Abstract
Little is known about how hematopoietic stem cells (HSCs) self-renew. We studied the regeneration of HSCs in culture. Effects of various cytokines on cell division of CD34−/low c-Kit+Sca-1+ lineage marker–negative (CD34−KSL) bone marrow cells of the mouse were first evaluated in serum-free single cell culture. We then performed a competitive repopulation assay on divided cells to ask if such cell division involved self-renewal of HSCs.
In the presence of stem cell factor (SCF), thrombopoietin (TPO) induced a first cell division of CD34−KSL cells more efficiently than did interleukin (IL)-3 or IL-6. Multilineage repopulating cells were detected in a significant proportion of cells derived from single cells in culture with TPO and SCF, although this culture condition led to a substantial decrease in HSC number. These regenerated repopulating cells could be further transplanted into secondary recipients. When paired daughter cells were separately studied, one of a pair gave rise to repopulating cells with self-renewal potential, suggesting asymmetric self-renewal division. This study provides evidence that one HSC regenerates at least one HSC in culture.
Keywords: mouse, bone marrow transplantation, hematopoiesis, self-renewal, cell division
Introduction
Hematopoietic stem cells (HSCs) are defined by their ability both to self-renew and to differentiate into all blood cell lineages throughout the life of an organism 1. The differentiation potential of HSCs has been extensively characterized over decades. However, the self-renewal potential of HSCs remains poorly understood, mostly because of the lack of culture conditions in which HSCs can self-renew in vitro without differentiation. HSC assays rely on in vivo transplantation, which also hampers study of the self-renewal of HSCs. For example, the frequency of HSCs in C57BL/6 mice has been estimated to be from 1 to 10 per 105 bone marrow cells 2 3 4 5. As variations in HSC measurement may be unavoidable, it is difficult to evaluate a small increase in HSC number.
In vivo self-renewal of HSCs has been studied by marking HSCs with retroviral integration. It has been shown that restricted clones expand in anemic 6 or myeloablated hosts after transplantation 7 8. Quantitative studies have also explored HSC expansion after transplantation. It has been calculated that one competitive repopulating unit (CRU) can regenerate up to 15 CRU on average after transplantation into lethally irradiated mice 9. We have recently measured the numbers of HSCs that one HSC can produce after transplantation; our unpublished data support in vivo expansion of HSCs.
Attempts to amplify HSCs in vitro have been made under various culture conditions. Clonal expansion of HSCs takes place in Dexter-type culture, albeit with a net loss in the number of HSCs 10 11. Recently, a modest net gain of HSCs has been reported using serum-free culture containing stem cell factor (SCF), IL-11, and Flt-3 ligand 12. Labeling experiments with carboxyfluorescein diacetate succinimidyl ester (CFSE) suggested that dividing cells in culture contributed to this expansion of HSC numbers 13. Thrombopoietin (TPO) is the primary regulator of megakaryopoiesis 14. Apart from its lineage-restricted effect, TPO has also been shown to promote the survival of HSCs 15. TPO acts synergistically with SCF 16 17 18. The addition of TPO to Dexter culture has been reported to support expansion of HSCs 19. Collectively, these data suggest that HSCs also are able to self-renew in vitro under certain conditions.
The purpose of this study was to obtain definite proof of in vitro self-renewal of HSCs to investigate a manner of HSC division. We used CD34−/lowKit+Sca-1+ lineage marker–negative (CD34−KSL) bone marrow cells, in which HSCs are significantly enriched 20. The strategies were designed as follows: (a) in vitro induction of cell division of single CD34−KSL cells by certain cytokines, (b) detection of HSCs among cells in culture derived from single cells, and (c) detection of HSC activity in each cell of paired daughter cells. We first screened various cytokines for mitogenic stimulation to CD34−KSL cells. SCF was necessary for the in vitro survival of CD34−KSL cells, as previously suggested 21 22. As TPO, IL-3, or IL-6 promoted cell division of CD34−KSL cells in the presence of SCF, cells derived from a single cell in these culture conditions were subjected to transplantation experiments. Significant stem cell activity was detected among progeny of single cells cultured with SCF and TPO. This activity was considered to belong to one of the separated daughter cells. Our findings constitute evidence for in vitro self-renewal of HSCs.
Materials and Methods
Mice.
C57BL/6 (B6-Ly5.2) mice were purchased from Charles River Japan, Inc. Mice congenic for the Ly5 locus (B6-Ly5.1) were bred and maintained at the University of Tsukuba Animal Research Center. The Animal Experiment Committee of the University of Tsukuba approved animal care and use. B6-Ly5.1/Ly5.2 (B6-F1) mice were obtained from mating pairs of B6-Ly5.1 and B6-Ly5.2 mice.
Cells.
CD34−KSL cells were purified from bone marrow cells of 2-mo-old B6-Ly5.1 mice as previously described 20. In brief, low-density cells were isolated on Lymphoprep (Nycomed). The cells were stained with an antibody cocktail consisting of biotinylated anti–Gr-1 (RB6-8C5), Mac-1 (M1/70), B220 (RA3-6B2), CD4 (GK1.5), CD8 (53-6.7), and Ter-119 mAbs. Lineage-positive cells were depleted with streptavidin–magnetic beads (M-280; Dynal). The cells were further stained with PE-conjugated anti–Sca-1, allophycocyanin (APC)-conjugated anti–c-Kit (ACK-2), and FITC-conjugated anti-CD34 (49E8) antibodies. Biotinylated antibodies were developed with streptavidin–Texas Red (Life Technologies). All antibodies except for ACK-2 (a gift of Dr. S.I. Nishikawa, Kyoto University, Kyoto, Japan) were purchased from PharMingen. Four-color analysis and sorting were performed on a FACS Vantage™ (Becton Dickinson). Dead cells stained with propidium iodide were excluded from analysis and sorting. Automated deposition of single cells was carried out by Clone-Cyt (Becton Dickinson). After cell sorting, the presence of one cell per well was verified under an inverted microscope.
Single Cell Culture.
A round-bottomed 96-well plate (Corning Inc.) was used for culture. A well contained 200 μl of 5 × 10−5 M 2-β-mercaptoethanol and 2 mM l-glutamine in StemPro-34 SFM (Life Technologies). Cytokines supplemented were: 10 ng/ml mouse SCF, 100 ng/ml human TPO, 10 ng/ml mouse IL-3, 100 ng/ml human IL-6 (PeproTech), 100 ng/ml human IL-11 (PeproTech), and 10 ng/ml human G-CSF. SCF, TPO, IL-3, and G-CSF were provided by Kirin Brewery Co., Takasaki, Japan. The cells were incubated at 37°C in a humidified atmosphere with 5% CO2 in air. The number of cells per well was monitored until day 7 of culture. Final evaluation of cell division and colony formation was conducted at day 14 of culture.
Competitive Repopulation Assay.
Competitive repopulation 23 24 using the Ly5 system was performed as described 25. In brief, single cells or cells derived from single cells in culture (B6-Ly5.1) were mixed with 2 × 105 bone marrow competitor cells (B6-F1) and were transplanted into B6-Ly5.2 mice irradiated at a dose of 9.5 Gy. After transplantation, peripheral blood cells of the recipients were stained with biotinylated anti-Ly5.1 (A20) and FITC-conjugated anti-Ly5.2 (104). The cells were simultaneously stained with PE-conjugated anti-B220 antibody or a mixture of PE-conjugated anti–Mac-1 and –Gr-1 antibodies or a mixture of PE-conjugated anti-CD4 and -CD8 antibodies. Biotinylated antibody was developed with streptavidin–APC (PharMingen). The cells were analyzed on a FACS®. Percentage chimerism was calculated as (percent Ly5.1 cells) × 100/(percent Ly5.1 cells + percent F1 cells). When percent chimerism was >1.0 with myeloid and B and T lymphoid lineage reconstitution, recipient mice were considered to be multilineage reconstituted (positive mice).
Secondary Transplantation.
2 × 106 bone marrow cells of the primary recipient mice were transferred into lethally irradiated B6-Ly5.2 mice. Competition between test (Ly5.1) and competitor (F1) donor cells in secondary recipients (Ly5.2) was evaluated as in primary recipients.
Paired Daughter Cells.
When a single cell gave rise to two daughter cells in culture, daughter cells were separated into different wells by using micromanipulation technique as described 26 27. Individual paired daughter cells were further incubated under the same conditions as before. Cultured cells were subjected to a competitive repopulation assay.
Results
Induction of CD34−KSL Cell Division by Cytokines.
Direct effects of various cytokines on cell division of CD34−KSL cells were evaluated at the single-cell level in serum-free culture. Representative results for cell division and colony formation are graphically demonstrated in Fig. 1. SCF alone stimulated ∼25% of these cells to divide during 2 wk but did not further support them to form colonies consisting of >50 cells. Fewer than 10 or 5% of the cells underwent cell division on stimulation with TPO or IL-3 alone. No cell division was detected in culture with IL-6, IL-11, or G-CSF (data for IL-11 and G-CSF not shown).
Figure 1.
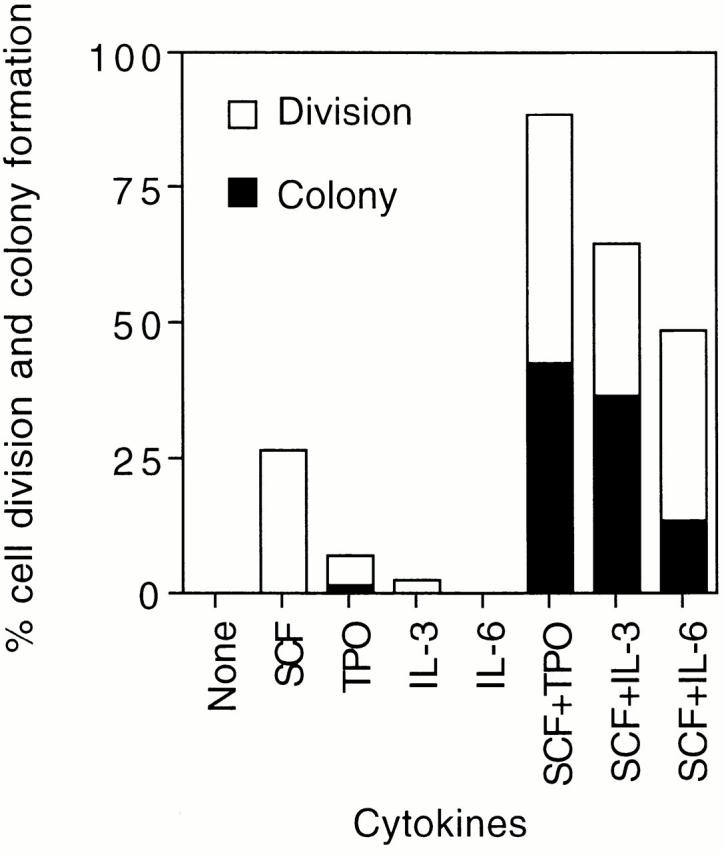
Cell division and colony formation of single CD34−KSL cells. A single-cell culture was performed in the presence of the indicated cytokines. The histogram shows the mean percentages of cells that underwent cell division at least once and of cells that gave rise to colonies during 2 wk of culture.
In combination with SCF, TPO induced cell division in nearly 90% of CD34−KSL cells. Half of them continuously divided and formed colonies. In response to SCF plus IL-3 or IL-6, ∼65 or 50% of these cells underwent cell division. Approximately half or one-fourth of the single cells that had undergone cell division at least once developed colonies in the presence of SCF plus IL-3 or IL-6.
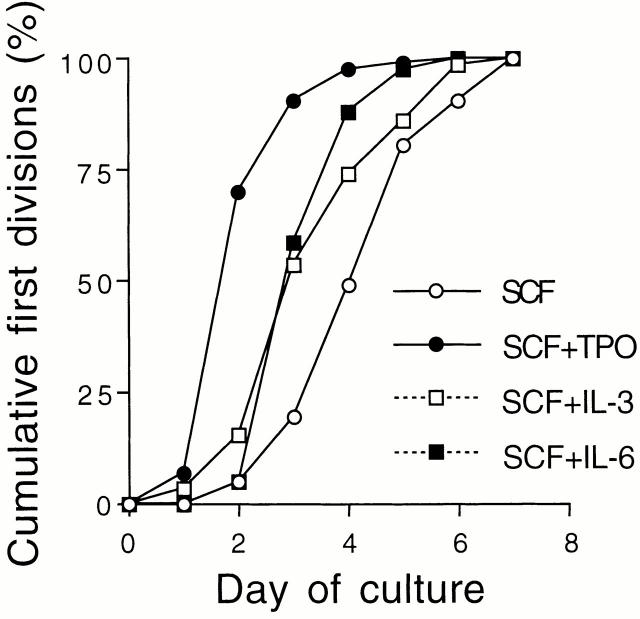
Fig. 2 shows cumulative percentages of first cell division within a week of culture. In the presence of SCF alone, CD34−KSL cells underwent a first cell division in a random fashion. In the presence of TPO and SCF, >50% of the cells underwent a first cell division between days 1 and 2, suggesting that these cytokines accelerate and presumably synchronize the entry of CD34−KSL cells into the cell cycle. The data support previous findings that this combination of cytokines shortens the time required for the first cell division of Hoechst 33342 low and rhodamine 123 low cells which have also been shown to represent an HSC population 17. IL-3 or IL-6 in combination with SCF showed a similar effect, but to a lesser extent, on induction of cell division. These data indicate that TPO with the support of SCF directly and efficiently acted on CD34−KSL cells as a mitogenic factor.
Figure 2.
Cumulative percentage of first cell division. Individual CD34−KSL cells were incubated in the presence of the indicated cytokines. A first day on which two or more cells were observed was recorded for each single cell. The cumulative distribution of first cell division among the cells that divided within a week is presented.
Regeneration of Repopulating Cells in Culture with Different Combinations of Cytokines.
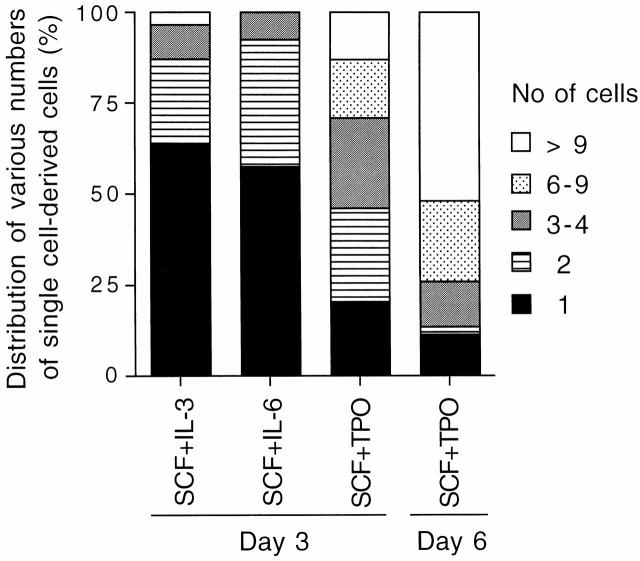
To clarify whether multilineage repopulating cells were present in culture after a single CD34−KSL cell gave rise to multiple cells, cultured cells were subjected to a competitive repopulation assay. As shown in Table , multilineage repopulating cells were detected in 41.2% of freshly isolated single CD34−KSL cells. When CD34−KSL cells were incubated in the presence of SCF plus IL-3 or IL-6 or TPO for 3 d, single cells gave rise to various numbers of cells (Fig. 3). Two cells at day 3 of culture were individually transplanted into lethally irradiated mice, together with competitor cells. Blood cells of the recipient mice were analyzed at 12 wk after transplantation. Multilineage reconstitution was observed in 10.5% of the mice transplanted with two cells generated in the presence of SCF and IL-3 (Table ). No repopulating cells were detected among 15 recipients of two cells generated in the presence of SCF and IL-6. In contrast, 30% of the mice transplanted with two cells cultured in the presence of SCF and TPO for 3 d showed multilineage reconstitution. Thus, in combination with SCF, TPO supported the generation of repopulating cells more efficiently than did IL-3 or IL-6.
Table 1.
Stem Cell Activity in Cells Derived from a Single CD34−KSL Cell
| Cytokine | Culture period | No. transplanted cells | No. positive mice (%) | Chimerism in positive individuals |
|---|---|---|---|---|
| d | % | |||
| none | 0 | 1 | 7/17 (41.2) | 1.3, 1.3, 3.0, 4.5, 8.5, 15.0, 23.7 |
| SCF + IL-3 | 3 | 2 | 2/19 (10.5) | 5.3, 19.4 |
| SCF + IL-6 | 3 | 2 | 0/15 (0.0) | 0.0 |
| SCF + TPO | 3 | 1 | 5/20 (25.0) | 1.2, 2.0, 5.9. 6.1, 7.6 |
| 3 | 2 | 3/10 (30.0) | 2.7, 2.7, 3.0 | |
| 6 | 2–7 | 7/47 (14.9) | 1.6, 3.0, 4.9, 6.5, 8.9, 16.7, 61.1 | |
| 6 | 9–16 | 2/29 (6.9) | 2.1, 5.0 |
CD34−KSL bone marrow cells were sorted at one cell per well. A single cell was mixed with 2 × 105 bone marrow competitor cells and transplanted into an irradiated mouse. A single cell was also incubated in the presence of the indicated cytokines. A cell underwent cell division in culture and gave rise to two or more cells. Clonal cells in culture were transplanted along with 2 × 105 competitor cells. Blood cells of the recipients were analyzed for the presence of cells derived from a single cell at 12 wk after transplantation. The number of mice reconstituted with single cells or their progeny (positive mice) is the numerator; the number of mice transplanted is the denominator.
Figure 3.
Frequency of the number of cells derived from single CD34−KSL cells. Single cells gave rise to varying numbers of cells by days 3 and 6 of culture. The histogram shows the distribution of the number of cells produced by single cells.
In culture with SCF and TPO, ∼20% of the cells remained without cell division for 3 d (Fig. 3). We transplanted these single cells in culture to examine their repopulating activity. As shown in Table , 25% of these cells showed reconstitution. Thus, repopulating cells still remained as single cells through 3 d of culture. However, their repopulating activity seemed comparable to that of the cells that had already undergone cell division by day 3 of culture.
Transplantation of Various Numbers of Cells in Culture with SCF and TPO.
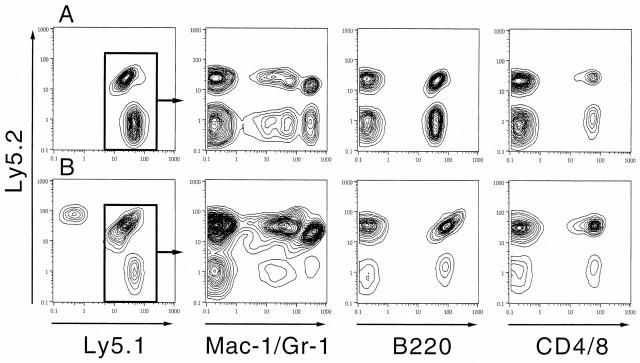
Single CD34−KSL cells gave rise to widely ranging numbers of cells in the presence of SCF and TPO when incubation time was extended through day 6 (Fig. 3). To study the relationship between the number of cells generated and the presence of repopulating cells among them, different numbers of cells in cultures were individually transplanted into irradiated mice. A group of mice transplanted with 2–7 cells was compared with a group of mice transplanted with 9–16 cells as shown in Table . Multilineage reconstitution occurred in ∼15% of the recipients of 2–7 cultured cells. Fig. 4 A shows data from FACS® analyses for one of the best reconstituted mice from this group. Myeloid and B and T lymphoid cells were reconstituted with cultured cells (Ly5.1) as well as with cells derived from competitor cells (F1). On the other hand, 7% of the recipients of 9–16 cells showed multilineage reconstitution. It should be noted that the percentage of positive mice decreased as incubation time increased. These data suggest that repopulating cells cease to persist as cells undergo more division with longer incubation time under the conditions used.
Figure 4.
Multilineage reconstitution with cultured cells. One of the best reconstituted cases is shown. An irradiated mouse was transplanted with six cells generated from one cell in culture with SCF and TPO by day 6 of incubation (see Table ). Blood cells of the recipients were analyzed for the presence of donor-derived cells 12 wk after transplantation (A). Secondary transplantation with bone marrow cells of this mouse resulted in multilineage reconstitution shown by analysis 16 wk after secondary transplantation (B). The cells derived from test and competitor donor cells (B6-Ly5.1 and F1 cells) were gated to display reconstitution in myeloid and B and T lymphoid lineages.
Secondary Transplantation of Bone Marrow Cells Reconstituted with Clonal Cultured Cells.
To evaluate the self-renewal potential of repopulating cells present in clonal cultured cells, a secondary transplantation was performed at 20 wk after primary transplantation. As shown in Table , mice 1, 2, 3, and 4 were reconstituted with 3, 4, 4, and 6 cells derived from a single cell cultured with SCF and TPO for 6 d. The number of bone marrow cells recovered per mouse was comparable to the number collected from a normal mouse. The percentages of chimerism in bone marrow cells were similar to those in peripheral blood cells. In all cases, 2 × 106 bone marrow cells from the reconstituted mice were transferred into each lethally irradiated mouse. Blood cells of the recipients were subjected to FACS® analysis another 20 wk after secondary transplantation.
Table 2.
Secondary Transplantation of Bone Marrow Cells Reconstituted with Clonal Cultured Cells
| Primary recipients | Secondary recipients | ||||||
|---|---|---|---|---|---|---|---|
| Percent chimerism | |||||||
| Mouse no. | No. cells injected | No. BM cells (% Ly5.1) | Positive mice | All WBCs | Myeloid | B lymphoid | T lymphoid |
| 1 | 3 | 3.6 × 107 (14.1) | 4/8 | 7.4 ± 4.2 (n = 4) | 5.2 ± 2.0 (n = 4) | 4.2 ± 2.4 (n = 4) | 7.9 ± 2.9 (n = 4) |
| 2 | 4 | 4.0 × 107 (6.3) | 0/10 | – | – | – | – |
| 3 | 4 | 3.0 × 107 (6.5) | 10/10 | 18.5 ± 11.7 (n = 10) | 27.3 ± 15.1 (n = 10) | 12.5 ± 8.9 (n = 10) | 14.9 ± 13.9 (n = 10) |
| 4 | 6 | 2.6 × 107 (63.0) | 9/9 | 16.7 ± 10.8 (n = 9) | 5.4 ± 2.6 (n = 9) | 13.4 ± 6.2 (n = 9) | 38.3 ± 22.0 (n = 9) |
At 20 wk after primary transplantation, bone marrow cells were collected from both tibiae and femora of mice reconstituted with cultured cells of single cell origin. 2 × 106 cells were transferred into each secondary recipient. At 20 wk after secondary transplantation, peripheral blood cells of the recipients were analyzed for percent chimerism of cells derived from a single cell. WBCs, white blood cells.
Table shows the result of analysis for secondary recipients. All secondary recipients of cells from mice 3 and 4 were multilineage reconstituted with culture-derived cells. Fig. 4 B demonstrates multilineage reconstitution in one of the secondary recipients of cells from mouse 4. Four out of eight recipients of cells from mouse 1 were reconstituted. None of the recipients of cell from mouse 2 showed reconstitution.
We concluded that self-renewing repopulating cells were present among the cells that had been generated from single cells cultured with SCF and TPO. Some repopulating cells among cultured cells were not capable of further repopulation in secondary recipients, suggesting heterogeneity of regenerated repopulating cells in terms of self-renewal potential.
Transplantation of Each Paired Daughter Cell.
We examined stem cell activity in paired daughter cells. To do this, when a single cell gave rise to two daughter cells by day 2 of culture with SCF and TPO, one of the daughter cells was transferred into a new well by micromanipulation. We selected pairs in which both cells subsequently gave rise to two cells by the following day. Two cells derived from each paired daughter cell at day 3 of culture were individually transplanted into an irradiated mouse. Thereby we wished to evaluate which daughter cell retained repopulating activity. As a control in this experiment, four cells generated directly from a single cell in culture by day 3 were transplanted.
The result of long-term reconstitution with paired daughter cells is shown in Table . When four unmanipulated cells were transplanted, multilineage long-term reconstitution was observed in 2 of 14 recipient mice (14.3%). On the other hand, when two cells derived from individual paired daughter cells were transplanted, 2 of 26 recipient mice showed a long-term reconstitution. Since these 2 positive mice received daughter cell–derived cells from different pairs, repopulating cells turned out to be detected in 2 of 13 pairs (15.4%) examined. These reconstituted mice, designated as mice 5 and 6, subsequently served as donors for secondary transplantation. As shown in Table , successful transplantation was observed for all recipients of bone marrow cells from mouse 5, but only 3 of 10 recipients of cells from mouse 6 showed multilineage reconstitution.
Table 3.
Transplantation of Paired Daughter Cells
| Cells transplanted | Positive cases | Percent chimerism |
|---|---|---|
| 4 | 2/14 mice | 2.1, 5.5 |
| 2 of 4 (paired daughters) | +,− pair: 2/13 +,+ pair: 0/13 | 8.6, 61.4 |
Four cells generated from a single cell by day 3 were transplanted. When a single cell gave rise to paired daughter cells at day 2 of culture, two cells were separated by micromanipulation. Two cells that were further generated from each paired daughter cell at day 3 were individually transplanted into an irradiated mouse. Peripheral blood cells were analyzed for the presence of test donor-derived cells at 20 wk after transplantation. The number of mice reconstituted is shown when 4 cells were transplanted, as is the number of positive pairs when 2 paired daughter-derived cells were transplanted. +,− pair, one of the two recipients was reconstituted. +,+ pair, both recipients were reconstituted.
Table 4.
Secondary Transplantation of Bone Marrow Cells Reconstituted with One of the Paired Daughter Cells
| Primary recipients | Secondary recipients | |||||
|---|---|---|---|---|---|---|
| Percent chimerism | ||||||
| Mouse no. | No. BM cells (% Ly5.1) | Positive mice | All WBCs | Myeloid | B lymphoid | T lymphoid |
| 5 | 3.5 × 107 (84.0) | 10/10 | 64.9 ± 21.1 (n = 10) | 36.4 ± 19.5(n = 10) | 60.2 ± 15.5 (n = 3) | 63.7 ± 21.2 (n = 10) |
| 6 | 4.8 × 107 (10.0) | 3/10 | 11.1 ± 6.2(n = 3) | 4.4 ± 1.6 (n = 3) | 9.4 ± 8.1 (n = 3) | 21.3 ± 8.5(n = 3) |
Two mice successfully reconstituted with daughter cell–derived cells (see Table ) served as donors for secondary transplantation. The indicated numbers of bone marrow (BM) cells were collected from these mice 20 wk after transplantation. Percent Ly5.1 shows the percentage of daughter cell–derived cells among bone marrow cells. In both cases, 2 × 106 bone marrow cells were transferred into each of 10 lethally irradiated mice. Peripheral blood cells of the recipients were analyzed 20 wk after secondary transplantation. WBCs, white blood cells.
Discussion
The experiments described here demonstrate in vitro self-renewal of HSCs. Both multilineage repopulating potential and self-renewing potential were detected in cells produced by a single CD34−KSL cell. It is evident that a single cell regenerated at least one HSC under the conditions used.
The combination of SCF and TPO was more effective for regeneration of HSCs than was the combination of SCF and IL-3 or SCF and IL-6. It has been shown that c-Mpl, the receptor for TPO, is expressed on HSCs 28. We have detected its expression in CD34−KSL cells at the messenger RNA level 29. TPO has been suggested to act directly on an HSC population 17. We conclude that the induction of self-renewal is a direct effect of TPO in support of SCF, as defined conditions were used for single-cell culture. Studies of c-kit mutant mice 30 and c-mpl knockout mice 31 have suggested that self-renewal ability of HSCs in these mice is reduced. Accordingly, SCF and TPO seem to play a principal role in HSC self-renewal.
We predicted that HSCs would retain their activity after they underwent cell division a few times in the presence of any cytokine combination. However, this was not the case. After single cells underwent cell division only once, repopulating potential appeared to be lost from some of these cells, and the maintenance of repopulating activity in divided cells was influenced by the cytokines used. Thus, depending on the culture condition used, HSCs could begin the differentiation process even at the first cell division. They tend to lose their self-renewal potential after repeated divisions under the in vitro conditions. Indeed, the number of multilineage repopulating cells substantially decreased as the numbers of their progeny in culture with SCF and TPO increased, although self-renewal cell division was certainly detected in these events (Table ). However, once these cells were restored to an in vivo environment, some of them proved to have maintained their potential, as shown in the transplantation experiments (Table and Table ).
The combination of SCF and IL-11 was less effective on cell division of CD34−KSL cells than was that of SCF and IL-6 (data not shown). However, because it has been described that SCF and IL-11 in combination with Flt-3 ligand expand HSCs by day 10 of culture 12 13, we are currently examining the effect of this cytokine combination on HSC self-renewal in our single-cell system.
Results of the paired daughter cell experiment confirmed the presence of one HSC in a pair. The incidence of reconstitution among mice transplanted with four cells derived from a single cell was similar to the incidence of positive pairs among the pairs consisting of two mice individually transplanted with two cells derived from paired daughter cells (14.3 and 15.4%). We interpret this result to mean that when the cells initially cultured were HSCs, a first cell division of these cells resulted in two daughter cells, of which one was an HSC and the other was committed to differentiation. It is suggested that self-renewal occurs by asymmetric cell division in the presence of SCF and TPO. To verify this possibility, the same experiment should be performed but with a large number of paired daughter cells. Otherwise, different culture conditions to efficiently induce symmetric self-renewal division should be identified.
We observed a variety of reconstitution levels among the recipients of freshly isolated single cells as well as of cells derived from single cells in culture, as presented in Table . This observation is likely to reflect the heterogeneity of HSCs and of regenerated HSCs in repopulating activity. The heterogeneity of HSCs may result from their asymmetric cell divisions during the development of hematopoiesis as has been suggested 32. It is possible that HSCs regenerate a variety of HSCs in self-renewal and differentiation potentials in vivo.
It has been suggested that slowly dividing cells have higher proliferative potential than do rapidly dividing cells in the case of human candidate HSCs 32. It is possible that rapidly dividing cells with less repopulating activity were selected for study of paired daughter cell transplantation. However, when two cells and single cells at day 3 of culture were examined by competitive repopulation, the frequencies of the presence of repopulating cells and their degrees of repopulation were comparable between the two groups (Table ). The mouse transplanted with daughter cell–derived cells showed one of the highest repopulation levels (61.4% total chimerism). Furthermore, when single cells that remained without cell division through day 6 of culture were transplanted, none of the six recipients showed repopulating activity (data not shown). More such cases should be examined for HSC activity. However, single cells at day 6 of culture constituted only a minor population (10%) of cultured cells. Therefore, we believe that a majority of repopulating cells in CD34−KSL cells underwent cell division by day 6 of culture under the conditions used. It is suggested that cell division of HSCs may be controllable to a certain extent by exogenous factors.
The single cell culture and transplantation system described in this paper may serve as a sensitive and powerful assay to investigate in vitro HSC self-renewal. It is possible to rigorously examine the effects of cytokines, as demonstrated herein, but also those of any sort of protein.
Acknowledgments
The authors thank Y. Morita for his excellent assistance in FACS® operation and A.S. Knisely for careful review of the manuscript.
This work was supported by grants from Core Research for Evolutional Science and Technology of Japan Science and Technology Corporation and the Ministry of Education, Science, Sports and Culture of Japan.
Footnotes
Abbreviations used in this paper: HSCs, hematopoietic stem cells; SCF, stem cell factor; TPO, thrombopoietin.
References
- Till J.E., McCulloch E.A., Siminovitch L. A stochastic model of stem cell proliferation, based on the growth of spleen colony-forming cells. Proc. Natl. Acad. Sci. USA. 1964;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D.E., Astle C.M., Stone M. Numbers and function of transplantable primitive immunohematopoietic stem cells. J. Immunol. 1989;142:3833–3840. [PubMed] [Google Scholar]
- Szilvassy S.J., Humphries R.K., Lansdorp P.M., Eaves A.C., Eaves C.J. Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proc. Natl. Acad. Sci. USA. 1990;87:8736–8740. doi: 10.1073/pnas.87.22.8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebel V.I., Miller C.L., Thornbury G.R., Dragowska W.H., Eaves C.J., Lansdorp P.M. A comparison of long-term repopulating hematopoietic stem cells in fetal liver and adult bone marrow from the mouse. Exp. Hematol. 1996;24:638–648. [PubMed] [Google Scholar]
- Zhong R.-K., Astle C.M., Harrison D.E. Distinct developmental patterns of short-term and long-term functioning lymphoid and myeloid precursors defined by competitive limiting dilution analysis in vivo. J. Immunol. 1996;157:138–145. [PubMed] [Google Scholar]
- Dick J.E., Magli M.C., Huszar D., Phillips R.A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hematopoietic system of WWv mice. Cell. 1985;42:71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Lemischka I.R., Raulet D.H., Mulligan R.C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986;45:917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Keller G., Snodgrass R. Life span of multipotential hematopoietic stem cells in vivo. J. Exp. Med. 1990;171:1407–1418. doi: 10.1084/jem.171.5.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawliuk R., Eaves C., Humphries R.K. Evidence of both ontogeny and transplant dose-regulated expansion of hematopoietic stem cells in vivo. Blood. 1996;88:2852–2858. [PubMed] [Google Scholar]
- Fraser C.C., Eaves C.J., Szilvassy S.J., Humphries R.K. Expansion in vitro retrovirally marked totipotent hematopoietic stem cells. Blood. 1990;76:1071–1076. [PubMed] [Google Scholar]
- Fraser C.C., Szilvassy S.J., Eaves C.J., Humphries R.K. Proliferation of totipotent hematopoietic stem cells in vitro with retention of long-term competitive reconstituting ability. Proc. Natl. Acad. Sci. USA. 1992;89:1968–1972. doi: 10.1073/pnas.89.5.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C.L., Eaves C. Expansion in vivo of adult murine hematopoietic stem cells with transplantable lympho-myeloid reconstituting ability. Proc. Natl. Acad. Sci. USA. 1997;94:13648–13653. doi: 10.1073/pnas.94.25.13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostendorp R.A.J., Audet J., Eaves C.J. High-resolution tracking of cell division suggests similar cell cycle kinetics of hematopoietic stem cells stimulated in vitro and in vivo. Blood. 2000;95:855–862. [PubMed] [Google Scholar]
- Kaushansky K. Thrombopoietinthe primary regulator of platelet production. Blood. 1995;86:419–431. [PubMed] [Google Scholar]
- Matsunaga T., Kato T., Miyazaki H., Ogawa M. Thrombopoietin promotes the survival of murine hematopoietic long-term reconstituting cellscomparison with the effects of Flt3/Flk-2 ligand and interleukin-6. Blood. 1998;92:452–461. [PubMed] [Google Scholar]
- Ku H., Yonemura Y., Kaushansky K., Ogawa M. Thrombopoietin, the ligand for the Mpl receptor, synergizes with steel factor and other early acting cytokines in supporting proliferation of primitive hematopoietic progenitors of mice. Blood. 1996;87:4544–4551. [PubMed] [Google Scholar]
- Sitnicka E., Lin N., Priestley G.V., Fox N., Broudy V.C., Wolf N.S., Kaushansky K. The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells. Blood. 1996;87:4998–5005. [PubMed] [Google Scholar]
- Ramsfjell V., Borge O.J., Veiby O.P., Cardier J., Murphy M.J., Jr., Lyman S.D., Lok S., Jacobsen S.E.W. Thrombopoietin, but not erythropoietin, directly stimulates multilineage growth of primitive murine bone marrow progenitor cells in synergy with early acting cytokinesdistinct interactions with the ligands for c-kit and FLT3. Blood. 1996;88:4481–4492. [PubMed] [Google Scholar]
- Yagi M., Ritchie K.A., Sitnicka E., Storey C., Roth G.J., Bartelmez S. Sustained ex vivo expansion of hematopoietic stem cells mediated by thrombopoietin. Proc. Natl. Acad. Sci. USA. 1999;96:8126–8131. doi: 10.1073/pnas.96.14.8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M., Harada K.-I., Hamada H., Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- Li C.L., Johnson G.R. Stem cell factor enhances the survival but not the self-renewal of murine hematopoietic long-term repopulating cells. Blood. 1994;84:408–414. [PubMed] [Google Scholar]
- Keller J.R., Ortiz M., Ruscetti F.W. Steel factor (c-kit ligand) promotes the survival of hematopoietic stem/progenitor cells in the absence of cell division. Blood. 1995;86:1757–1764. [PubMed] [Google Scholar]
- Ogden D.A., Micklem H.S. The fate of serially transplanted bone marrow cell populations from young and old donors. Transplantation. 1976;22:287–293. doi: 10.1097/00007890-197609000-00010. [DOI] [PubMed] [Google Scholar]
- Harrison D.E., Astle C.M., Delaittre J.A. Loss of proliferative capacity in immunohemopoietic stem cells caused by serial transplantation rather than aging. J. Exp. Med. 1978;147:1526–1531. doi: 10.1084/jem.147.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema H., Nakauchi H. Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood. 2000;95:2284–2288. [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Disparate differentiation in mouse hemopoietic colonies derived from paired progenitors. Proc. Natl. Acad. Sci. USA. 1984;81:2520–2524. doi: 10.1073/pnas.81.8.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda J., Suda T., Ogawa M. Analysis of differentiation of mouse hemopoietic stem cells in culture by sequential replating of paired progenitors. Blood. 1984;64:393–399. [PubMed] [Google Scholar]
- Solar G.P., Kerr W.G., Zeigler F.C., Hess D., Donahue C., Sauvage F.J., Eaton D.L. Role of c-mpl in early hematopoiesis. Blood. 1998;92:4–10. [PubMed] [Google Scholar]
- Nakauchi H., Takano H., Ema H., Osawa M. Further characterization of CD34-low/negative mouse hematopoietic stem cells. Ann. NY Acad. Sci. 1999;872:57–66. doi: 10.1111/j.1749-6632.1999.tb08453.x. [DOI] [PubMed] [Google Scholar]
- Miller C.L., Rebel V.I., Helgason C.D., Lansdorp P.M., Eaves C.J. Impaired steel factor responsiveness differentially affects the detection and long-term maintenance of fetal liver hematopoietic stem cells in vivo. Blood. 1997;89:1214–1223. [PubMed] [Google Scholar]
- Kimura S., Roberts A.W., Metcalf D., Alexander W.S. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc. Natl. Acad. Sci. USA. 1998;95:1195–1200. doi: 10.1073/pnas.95.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummendorf T.H., Dragowska W., Zijlmans J.M., Thornbury G., Lansdorp P.M. Asymmetric cell divisions sustain long-term hematopoiesis from single-sorted human fetal liver cells. J. Exp. Med. 1998;188:1117–1124. doi: 10.1084/jem.188.6.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]