTH responses were measured, spleens from UVR-exposed animals and NR control animals were harvested and transferred into naive recipient mice. Recipient mice were immediately sensitized with the indicated antigen and received antigenic challenge 6–10 d later. Mice receiving NR, antigen-primed spleen cells from either C3H or C3H/gld donors showed potent CHS and DTH responses to FITC and C. albicans, respectively. Animals that received spleen cells from UVR-exposed, antigen-primed C3H mice demonstrated a suppression of both CHS and DTH responses, confirming the presence of UV-induced splenic suppressor cells as expected (Fig. 5). In contrast, adoptively transferred UVR-exposed spleen cells from C3H/gld mice primed with either FITC or C. albicans failed to inhibit antigen sensitization in naive mice. Although these experiments suggest a loss of transferable suppressor cell activity in the absence of donor-derived FasL, they are not definitive, as such mice also lack primary UVR-mediated suppression, which may be essential for the subsequent generation of transferable suppressor cells.
Figure 5.
Adoptive transfer of UV-induced suppression by spleen cells requires FasL. 108 spleen cells from indicated donors were injected intravenously into naive hosts, sensitized, and challenged with FITC, C. albicans, and Balb/c spleen cells as described in Materials and Methods. Values shown represent mean ± SE for five mice per group using measurements from two footpads per mouse. −, negative (challenge only); +, positive (sensitized and challenged); NR, recipient of spleen cells from NR sensitized donors, sensitized, and challenged; and UV, recipient of spleen cells from UV-irradiated sensitized donors, sensitized, and challenged. **P < 0.0001 vs. positive control.
To this end, transferable suppression was also evaluated in C3H/gld mice immunized with allogeneic spleen cells in which primary, UVR-mediated immune suppression was intact. For these experiments, spleen cells from UVR-exposed, FasL-deficient C3H/gld (data not shown) or B6/gld mice immunized with allogeneic spleen cells were transferred to naive, matched wild-type recipients (Fig. 5). As expected, spleen cells from wild-type mice that received UVR before antigen priming showed potent suppressor cell activity when transferred into matched wild-type recipients (Fig. 4). Consistent with our previous experiments, spleen cells from FasL-deficient mice that received UVR before antigen priming showed no such transferable suppressor cell activity. That C3H/gld and B6/gld mice exhibited potent UVR-induced primary suppression but not splenic suppressor cell activity when immunized with allogeneic whole spleen cells (Fig. 3) suggests that these events are mechanistically dissimilar and that transferable suppression is strictly dependent upon FasL expression on the donor population. In further support of this premise, wild-type mice that received UVR before immunization with disrupted allogeneic spleen cells exhibited transferable suppressor cell activity comparable to that of C3H mice immunized with intact allogeneic spleen cells. Spleen cells from FasL-deficient mice that received UVR before immunization with disrupted alloantigen, however, again demonstrated no such transferable suppressor cell activity (data not shown). These results substantiate that donor-derived FasL expression is uniformly required for the generation and/or effector activity of UV-induced transferable suppressor cells for all antigen systems tested.
Transfer of UV-induced Suppression Does Not Require Recipient-derived FasL.
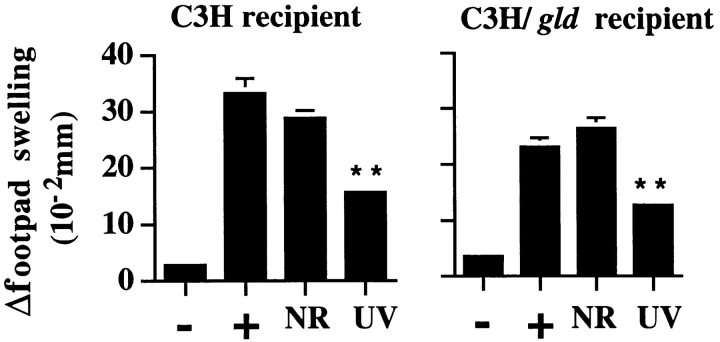
In the experimental models above, we have shown a stringent requirement for donor-derived FasL in UV-mediated transferable suppression. To discern whether FasL was also required in recipient mice, we evaluated UV-induced transferable suppression in FasL-deficient recipients. Such experiments were carried out by transferring spleen cells from wild-type mice that received UVR before immunization with C. albicans into either wild-type or FasL-deficient recipient mice (C3H or C3H/gld). Results from one such experiment are shown in Fig. 6. Consistent with our previous findings (Fig. 5), spleen cells from UVR- exposed, C. albicans–immunized C3H donor mice suppressed subsequent antigen responsiveness in naive C3H recipients (58% suppression relative to positive control mice; P < 0.0001). Similarly, UVR-exposed, C. albicans–immunized C3H donor spleen cells markedly suppressed subsequent antigen responsiveness in C3H/gld recipients (53% suppression relative to positive control mice; P < 0.0001), ruling out a requirement for recipient-derived FasL in transferable suppression induced by UVR. No suppression was observed when NR, C. albicans–immunized C3H splenocytes were transferred to either C3H or C3H/gld recipients, confirming a requirement for both UVR and antigen exposure in the generation of suppressor cell activity (16, 17).
Figure 6.
Suppression of DTH responses by adoptively transferred T suppressor cells does not require host-derived FasL. 108 spleen cells from indicated donors were injected intravenously into naive C3H or C3H/gld hosts, sensitized, and challenged with C. albicans as described in Materials and Methods. Values shown represent mean ± SE for five mice per group using measurements from two footpads per mouse. −, negative (challenge only); +, positive (sensitized and challenged); NR, recipient of spleen cells from NR-sensitized donors, sensitized, and challenged; and UV, recipient of spleen cells from UV-irradiated sensitized donors, sensitized, and challenged. **P < 0.0001 vs. positive control.
Discussion
Fas and FasL are complementary receptor–ligand proteins that induce apoptosis in many cell types (30). Fas is constitutively expressed in numerous tissues (56) and is rapidly upregulated in activated lymphocytes (31, 32). Although constitutive FasL expression is restricted to only a few tissues (57), transient FasL upregulation has been observed in a variety of cell types after genotoxic damage or cellular injury (3, 36, 58). Activated Fas+ lymphocytes can upregulate FasL upon T cell receptor engagement (39–41). Fas/FasL-induced apoptosis has been shown to play a critical role in the control of lymphocyte proliferation, peripheral tolerance, and specific immune responses occurring in discrete organ environments (35, 59). The effect of UVR on immune function shares several commonalities with Fas/ FasL-driven immunoregulation. UVR (6, 10), like FasL (35, 37), can mediate antigen-specific immune suppression. Moreover, UVR (19–23), like FasL (42), has been shown to shift the activation of T cells from a Th1- to a Th2-type immune response. Therefore, we were prompted to examine the role of Fas/FasL interactions in UVR-induced immune suppression.
Our observations are important in that they identify FasL as a fundamental constituent of both primary and transferable antigen-specific suppression induced by UVR (Table I). The central role of FasL in UV-induced systemic suppression of CHS and DTH responses varies from that of Th2-like immunomodulatory cytokines and cis-urocanic acid, which are not shared conjointly in the suppression of CHS and DTH responses (19–21; Kripke, M.L., unpublished observations). For example, IL-10 appears to be essential for systemic UVR-induced suppression of DTH responses (20), whereas TNF-α is essential for CHS suppression (19, 60). The selective requirement of FasL for UVR- mediated suppression of CHS responses to FITC and DTH responses to Candida, but not alloantigen, is reminiscent of our previous finding that repair of UV-induced DNA damage could restore immune responses to FITC and Candida but not alloantigen (25–27; Kripke, M.L., unpublished observations). Taken with the recent report that DNA damage can activate the FasL promoter and upregulate FasL expression (61), our findings raise the interesting possibility that UVR-induced DNA damage and FasL are interrelated in the induction of UVR-induced immune suppression. The potent induction of FasL mRNA in skin-draining lymph nodes after UVR lends additional credence to this premise (Fig. 2).
Table I.
Immunologic Effects of UV Exposure on FasL-deficient Mice
| Antigen | UV-induced immunosuppression | UV-induced suppressor cells | ||||||
|---|---|---|---|---|---|---|---|---|
| Wild-Type | gld/gld | Wild-Type | gld/gld | |||||
| FITC | + | − | + | − | ||||
| Candida | + | − | + | − | ||||
| Alloantigen (whole) | + | + | + | − | ||||
| Alloantigen (disrupted) | + | − | + | − | ||||
+ denotes significant suppression (P < 0.05) of antigen responses. − denotes an absence of suppression.
How might DNA damage, FasL, and immunomodulatory cytokines interrelate in the induction of UVR-induced immune suppression? First, DNA damage by a variety of agents, including UV light, has been shown to induce the expression of both Fas and FasL (3, 36, 61). Our laboratory has previously demonstrated that cyclobutane pyrimidine dimer–containing APC are present in the lymph nodes of UVR-exposed mice (27). Such APC may upregulate FasL and eliminate responding Fas+ T cells as has been recently reported for dendritic cells in vitro (42, 54). Second, aberrant FasL upregulation coupled with alterations in Fas sensitivity as a result of UVR-induced immunomodulatory cytokine production may contribute to the termination of immune responses by inappropriate apoptosis of T cells and/or APC (38, 40, 41). Third, suppressor T cells responding to antigen in the context of the UVR cytokine milieu may differentiate along a novel pathway requiring FasL as a growth factor (55, 62). Published observations, along with this report, collectively favor a model in which UV-induced changes in APC phenotype/function are pivotal in the induction of antigen-specific immune suppression. Our data involving intact and disrupted alloantigen point toward a requirement for FasL on UVR-exposed host APC. While such APC are required for the antigen presentation of FITC, C. albicans, and disrupted alloantigen, intact allogeneic spleen cells may circumvent this requirement by providing a source of FasL while acting as their own APC. These results suggest that the nature of the antigen (and thus the APC involved) critically determines the requirement for host-derived FasL in primary UVR-induced immune suppression.
What then might be the requirement for FasL in the generation and activity of transferable suppressor cells? Considering the low penetrance of UVR in the skin, it appears unlikely that direct UVR exposure and UV-induced DNA damage occurs on T cells. It is conceivable, however, that DNA-damaged APC may influence the development of such T cells. In this regard, we have previously shown that DNA-damaged APC cluster with suppressor T cells in the draining lymph nodes after UVR and antigen exposure (27, 43, 63). Taken with the observation that Fas ligation can induce proliferation in some T cells (62), it is possible that FasL on DNA-damaged APC may act as a growth factor for UV-induced T suppressor cells in the context of the UVR-treated animal. Interestingly, Groux et al. (64) have recently described an IL-10 driven, antigen-specific CD4+ T cell that can potently suppress antigen-specific immune responses in vivo. Such findings suggest that UVR-induced immunoregulatory Th2 cytokines such as IL-10 (18–20) may also participate in the differentiation and maintenance of the suppressor cell population. On the other hand, FasL may be required for effector activity of the UV-induced suppressor cells, perhaps by inducing apoptosis in the responding recipient T cell population. Experiments are currently in progress to test these possibilities.
Recent studies highlight the complexity of the immunomodulatory effects of UV in vivo. Hart et al. have documented mast cell–derived histamine as a component of the UV-induced systemic immunosuppression of DTH responses to alloantigen (65). In contrast to our studies on UV-induced systemic immune suppression, Schwartz et al. have shown a nonessential role for FasL in UV-induced local immune suppression (66). The local model of UV-induced immune suppression differs markedly from the systemic model in both the route of administration (antigen is administered through the UV-irradiated site), specific cytokine involvement, and the requirement for FasL. For example, UVR-induced suppression of local responses is TNF-α dependent and IL-10 independent and involves the production of cis-urocanic acid (67). In contrast, UVR-induced systemic immune suppression is independent of both TNF-α and cis-urocanic acid but dependent upon IL-10 production (19, 20; Kripke, M.L., unpublished observations). Collectively, these findings emphasize mechanistic differences between UVR-mediated local and systemic suppression and suggest the existence of at least two nonoverlapping pathways in the generation of systemic UVR-induced immune suppression. One pathway requires FasL on host-derived APC and is sensitive to reversal by the repair of UV-induced DNA damage (25–27; Kripke, M.L., unpublished observations); the other requires histamine (65) and is independent of host-derived FasL. Interestingly, both pathways require FasL for the generation of transferable suppression but may differ in their requirement for FasL in the host (66).
In summary, our experiments document that Fas/FasL interactions are essential for UVR-induced systemic suppression of CHS and DTH responses to antigens presented by host-derived APC (Table I). The requirement for Fas/ FasL in UVR-induced immune suppression can be eliminated if antigen presentation bypasses the requirement for host-derived APC (intact alloantigen). Moreover, host- derived, but not recipient-derived, FasL expression is critically required for the generation and/or function of UVR-induced suppressor cells. The crucial role of FasL in both systemic primary and transferable UV-induced immune suppression suggests that the dysregulation of Fas-mediated apoptosis may ultimately underlie both processes.
Acknowledgments
We would like to thank Lauri Eichler and Katherine Rath for expert technical assistance and Dr. Kathleen M. McAveney for critical scientific discussions.
This work was supported by a grant from the Skin Cancer Foundation Research Program, University of Texas M.D. Anderson Cancer Center (to L. Owen-Schaub), American Cancer Society grant CIM-88929 (to L. Owen-Schaub), National Institutes of Health (NIH) grant CA52457 (to M.L. Kripke), and institutional core grant CA16672 from the National Cancer Institute. L.L. Hill is the recipient of an NIH postdoctoral fellowship and a Cockrell Foundation University Cancer Fighters Scientific Achievement Fellowship.
Abbreviations used in this paper
- CHS
contact hypersensitivity
- DTH
delayed type hypersensitivity
- FasL
Fas ligand
- NR
nonirradiated
- RT
reverse transcriptase
- UVR
UV-irradiated
Footnotes
V.K. Shreedhar's current address is the Department of Medicine, Harvard Medical School, 300 Longwood Ave., Elders 1220, Boston, MA 02115.
References
- 1.Whittaker S. Sun and skin cancer. Br J Hosp Med. 1996;56:515–518. [PubMed] [Google Scholar]
- 2.Fisher MS, Kripke ML. Systemic alteration induced in mice by ultraviolet light irradiation and its relationship to ultraviolet carcinogenesis. Proc Natl Acad Sci USA. 1977;74:1688–1692. doi: 10.1073/pnas.74.4.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutierrez-Steil C, Wrone-Smith T, Sun X, Krueger JG, Coven T, Nickoloff B. Sunlight-induced basal cell carcinoma tumor cells and ultraviolet-B-irradiated psoriatic plaques express Fas ligand (CD95L) J Clin Investig. 1998;101:33–39. doi: 10.1172/JCI1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orlin JB, Ellis MH. Transfusion-associated graft-versus-host disease. Curr Opin Hematol. 1997;4:442–448. doi: 10.1097/00062752-199704060-00015. [DOI] [PubMed] [Google Scholar]
- 5.Rook AH, Gottlieb SL, Wolfe JT, Vowels BR, Sood SS, Niu Z, Lessin SR, Fox FE. Pathogenesis of cutaneous T cell lymphoma: implications for the use of recombinant cytokines and photopheresis. Clin Exp Immunol. 1997;107:16–20. [PubMed] [Google Scholar]
- 6.Kripke ML. Immunological unresponsiveness induced by ultraviolet radiation. Immunol Rev. 1984;80:87–102. doi: 10.1111/j.1600-065x.1984.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 7.Yoshikawa T, Streilein JW. Genetic basis of the effects of ultraviolet-B on cutaneous immunity. Evidence that polymorphism at the TNFα and Lps loci governs susceptibility. Immunogenetics. 1990;32:398–405. doi: 10.1007/BF00241633. [DOI] [PubMed] [Google Scholar]
- 8.Streilein JW, Bergstresser PR. Genetic basis of ultraviolet-B on contact hypersensitivity. Immunogenetics. 1988;27:252–258. doi: 10.1007/BF00376119. [DOI] [PubMed] [Google Scholar]
- 9.Denkins Y, Fidler IJ, Kripke ML. Exposure of mice to UV-B radiation suppresses delayed hypersensitivity to Candida albicans. . Photochem Photobiol. 1989;49:615–619. doi: 10.1111/j.1751-1097.1989.tb08432.x. [DOI] [PubMed] [Google Scholar]
- 10.Kripke ML. Ultraviolet radiation and immunology: something new under the sun—Presidential Address. Cancer Res. 1994;54:6102–6105. [PubMed] [Google Scholar]
- 11.Elmets CA, Bergstresser PR, Tigelaar RE, Wood PJ, Streilein JW. Analysis of the mechanism of unresponsiveness produced by haptens painted on the skin exposed to low dose ultraviolet radiation. J Exp Med. 1983;158:781–794. doi: 10.1084/jem.158.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noonan FP, DeFabo EC, Kripke ML. Suppression of contact hypersensitivity by UV radiation and its relation to UV-induced suppression of tumor immunity. Photochem Photobiol. 1981;34:683–689. [PubMed] [Google Scholar]
- 13.Fisher MS, Kripke ML. Suppressor T lymphocytes control the development of primary skin cancers in ultraviolet-irradiated mice. Science. 1982;216:1133–1134. doi: 10.1126/science.6210958. [DOI] [PubMed] [Google Scholar]
- 14.Ullrich SE, Kripke ML. Mechanisms in the suppression of tumor rejection produced in mice by repeated UV irradiation. J Immunol. 1984;133:2786–2790. [PubMed] [Google Scholar]
- 15.Ullrich SE, Magee MJ. Specific suppression of allograft rejection after treatment of recipient mice with UV radiation and allogeneic spleen cells. Transplantation. 1988;46:115–119. doi: 10.1097/00007890-198807000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Kripke ML, Morison WL, Parrish JA. Systemic suppression of contact hypersensitivity in mice by psoralen plus UVA (PUVA) J Invest Dermatol. 1983;81:87–92. doi: 10.1111/1523-1747.ep12542071. [DOI] [PubMed] [Google Scholar]
- 17.Ullrich SE. Suppression of lymphoproliferation by hapten-specific suppressor T lymphocytes from mice exposed to ultraviolet radiation. Immunology. 1985;54:343–348. [PMC free article] [PubMed] [Google Scholar]
- 18.Ullrich SE. The effect of ultraviolet radiation-induced suppressor cells on T-cell activity. Immunology. 1987;60:353–360. [PMC free article] [PubMed] [Google Scholar]
- 19.Rivas JM, Ullrich SE. The role of IL-4, IL-10, and TNF-α in the immune suppression induced by ultraviolet radiation. J Leuk Biol. 1994;56:769–775. doi: 10.1002/jlb.56.6.769. [DOI] [PubMed] [Google Scholar]
- 20.Rivas J, Ullrich SE. Systemic suppression of DTH by supernatants from UV-irradiated keratinocytes: an essential role for interleukin 10. J Immunol. 1992;149:3865–3871. [PubMed] [Google Scholar]
- 21.Araneo B, Dowell T, Moon HB, Daynes RA. Regulation of murine lymphocyte production in vivo. UV radiation depresses IL-2 and enhances IL-4 production by T cells through an IL-1 dependent mechanism. J Immunol. 1989;143:1737–1742. [PubMed] [Google Scholar]
- 22.Simon JC, Cruz PC, Bergstresser PR, Tigelaar RE. Low dose ultraviolet B-irradiated Langerhans cells preferentially activate CD4+cells of the T helper 2 subset. J Immunol. 1990;145:2087–2091. [PubMed] [Google Scholar]
- 23.Simon JC, Tigelaar RE, Bergstresser PR, Edelbaum D, Cruz PD. Ultraviolet B radiation converts Langerhans cells from immunogenic to tolerogenic antigen-presenting cells. J Immunol. 1991;146:485–491. [PubMed] [Google Scholar]
- 24.Schmitt DA, Owen-Schaub L, Ullrich SE. Effect of IL-12 on immune suppression and suppressor cell induction by ultraviolet radiation. J Immunol. 1995;154:5114–5120. [PubMed] [Google Scholar]
- 25.Kripke ML, Cox PA, Alas PG, Yarosh DB. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci USA. 1992;89:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kripke ML, Cox PA, Bucana CB, Vink AA, Alas L, Yarosh DB. Role of DNA damage in local suppression of contact hypersensitivity in mice by UV radiation. Exp Dermatol. 1996;5:173–180. doi: 10.1111/j.1600-0625.1996.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 27.Vink AA, Strickland FM, Bucana C, Cox PA, Roza L, Yarosh DB, Kripke ML. Localization of DNA damage and its role in altered antigen presenting cell function in ultraviolet-irradiated mice. J Exp Med. 1996;183:1491–1500. doi: 10.1084/jem.183.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishigori C, Yarosh D, O'Connor A, Shreedhar VK, Ullrich SE, Cox P, Kripke ML. HindIIIliposomes suppress delayed-type hypersensitivity responses in vivo and induce epidermal IL-10 in vitro. J Immunol. 1998;161:2684–2691. [PubMed] [Google Scholar]
- 29.O'Connor A, Nishigori C, Yarosh D, Alas L, Kibitel J, Burley L, Cox P, Bucanc C, Ullrich S, Kripke M. DNA double strand breaks in epidermal cells cause immune suppression in vivo and cytokine production in vitro. J Immunol. 1996;157:271–278. [PubMed] [Google Scholar]
- 30.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 31.Owen-Schaub LB, Yonehara S, Crump WL, Grimm EA. DNA fragmentation and cell death is selectively triggered in activated human lymphocytes by Fas antigen engagement. Cell Immunol. 1992;140:197–205. doi: 10.1016/0008-8749(92)90187-t. [DOI] [PubMed] [Google Scholar]
- 32.Miyawaki T, Uehara T, Nibu R, Tsuji T, Yachie A, Yonehara S, Taniguchi N. Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral blood. J Immunol. 1992;149:3753–3758. [PubMed] [Google Scholar]
- 33.Singer GG, Abbas AK. The Fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1:365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 34.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 35.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 36.Müller M, Strand S, Hug H, Heinemann E-M, Walczak H, Hoffmann WJ, Stremmel W, Krammer PH, Galle PR. Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53. J Clin Investig. 1997;99:403–413. doi: 10.1172/JCI119174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milik AM, Buechner-Maxwell VA, Sonstein J, Kim S, Seitzman GD, Beals TF, Curtis JL. Lung lymphocyte elimination by apoptosis in the murine response to intratracheal particulate antigen. J Clin Investig. 1997;99:1082–1091. doi: 10.1172/JCI119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ju ST, Panka DJ, Cul H, Ettinger R, El-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 39.Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, Green DR. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 41.Dhein J, Walczak H, Bäumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Brunner T, Carter L, Dutton RW, Rogers P, Bradley L, Sato T, Reed JC, Green D, Swain SL. Unequal death in T helper (Th)1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J Exp Med. 1997;185:1837–1849. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saijo S, Bucana CD, Rameriz KM, Cox PA, Kripke ML, Strickland FM. Deficient antigen presentation and Ts induction are separate effects of ultraviolet irradiation. Cell Immunol. 1995;164:189–202. doi: 10.1006/cimm.1995.1161. [DOI] [PubMed] [Google Scholar]
- 44.Wu J, Zhou T, He J, Mountz JD. Autoimmune disease in mice due to integration of an endogenous retrovirus in an apoptosis gene. J Exp Med. 1993;178:461–468. doi: 10.1084/jem.178.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 46.Chu J-L, Drappa J, Parnassa A, Elkon KB. The defect in Fas mRNA expression in MRL/lpr mice is associated with insertion of the retrotransposon, ETn. . J Exp Med. 1993;178:723–730. doi: 10.1084/jem.178.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adachi M, Watanabe-Fukunaga R, Nagata S. Aberrant transcription caused by the insertion of an early transposable element in an intron of the Fas antigen gene of lprmice. Proc Natl Acad Sci USA. 1993;90:1756–1760. doi: 10.1073/pnas.90.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mariani SM, Matiba B, Armandola EA, Krammer PH. The APO-1/Fas (CD95) receptor is expressed in homozygous MRL/lpr mice. Eur J Immunol. 1994;24:3119–3123. doi: 10.1002/eji.1830241231. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 50.Hahne M, Peitsch MC, Irmler M, Schröter M, Lowin B, Rousseau M, Bron C, Renno T, French L, Tschopp J. Characterization of the non-functional Fas ligand of gldmice. Int Immunol. 1995;7:1381–1386. doi: 10.1093/intimm/7.9.1381. [DOI] [PubMed] [Google Scholar]
- 51.Ramsdell F, Seaman MS, Miller RE, Tough TW, Alderson MR, Lynch DH. gld/gldmice are unable to express a functional ligand for Fas. Eur J Immunol. 1994;24:928–933. doi: 10.1002/eji.1830240422. [DOI] [PubMed] [Google Scholar]
- 52.Booker JK, Reap EA, Cohen PL. Expression and function of Fas on cells damaged by gamma irradiation in B6 and B6/lprmice. J Immunol. 1998;161:4536–4541. [PubMed] [Google Scholar]
- 53.Watanabe D, Suda T, Hashimoto H, Nagata S. Constitutive activation of the Fas ligand gene in mouse lymphoproliferative disorders. EMBO (Eur Mol Biol Organ) J. 1995;14:12–18. doi: 10.1002/j.1460-2075.1995.tb06970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Süss G, Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas ligand–induced apoptosis. J Exp Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki I, Fink PJ. Maximal proliferation of cytotoxic T lymphocytes requires reverse signaling through Fas ligand. J Exp Med. 1998;187:123–128. doi: 10.1084/jem.187.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leithäuser F, Dhein J, Mechtersheimer G, Koretz K, Brüderlein S, Henne C, Schmidt A, Debatin K-M, Krammer PH, Möller P. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest. 1993;69:415–429. [PubMed] [Google Scholar]
- 57.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 58.Reap EA, Roof K, Maynor K, Borrero M, Booker J, Cohen PL. Radiation and stress-induced apoptosis: a role for Fas/Fas ligand interactions. Proc Natl Acad Sci USA. 1997;94:5750–5755. doi: 10.1073/pnas.94.11.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griffith TS, Yu X, Herndon JM, Green DR, Ferguson TA. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1996;5:7–16. doi: 10.1016/s1074-7613(00)80305-2. [DOI] [PubMed] [Google Scholar]
- 60.Yoshikawa T, Streilein JW. Tumor necrosis factor-alpha and ultraviolet light have similar effects on contact hypersensitivity in mice. Reg Immunol. 1990;3:139–144. [PubMed] [Google Scholar]
- 61.Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green DR. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-κB and AP-1. Mol Cell. 1998;1:543–551. doi: 10.1016/s1097-2765(00)80054-4. [DOI] [PubMed] [Google Scholar]
- 62.Alderson MR, Armitage RJ, Marakovsky E, Tough TW, Roux E, Schooley K, Ramsdell F, Lynch DH. Fas transduces activation signals in normal human T lymphocytes. J Exp Med. 1993;178:2231–2235. doi: 10.1084/jem.178.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okamoto H, Kripke ML. Effector and suppressor circuits of the immune response are activated in vivoby different mechanisms. Proc Natl Acad Sci USA. 1987;84:3841–3845. doi: 10.1073/pnas.84.11.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 65.Hart PH, Grimbaldeston MA, Swift GJ, Jaksie A, Noonan FP, Finlay-Jones JJ. Dermal mast cells determine susceptibility to ultraviolet B–induced systemic suppression of contact hypersensitivity responses in mice. J Exp Med. 1998;187:2045–2053. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwartz A, Grabbe S, Grosse-Heitmeyer K, Roters B, Reimann H, Luger TA, Trinchieri G, Schwartz T. Ultraviolet light-induced immune tolerance is mediated via the Fas/Fas-ligand system. J Immunol. 1998;160:4262–4270. [PubMed] [Google Scholar]
- 67.Niizeki H, Streilein JW. Hapten-specific tolerance induced by acute, low-dose ultraviolet B radiation of skin is mediated via interleukin-10. J Invest Dermatol. 1997;109:25–30. doi: 10.1111/1523-1747.ep12276415. [DOI] [PubMed] [Google Scholar]