Abstract
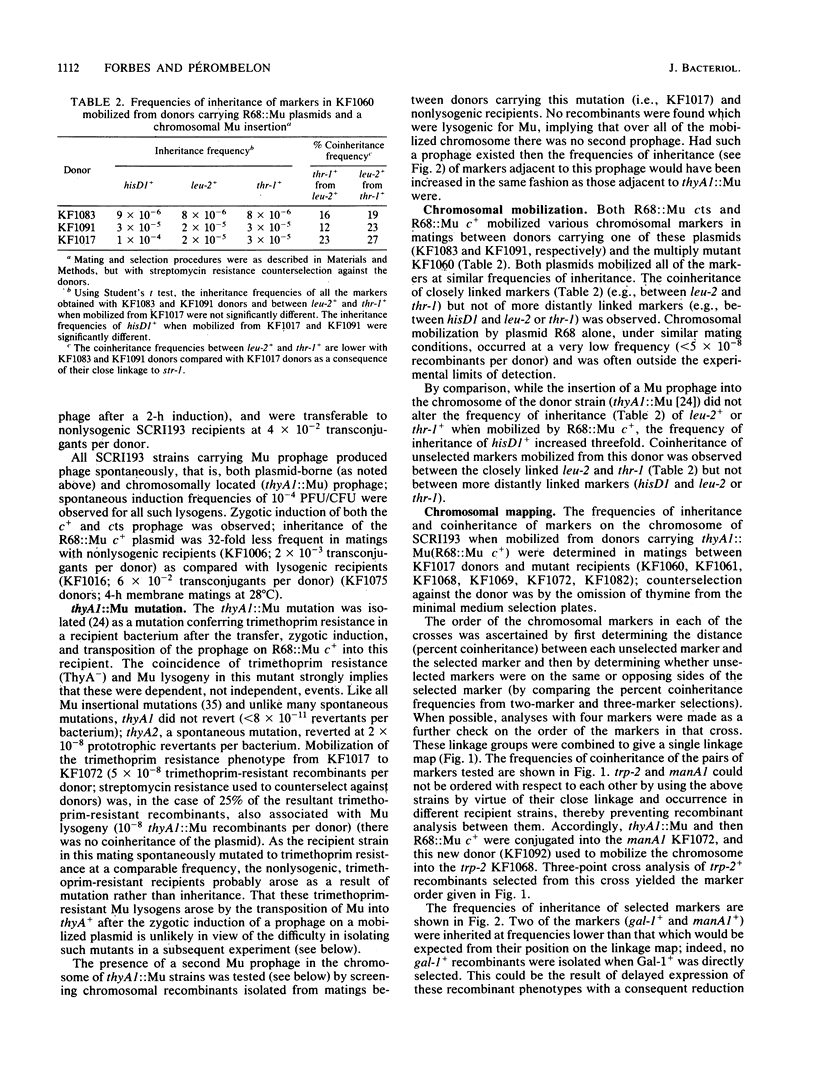
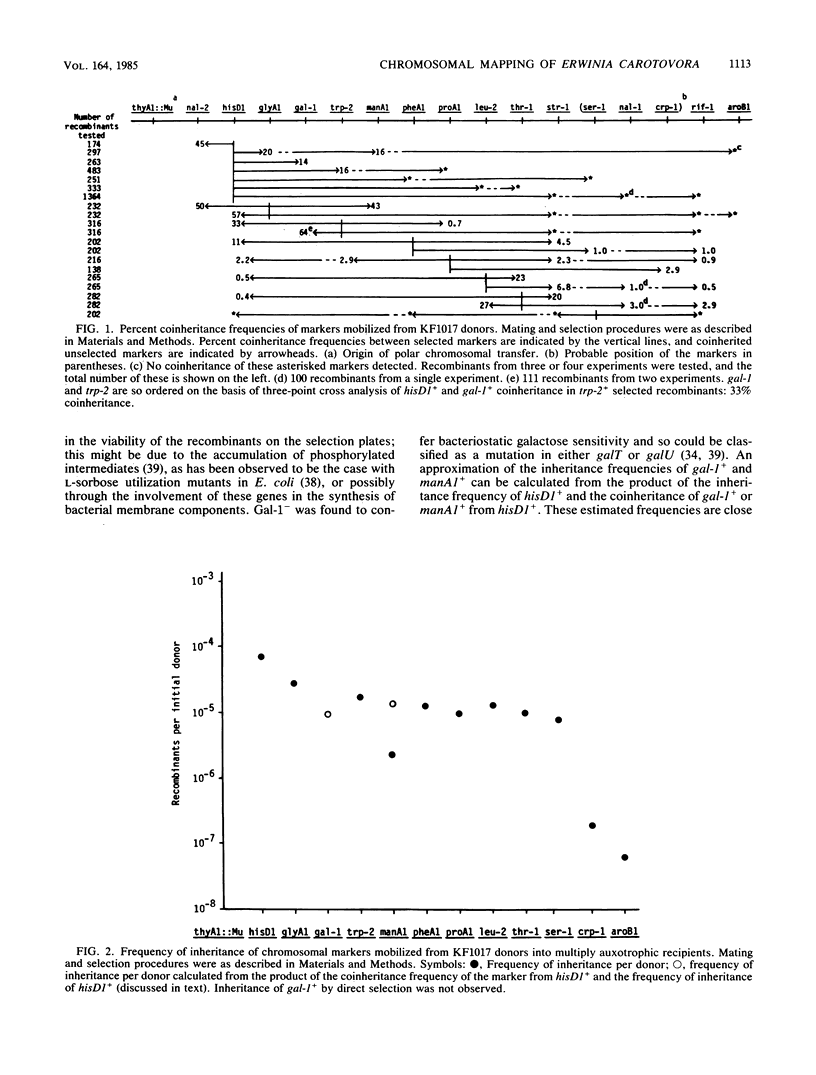
Conjugational gene transfer was established in Erwinia carotovora subsp. carotovora SCRI193 by using plasmid R68::Mu c+ to mobilize the chromosome into multiply mutant recipients. It was observed that although the plasmid alone mobilized markers randomly at a frequency of ca. 10(-5) chromosomal recombinants per donor, the presence of a Mu prophage on the chromosome of the donor increased the frequency of mobilization of markers adjacent to the prophage by up to 10-fold. Using this system it was possible to order 17 chromosomal mutations. The behavior of Mu in E. carotovora subsp. carotovora was also studied.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K. Acceptance by Erwinia spp. of R plasmid R68.45 and its ability to mobilize the chromosome of Erwinia chrysanthemi. J Bacteriol. 1980 Apr;142(1):111–119. doi: 10.1128/jb.142.1.111-119.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Starr M. P. Donor strains of the soft-rot bacterium Erwinia chrysanthemi and conjugational transfer of the pectolytic capacity. J Bacteriol. 1977 Dec;132(3):862–869. doi: 10.1128/jb.132.3.862-869.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Starr M. P. Genetics of Erwinia species. Annu Rev Microbiol. 1980;34:645–676. doi: 10.1146/annurev.mi.34.100180.003241. [DOI] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Faelen M., Toussaint A., De Lafonteyne J. Model for the enchancement of lambde-gal integration into partially induced Mu-1 lysogens. J Bacteriol. 1975 Mar;121(3):873–882. doi: 10.1128/jb.121.3.873-882.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. W., Harnish B. W. Transposition of a chromosomal segment bounded by redundant rRNA genes into other rRNA genes in Escherichia coli. J Bacteriol. 1982 Feb;149(2):449–457. doi: 10.1128/jb.149.2.449-457.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaswal R. K., Bressan R. A., Handa A. K. Mutagenesis of Erwinia carotovora subsp. carotovora with bacteriophage Mu d1 (Apr lac cts62): construction of his-lac gene fusions. J Bacteriol. 1984 May;158(2):764–766. doi: 10.1128/jb.158.2.764-766.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotoujansky A., Lemattre M., Boistard P. Utilization of a thermosensitive episome bearing transposon TN10 to isolate Hfr donor strains of Erwinia carotovora subsp. chrysanthemi. J Bacteriol. 1982 Apr;150(1):122–131. doi: 10.1128/jb.150.1.122-131.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murooka Y., Takizawa N., Harada T. Introduction of bacteriophage Mu into bacteria of various genera and intergeneric gene transfer by RP4::Mu. J Bacteriol. 1981 Jan;145(1):358–368. doi: 10.1128/jb.145.1.358-368.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. H., Shipley P. Host range and properties of the Pseudomonas aeruginosa R factor R1822. J Bacteriol. 1973 Feb;113(2):772–780. doi: 10.1128/jb.113.2.772-780.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugashetti B. K., Chatterjee A. K., Starr M. P. Isolation and characterization of Hfr strains of Erwinia amylovora. Can J Microbiol. 1978 Apr;24(4):448–454. doi: 10.1139/m78-074. [DOI] [PubMed] [Google Scholar]
- Riley M., Anilionis A. Evolution of the bacterial genome. Annu Rev Microbiol. 1978;32:519–560. doi: 10.1146/annurev.mi.32.100178.002511. [DOI] [PubMed] [Google Scholar]
- SUNDARARAJAN T. A., RAPIN A. M., KALCKAR H. M. Biochemical observations on E. coli mutants defective in uridine diphosphoglucose. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2187–2193. doi: 10.1073/pnas.48.12.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonejans E., Toussaint A. Utilization of plasmid pULB113 (RP4::mini-Mu) to construct a linkage map of Erwinia carotovora subsp. chrysanthemi. J Bacteriol. 1983 Jun;154(3):1489–1492. doi: 10.1128/jb.154.3.1489-1492.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher S. L., Bender R. A., Magasanik B. Genetic control of glutamine synthetase in Klebiella aerogenes. J Bacteriol. 1975 Jan;121(1):320–331. doi: 10.1128/jb.121.1.320-331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR A. L. BACTERIOPHAGE-INDUCED MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Dec;50:1043–1051. doi: 10.1073/pnas.50.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint A., Schoonejans E. Production and modification of Mu (G-) phage particles in E. coli K12 and Erwinia. Genet Res. 1983 Apr;41(2):145–154. doi: 10.1017/s0016672300021182. [DOI] [PubMed] [Google Scholar]
- Woodward M. J., Charles H. P. Genes for l-sorbose utilization in Escherichia coli. J Gen Microbiol. 1982 Sep;128(9):1969–1980. doi: 10.1099/00221287-128-9-1969. [DOI] [PubMed] [Google Scholar]
- Yarmolinsky M. B., Wiesmeyer H., Kalckar H. M., Jordan E. HEREDITARY DEFECTS IN GALACTOSE METABOLISM IN ESCHERICHIA COLI MUTANTS, II. GALACTOSE-INDUCED SENSITIVITY. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1786–1791. doi: 10.1073/pnas.45.12.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldis J. B., Bukhari A. I., Zipser D. Orientation of prophage Mu. Virology. 1973 Sep;55(1):289–294. doi: 10.1016/s0042-6822(73)81033-5. [DOI] [PubMed] [Google Scholar]
- Zink R. T., Kemble R. J., Chatterjee A. K. Transposon Tn5 mutagenesis in Erwinia carotovora subsp. carotovora and E. carotovora subsp. atroseptica. J Bacteriol. 1984 Mar;157(3):809–814. doi: 10.1128/jb.157.3.809-814.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Putte P., Gruijthuijsen M. Chromosome mobilization and integration of F-factors in the chromosome of RecA strains of E. coli under the influence of bacteriophage Mu-1. Mol Gen Genet. 1972;118(2):173–183. doi: 10.1007/BF00267086. [DOI] [PubMed] [Google Scholar]


