Abstract
Previous studies in mice and humans have suggested an important role for CD8+ T cells in host defense to Mtb. Recently, we have described human, Mtb-specific CD8+ cells that are neither HLA-A, B, or C nor group 1 CD1 restricted, and have found that these cells comprise the dominant CD8+ T cell response in latently infected individuals. In this report, three independent methods are used to demonstrate the ability of these cells to recognize Mtb-derived antigen in the context of the monomorphic HLA-E molecule. This is the first demonstration of the ability of HLA-E to present pathogen-derived antigen. Further definition of the HLA-E specific response may aid development of an effective vaccine against tuberculosis.
Keywords: CD8-positive T lymphocytes, HLA-E, human, Mycobacterium tuberculosis
Introduction
CD8+ T Cells in the Host Response to Tuberculosis.
Mycobacterium tuberculosis (Mtb)*, the etiological agent of tuberculosis (TB), remains a leading cause of infectious disease morbidity and mortality worldwide, with WHO (1) estimates of 8.4 million TB cases and two million TB-related deaths in 1999. In spite of these sobering statistics, the host cellular immune response successfully contains 90% of Mtb infections. Without doubt, HLA-II–mediated recognition of Mtb-derived antigens plays an essential role in the host response, due at least in part to the release of proinflammatory cytokines such as IFN-γ and TNF-α, and the subsequent activation of macrophages (2, 3).
While CD4+ T lymphocytes are essential for the containment of mycobacterial infection, there is increasing evidence to suggest that they are not sufficient. First, mice deficient in β2M, and hence MHC class I–dependent immunity, are impaired in their ability to control infection (4, 5). These observations are corroborated by data obtained in mice deficient in transporter associated with antigen processing (TAP), and hence MHC class I antigen processing (6). Second, activation of human macrophages by CD4-derived cytokines such as IFN-γ and TNF-α results in only modest containment of Mtb growth in vitro, due in part to a relative deficiency of inducible nitrous oxide. Third, granulysin, a constituent of the CTL granule has a direct mycobacteriostatic effect (7).
To explore the role of human CD8+ T cells in the host response to TB, we have used Mtb-infected dendritic cells (DCs) to derive both classically and nonclassically HLA-restricted CD8+ T cell clones from a latently infected individual (8, 9). Using a modified LDA analysis with Mtb-infected DCs as the APC, these nonclassically restricted clones were found to comprise the majority of Mtb-specific CD8+ T cells in two latently infected subjects (9).
Nonclassically Restricted CD8+ T Cells in the Host Response to TB.
Classical or HLA-Ia restricted CTL are defined as those restricted by the polymorphic MHC molecules HLA-A, B, and C. HLA-Ia–restricted T cells are capable of recognizing peptide antigens processed and presented from Mtb-infected APCs (2). Additionally, the effector cell frequencies to some Mtb proteins such as Ag85, 19 kD, ESAT-6, and CFP10/Mtb11 would suggest that these responses represent a robust recall response to mycobacterial infection (10–13).
Nonclassical or HLA-Ib–restricted CTL are defined as those restricted by monomorphic molecules with sequence homology to the classical HLA-Ia molecules. By presenting molecules uniquely derived from a bacterial source, these molecules may bridge traditional definitions of innate and acquired immunity. Examples include (i) the human group 1 CD1 (CD1a, b, and c) molecules, that process and present mycobacterially derived lipid and glycolipid antigens by virtue of an unusually deep-binding pocket (14), (ii) the murine H2M3 molecule which presents short, bacterially derived peptides possessing an NH2 terminal, formyl-methionine (15, 16), and (iii) the murine Qa1 molecule that can present GroEL-derived peptides from Salmonella (17, 18). The importance of nonclassically restricted T cells in the host response to infection with Mtb remains poorly defined, although it is intriguing that mice deficient in MHC class Ia molecules (H2-Kb/H2-Db double knockout mice) were better able to control infection than those deficient in β2M (MHC-Ia and MHC-Ib deficient, reference 19). Our data showing that nonclassically restricted T cells comprised the majority of Mtb-specific CD8+ T cells in two latently infected subjects suggested that this subset of T cells plays a significant role in the human host response to infection with Mtb. Two nonclassically restricted clones have been extensively characterized. These clones (23, 29) responded to APCs infected with the closely related Mtb and Mycobacterium bovis species, but not to atypical mycobacteria such as Mycobacterium avium. While phenotypically similar to other antigen-specific CD8+ T cell clones (CD8+, αβ TCR+, negative for NK cell markers CD16 and CD56), these clones were neither HLA-A, B, or C nor CD1 restricted. Studies with anti–HLA-I– and –HLA-II–blocking antibodies showed partial blockade with the pan–HLA-I antibody W6/32, but not with anti–HLA-II or anti–HLA-A antibodies. Mtb-derived antigen presentation was found to require proteasomal processing, but to be presented in a manner that was Brefeldin and hence TAP independent (8). In this work, we define HLA-E as the restricting allele for the nonclassically restricted clones.
Materials and Methods
Human Subjects.
Subjects were recruited from employees at Harborview Medical Center, Seattle, WA and Oregon Health Sciences University, Portland, OR. Protocols for venipuncture and apheresis have been IRB approved. The T cell clones studied herein were derived from a single latently infected individual (D160, documented TST conversion, no evidence of active disease, HLA-A2, A3, B14, B44).
Media and Reagents.
Culture medium consisted of RPMI 1640 supplemented with 10% Serva (Bio Whittaker), 50 μg/ml gentamicin sulfate (Bio Whittaker), 5 × 10−5 M 2 ME (Sigma-Aldrich), and 2 mM glutamine (GIBCO BRL). For the growth and assay of Mtb-reactive T cell clones, RPMI 1640 was supplemented with 10% human serum. Mtb strain H37Rv was obtained from American Type Culture Collection and grown in modified Middlebrook 7H9 media (Difco). After the preparation of glycerol stocks, aliquots were frozen, and subsequently titered on Middlebrook 7H10 plates (Becton Dickinson). H37Rv mannose lipoarabinomannan, Triton X-114 lipoprotein/membrane protein fraction (TX-114), culture filtrate proteins (CFPs), cell wall fraction, cell membrane fraction, cytosol fraction, mycolylarabinogalactan peptidoglycan, or Mtb genomic DNA were provided through NIAID contract N01 AI-75320 and Colorado State University, Fort Collins, CO.
Cell Lines and T Cell Clones.
EBV-transformed B cell lines were generated in our laboratory using supernatants from the cell line 9B5–8 (American Type Culture Collection). In the experiments presented herein, LCL were from HLA-A2 positive donors (D160 & KA). The HLA-I–deficient LCL line 721.221 as well as transfectants expressing HLA-E (721.221 E), HLA-E that is expressed with the HLA-A2 leader peptide required for cell surface expression (721.221 AEH), and HLA-F (721.221 F) were provided by D. Geraghty, Fred Hutchinson Cancer Research Center, Seattle, WA (20). 721.221 transfectants were cultured in the presence of hygromycin B (Calbiochem) in the medium at 200 mU/ml. HLA-A and -B null C1R transfectants expressing group 1 CD1 antigens were provided by W. Storkus, University of Pittsburgh Medical Center and University of Pittsburgh Cancer Institute, Pittsburgh, PA. CD1 transfectants of C1R were maintained in medium containing 1.8 mg/ml G418 (Genetecin, GIBCO BRL) as described previously (21). Cell lines were maintained by continuous passage in RPMI 1640 culture medium supplemented with 10% FBS. The TAP-deficient cell line T2- and β2m-deficient cell line Daudi were obtained from American Type Culture Collection.
Clones 23, 29, and 1–1B were derived from the same latently infected individual using Mtb-infected DCs as APCs. Clone 1–1B, is an Mtb CPF10/Mtb11 specific CD8+ T cell clone that is HLA-44 restricted and responds to the cognate peptide AEMKTDAATL (AA 2–11; reference 13).
Expansion of T Cell Clones.
To expand the CD8+ T cell clones, a rapid expansion protocol using anti-CD3 mAb stimulation was used (22). T cell clones were cultured in the presence of irradiated allogeneic PBMCs (25 × 106), irradiated allogeneic LCL (5 × 106), and anti-CD3 mAb (30 ng/ml; Chiron) in RPMI 1640 media with 10% human serum in a T-25 upright flask in a total volume of 30 ml. The cultures were supplemented with IL-2 (1 ng/ml; Chiron) on days +1, +4, +7, and +10 of culture. The cell cultures were washed on day +4 to remove remaining soluble anti-CD3 mAb.
Generation of Peripheral Blood DCs and Macrophages.
Monocyte-derived DCs were prepared according to the method of Romani et al. (23). In brief, PBMCs were isolated from heparinized blood by centrifugation over Ficoll-Hypaque (Sigma-Aldrich) and washed three times with culture medium. Alternatively, PBMCs were obtained via leukapheresis. Cells were resuspended in 1% HS medium and allowed to adhere to a T-75 (Costar) flask at 37° for 1 h in the presence of 10 ng/ml of GM-CSF (Immunex Corp.). After gentle rocking, nonadherent cells were removed, and 30 ml of 10% HS medium containing 10 ng/ml of IL-4 (Immunex Corp.) and 30 ng/ml of GM-CSF (Immunex Corp.) was added. After 18 h, the media was removed, centrifuged, and the cell-conditioned media placed on the adherent cells. After 5–7 d, cells were harvested with cell-dissociation media (Sigma-Aldrich).
Mtb/DC-conditioned Media.
106 monocyte-derived DCs were cultured overnight in the presence of Mtb (H37Rv; MOI = 100) in low-adherence 16-mm wells (model no. 3473, Costar). After 18 h, supernatants were harvested and passed through a 0.2 μM filter (Gelman Sciences) to remove viable Mtb. Where indicated, conditioned media was treated with either lipase (3,000 U/ml; Sigma-Aldrich L-8525), DNase (2 U/ml; Sigma-Aldrich D5793), or proteinase K (0.2 mg/ml; Sigma-Aldrich P-0390). After overnight incubation, PMSF (60 μg/ml, Sigma-Aldrich) was added to all samples to inactivate proteinase K and to control for PMSF toxicity. To determine whether or not proteasomal function is required for the generation of the peptide antigen, DCs were incubated with lactacystin either before or 18 h after the addition of Mtb. To assay the activity of the supernatant, DCs were seeded at 5 × 104 cells per well in 96-well, flat-bottomed plates in 50 μl of media. 5 × 104 T cells in 50 μl of media and 100 μl of conditioned media were then added, and IFN-γ assayed by ELISA after 18 h incubation at 37°C.
Flow Cytometry.
Cells to be analyzed for cell surface marker expression were first incubated at 4°C in a blocking solution of PBS containing 2% normal rabbit serum (Sigma-Aldrich), 2% normal goat serum (Sigma-Aldrich), and 2% human serum to prevent nonspecific binding of mouse Ig. Cells were washed in FACS® buffer (PBS containing 0.5% FBS and 0.02% sodium azide) and incubated with either anti–HLA-E 3D12 (5 μg/ml; reference 21), anti–HLA-A2, anti–HLA-DR, or isotype control (5 μg/ml, Becton Dickinson) for 30 min at 4°C in a total volume of 50 μl. Cells were then washed, stained with an anti–mouse FITC antibody (Becton Dickinson) for 30 min at 4°C, and after washing, flow cytometry was performed using a FACSCalibur™ (Becton Dickinson). Data were collected on 104 viable cells.
IFN-γ ELISPOT Assay.
Mtb-specific effectors were detected from purified CD8+ T cells by ELISPOT, as described with minor modifications (24). In brief, 96-well nitrocellulose-backed plates (MAHA S4510; Millipore) were coated as recommended by the manufacturer with 10 μg/ml capture mouse anti-IFN (1-D1K, Mabtech AB) overnight at 4°C. Plates were then washed six times with PBS/0.05% Tween 20 (Sigma-Aldrich), blocked with RPMI 1640/10% HS for 1 h at room temperature. T cell clones (200) and APC (20,000) are added, and the plate incubated overnight at 37°C. After washing with PBS/0.05% Tween 20, 100 μl of 1 μg/ml biotinylated secondary anti–IFN-γ mAb (7B6–1, Mabtech AB) was added. After 2 h of incubation at room temperature, plates were washed six times and 100 μl Avidin/Biotinylated Enzyme (HRP) Complex (Vectastain ABC Elite kit; Vector Laboratories) was added to wells, and the plates were incubated for a further 1 h. Then, plates were washed six times, and 100 μl AEC substrate (Vectastain AEC substrate kit; Vector Laboratories) was added. After 4–7 min, the colorimetric reaction was stopped by washing with distilled water. Spots were quantitated using a Zeiss Axioplan 2 microscope with 3,200 K incident illumination equipped with a Epiplan Neofluar 5×/0.15 objective, Sony DXC 950 CCD camera, Märzhäuser scanning stage, MCP4 control unit, Pentium PC computer, and KS ELISPOT software (Carl Zeiss Vision).
Adenovirus Infection of APC.
To generate adenovirally infected APCs, DCs were harvested after 18 h of culture, and 5 × 105 DCs were seeded in Optimem (125 μl; Gibco Invitrogen Corp.) into low-adherence 16-mm wells (Costar). Adenoviral liposomes were prepared by coincubating the adenovirus and lipofectamine (6 μg/ml; Gibco Invitrogen Corp.) for 15 min and then added to the DC cultures. After 4–6 h, 1 ml fresh media containing GM-CSF (10 ng/ml; Immunex Corp.) and IL-4 (10 ng/ml; Immunex Corp.) was added.
Preparation and Manipulation of Triton X-114 Soluble Proteins.
Mtb H37Rv cells were disrupted by French press in a buffer of 10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, and 4% Triton X-114. The lysate was rocked overnight at 4°C, followed by centrifugation at 27,000× g (4°C) for 1 h. The supernatant was collected and placed at 37°C for 40 min to allow for biphasic partitioning and centrifuged at 27,000× g (20°C) for 30 min. The aqueous phase was removed and the detergent phase was back extracted three times with 10 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 1 mM EDTA (25). Precipitation of proteins from the final aqueous phase was achieved by the addition of 10 vol cold acetone and centrifugation at 27,000× g. The protein pellet was suspended in PBS, pH 7.5. To remove glycans and lipoglycans from the TX-114 protein extract, an equal vol of PBS saturated phenol was added to the protein suspension and rocked at room temperature for 2 h, followed by centrifugation at 27,000× g for 30 min. The aqueous phase was removed, and the phenol phase and interface was back extracted three times with an equal vol of PBS, pH 7.5. The final phenol phase was dialyzed extensively (2–3 d) against H20, and the protein concentration was determined using the bicinchoninic acid assay (26). 1 mg aliquots of the TX-114 soluble protein pool were digested with 10 μg/ml pronase at 37°C for 18 h. The removal of lipophilic material from the pronase digested sample was achieved by organic extraction with CHCl3/CH3OH 2:1 (27).
Statistical Analysis.
Where indicated, error bars denote the SEM of the mean. To compare each experimental mean with the control mean the “Dunnett's test” was employed. Statistical analysis performed using JMP software (SAS Institute Inc., Cary, NC).
Results
Mtb-derived Antigen Is Present in Mtb/DC-conditioned Media.
Recent analysis of the Mtb-derived 19-kD lipoprotein has demonstrated that presentation to HLA-Ia–restricted T cells can occur in a proteasome-dependent, but TAP-independent fashion using a paracrine-processing mechanism by which Mtb-derived lipopeptides gain access to an early endosomal compartment and are then potentially exported in a lipid vesicle (28, 29). This pathway would be consistent with the proteasome-dependent, TAP-independent pathway that we had observed, and led to the speculation that Mtb-derived antigen would be found within media conditioned by Mtb-infected DCs.
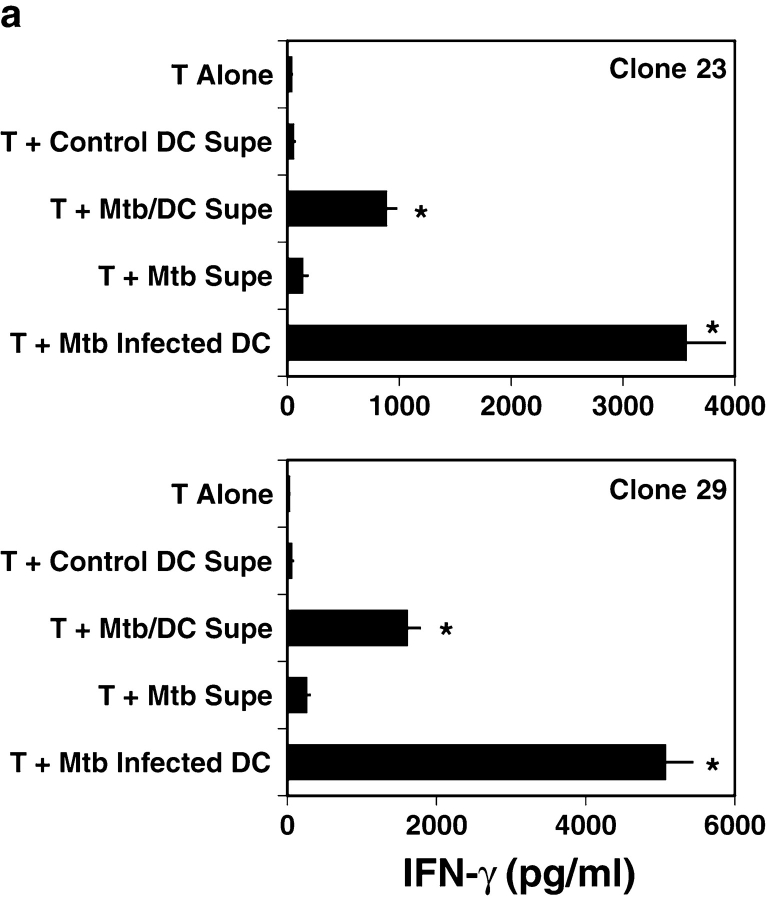
To test this hypothesis, human DCs were infected overnight with Mtb (H37Rv; MOI = 50), and the resulting supernatants twice filtered through a 0.2-μ filter to remove viable bacteria. These supernatants were then used to sensitize fresh DCs, and reactivity of the nonclassical CD8+ clones assessed by IFN-γ release. Mtb/DC-conditioned media was able to elicit T cell–dependent cytokine release (Fig. 1 a).
Figure 1.
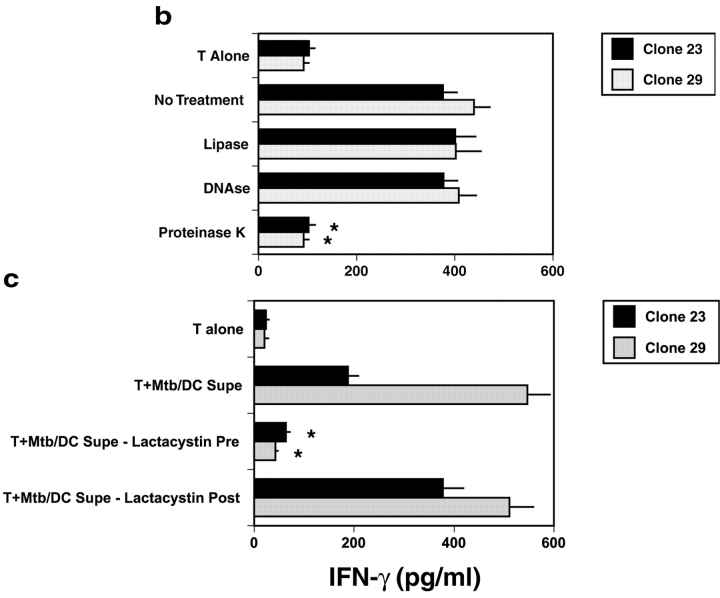
Mtb-derived antigen in Mtb/DC-conditioned media. (A) To assay the activity of the supernatant, DCs were seeded at 5 × 104 cells per well in 96-well, flat-bottomed plates in 50 μl of media. 5 × 104 T cells in 50 μl of media and 100 μl of conditioned media were then added, and IFN-γ assayed by ELISA after 18 h incubation at 37°C. Results represent the mean of duplicate determinations. Error bars denote the SEM. *P < 0.05, where the “Dunnett's test” has been used to compare each experimental mean with the control mean (T plus Control DC Supe). This experiment is representative of three such experiments. (B) Where indicated, Mtb/DC-conditioned media was treated with either lipase (3,000 U/ml L-8525, Sigma-Aldrich), DNase (2 U/ml D5793, Sigma-Aldrich), or proteinase K (0.2 mg/ml P-0390, Sigma-Aldrich). After overnight incubation, PMSF (60 μg/ml; Sigma-Aldrich) was added to all samples to inactivate the proteinase K. Results represent the mean of duplicate determinations. Error bars denote the SEM. Asterisks denote a significant loss of antigenic activity (P < 0.05) where the “Dunnett's test” has been used to compare each experimental mean with the control mean (No Treatment). This experiment is representative of two such experiments. (C) To determine whether or not proteasomal function is required for the generation of the peptide antigen, DCs were incubated with lactacystin (40 μM; E.J. Corey; Harvard Biolabs, Harvard, MA) added either before or 18 h after the addition of Mtb. T cell–dependent IFN-γ release was assessed as described in A. Results represent the mean of duplicate determinations. Error bars denote the SEM. Asterisks denote significant inhibition of antigenic activity (P < 0.05) where the “Dunnett's test” has been used to compare each experimental mean with the control mean (T plus Mtb/DC Supe). This experiment is representative of three such experiments.
Previously, we demonstrated that treatment of DCs with Escherichia coli, Staphylococcus aureus, LPS, or atypical mycobacteria could not elicit IFN-γ release from the nonclassically restricted clones (8), arguing against a nonspecific effect of DC activation. Here we have undertaken a series of experiments to further validate the relationship of naturally processed and presented Mtb antigen and the activity seen in the Mtb/DC supernatants. First, Mtb/DC supernatants were treated with either proteinase K, DNase, or lipase. Proteinase K abolished the ability of the supernatants to sensitize fresh DCs (Fig. 1 b), whereas lipase and DNase had no effect. Then, we sought to determine whether or not antigen processing was required for supernatant activity. Prior experiments were performed with fixed DCs, eliminating any possible effect of residual inhibitor on T cell activation. Previously, we have found that overnight incubation with lactacystin did not interfere with the ability to process and present HLA-II–associated antigen (8), and have found that HLA-Ia–dependent presentation of peptide antigen is not impaired (unpublished data). Because the supernatants would also contain the inhibitor, the proteasomal blocker lactacystin was added either before or after Mtb infection to control for possible toxicity. Lactacystin added before but not after infection with Mtb abolished antigenic activity (Fig. 1 c). These data suggested that proteasomal processing was required for the antigenic activity seen in the Mtb/DC supernatants.
HLA-E–dependent Antigen Presentation.
Previously, we had found that the pan–HLA-I antibody W6/32 could partially inhibit the recognition of Mtb-infected DCs by the nonclassical clones (8). These data were consistent with the hypothesis than an HLA-Ib molecule might restrict the nonclassical T cells. In this regard, W6/32 is capable of binding to HLA-E, F, and G in addition to classical HLA. HLA-E is known to bind HLA-Ia leader peptides, and to serve as an NK inhibitory molecule through its interaction with the CD94/NKG2A heterodimer (30, 31). However, several pieces of data suggested that it could also serve to directly present antigen to CD8+ T cells. First, Martinozzi and colleagues demonstrated that mouse CD8+ T cells can recognize HLA-A2 leader peptide in the context of HLA-E (32). Second, recombinant HLA-E is capable of binding to peptides from a combinatorial peptide library distinct from the known HLA-Ia leader peptides (33). Finally, HLA-I and virus-derived peptides can be recognized by human NK-like αβ TCR, CD8+ T cell clones in the context of HLA-E (34, 35). While these studies demonstrate the ability of T cells to recognize peptide presented in the context of HLA-E, the physiological relevance of this observation remains unknown.
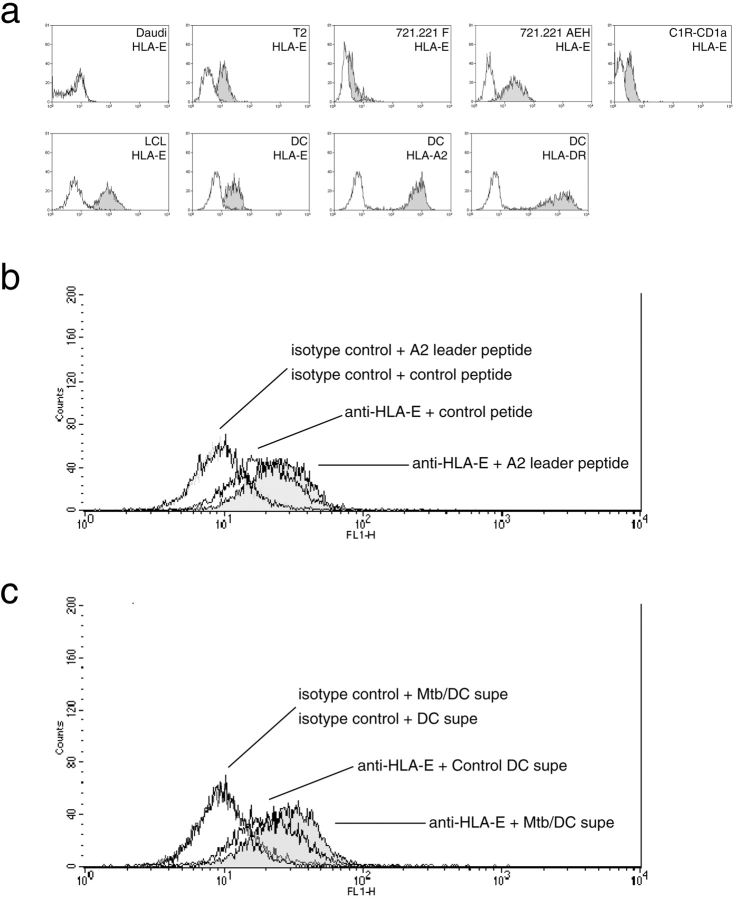
To test the hypothesis that the Mtb-specific nonclassical T cells were restricted by HLA-E, we sought to determine if the antigen found within the supernatants could stabilize cell-surface expression of HLA-E. For reference, FACS® staining for cell surface expression HLA-E of the cell lines used herein is provided (Fig. 2 a). The cell line T2 is TAP deficient, and hence expresses little cell surface HLA. Incubation of these cells overnight at 27°C facilitates the cell-surface expression and possible loading of empty HLA-I (36). Addition of the HLA-A2 leader peptide (VMAPRTLVL), in comparison to control peptide, stabilizes HLA-E expression (MFI = 32 vs. 19; Fig. 2 b); Similarly, Mtb/DC-conditioned medium, in comparison to DC control supernatant, stabilized the cell-surface expression of HLA-E (MFI = 33 vs. 22; Fig. 2 c). Stabilization of HLA-E was not observed at 37°C (unpublished data). Thus, these data suggested that Mtb/DC supernatants could contain a high-affinity HLA-E–binding activity capable of stabilizing HLA-E.
Figure 2.
Cell surface expression of HLA-E and stabilization by Mtb/DC-conditioned media. (A) The cell lines Daudi, T2, 721.221 F, 721.221 AEH, C1R-CD1a, LCL, and human monocyte-derived DCs were assessed for cell surface expression of HLA-E using the anti–HLA-E antibody 3D12 (shaded histogram). Isotype control staining is shown in the white histogram. For reference, cell surface expression of HLA-A2 and HLA-DR is shown on human monocyte-derived DCs. (B) TAP-deficient T2 cells were incubated overnight at 27°C with control DC conditioned media in the presence of either the HLA-A2 leader peptide VMAPRTLVL or control peptide AEMKTDAATL and stained for cell surface expression of HLA-E. Results represent the mean of duplicate determinations. These data are representative of two experiments.(C) TAP-deficient T2 cells were incubated overnight at 27°C with either control DC-conditioned media or Mtb/DC-conditioned media and stained for cell surface expression of HLA-E. Results represent the mean of duplicate determinations. These data are representative of two experiments.
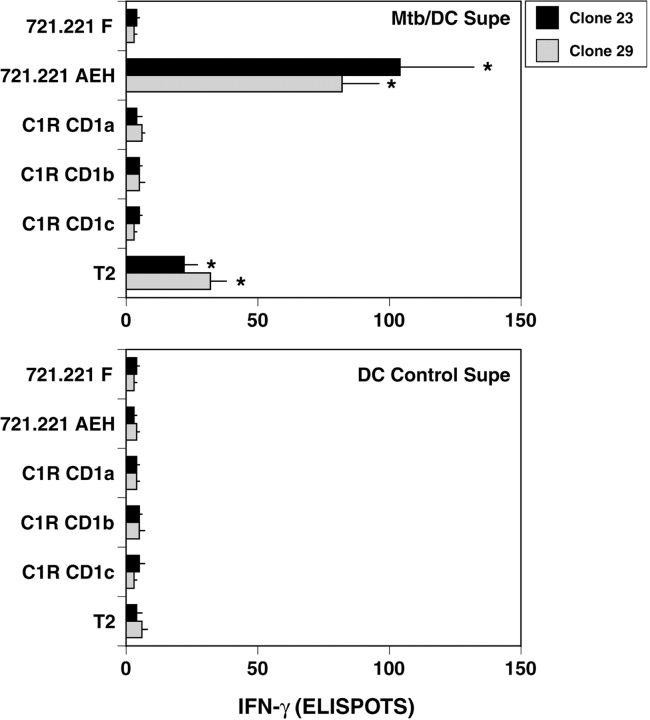
We then sought to directly test the hypothesis that the nonclassical T cell clones 23 and 29 are restricted by HLA-E. Because T cells express HLA-E, and hence would potentially be capable of self-presentation of preprocessed antigen, we sought to define conditions where each T cell would come in contact with a well-characterized APC. For this purpose, we used the ability of the sensitive IFN-γ ELISPOT to detect small numbers of T cells. Here, 200–250 nonclassical T cells were incubated with 20,000 APCs transfected to express defined restriction molecules. In the presence of Mtb/DC-conditioned media, the 721.221-AEH cell line (which has been stably transfected to express HLA-E along with the stabilizing HLA-A2 leader peptide; reference 31) and to a lesser extent T2 were able to present antigen to the nonclassical CD8+ T cells 23 and 29, while the 721.221 F, C1R-CD1a, C1R-CD1b, or C1R-CD1c functioned far less efficiently as APC (Fig. 3) . The ability of each cell line to present Mtb/DC conditioned media correlated well with cell surface expression of HLA-E (Fig. 2 a), and was further corroborated by the observation that the cell line 721.221 E, transfected to express HLA-E, but deficient in cell surface expression by virtue of absence of HLA leader peptide, failed to function as an APC (unpublished data). Less than 10 spots were observed when T cells and APC were coincubated in the presence of control DC supernatant excluding the possibility that the presence of HLA-E, or HLA-E stabilized by the HLA-A2 leader peptide alone was sufficient to activate the clones (Fig. 3).
Figure 3.
Preferential presentation of Mtb/DC-conditioned media by HLA-E. 200 nonclassical T cell clones were coincubated overnight with 20,000 stably transfected cells in the presence of either Mtb/DC or DC control conditioned media. IFN-γ release was determined by ELISPOT. Less than 10 ELISPOTS are observed with DC control media. Results represent the mean of duplicate determinations. Error bars denote the SEM. *P < 0.05, where the “Dunnett's test” has been used to compare each experimental mean with the control mean (721.221 F). These data are representative of three experiments.
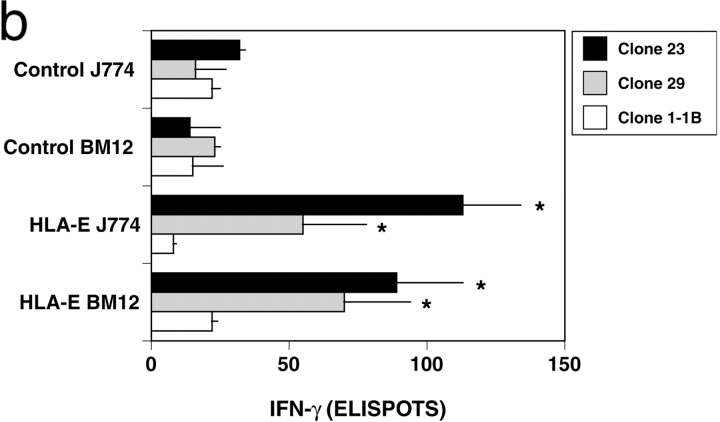
While compelling, interpretation of these data is complicated by the fact that all of the cell lines tested express HLA-E, with surface expression limited by the availability of the HLA-Ia leader peptides. In contrast, HLA-E is not present in the mouse. Thus, we asked whether or not the mouse phagocytic cell lines BM1 and J774 could process and present Mtb-derived antigen in the context of HLA-E. Initially, stable expression of human β2M was achieved by retroviral transduction. These cells were then infected with HLA-E expressing or control adenovirus (MOI = 50; HLA-E RAd270, reference 37, control RAd35, reference 38), and then infected with Mtb 1 d later. After overnight infection with Mtb (H37Rv; MOI = 2), the IFN-γ ELISPOT assay was used to assess the ability of the nonclassical T cell clones to recognize Mtb in the context of HLA-E. Cell surface expression of HLA-E could be demonstrated on the β2M-expressing cell lines via stabilization with the HLA-A2 leader peptide (Fig. 4 a, MFI = 22 vs. 9). Previously, we have found that a minority (<1:50) of Mtb-infected DCs are recognized by either classically or nonclassically restricted CD8+ T cells, and that recognition is correlated with the degree of intracellular infection (unpublished data). As a result, we postulated that HLA-E–dependent antigen processing and presentation would be relatively inefficient in the context of the murine cell lines. To this end, a saturating number of T cell clones (20,000) were used to ensure maximum detection of Mtb-infected APC. Both HLA-E and Mtb were required for T cell–dependent IFN-γ release by the nonclassically restricted clones (Fig. 4 b). The relatively modest number of Mtb-specific spots observed likely reflects the limiting number of functional APC in each well. As a control, the HLA-B44, CFP10/Mtb11 specific T cell clone 1–1B demonstrated no response to these cell lines in the presence or absence of Mtb.
Figure 4.
Preferential presentation by mouse macrophage cell lines expressing HLA-E. The pBIB retroviral vector containing human β2m was provided by S. Fling (Corixa Corp., Seattle, WA) and used to transduce murine J774 and BM12 cell lines under blastocidin selection (reference 40) and cell surface expression of human β2m confirmed via FACS®. (A) β2m-expressing J774 cells were infected with either control (Rad35) or HLA-E (Rad270) adenovirus. After 24 h, cells were incubated overnight at 27°C in the presence of HLA-A2 leader peptide VMAPRTLVL. Cell surface expression of HLA-E was assessed using the anti–HLA-E antibody 3D12. These data are representative of two experiments. (B) Murine J774 and BM12 cell lines expressing human β2M were infected with either control or HLA-E adenovirus, MOI = 50:1. After 24 h, cells were infected with Mtb (H37Rv, MOI = 2). To ensure detection of Mtb-infected cells, a saturating number (20,000) of T cells were added and after 18 h of coincubation IFN-γ release assessed by ELISPOT. All data represent the mean of duplicate determinations. Error bars denote the SEM. *P < 0.05, where the “Dunnett's test” has been used to compare each experimental mean with the control mean. These data are representative of two experiments.
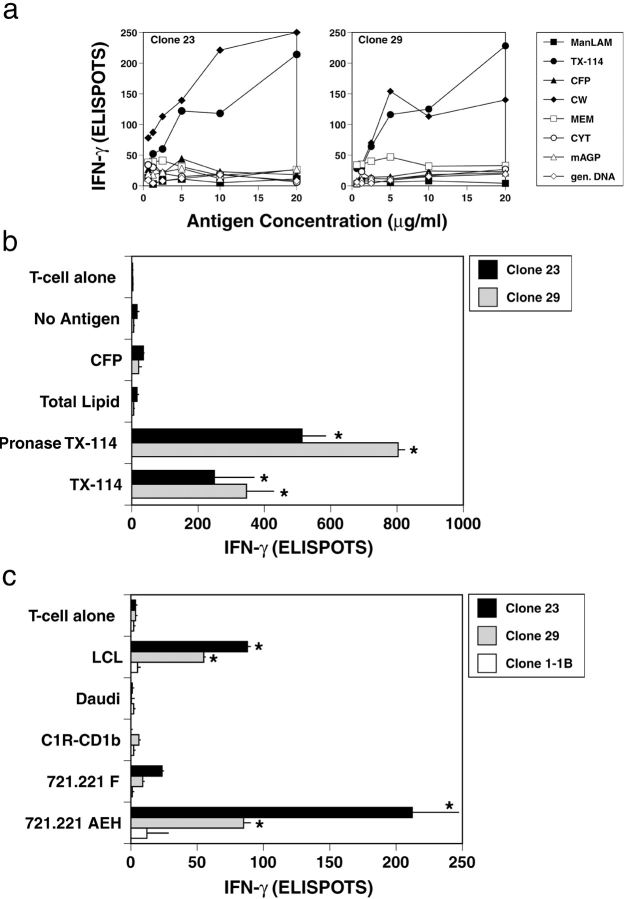
The crystal structure of HLA-E has been solved, and it has been suggested that the peptide-binding groove is uniquely able to present a limited set of hydrophobic peptides (30). We speculated, that the antigen presented by HLA-E might be a hydrophobic peptide or protein associated with the mycobacterial cell wall. To test this hypothesis, DCs were pulsed with partially-purified Mtb fractions. In these experiments, a saturating number of T cells (20,000) and APCs (20,000) were used to maximize the sensitivity of the assay for the detection of the antigenic activity. Neither secreted proteins, purified lipids from Mtb, nor Mtb DNA could elicit T cell dependent release of IFN-γ (Fig. 5 a). In contrast, both the cell wall fraction, and the lipoprotein-enriched TX-114 fraction were capable of sensitizing DC targets for T cell recognition. The response seen was dependent on the concentration of antigen added, confirming that neither T cells nor APCs were limiting under these experimental conditions. Treatment of the TX-114 fraction with the serine endopeptidase proteinase K abolished the antigenic activity in a manner similar to that shown for the Mtb/DC-conditioned media (unpublished data). Interestingly, treatment of the TX-114 fraction with pronase, a mixture of bacterial endo and exo proteinases, resulted in augmented antigenic activity (Fig. 5 b). We speculate that this differential susceptibility to proteolytic cleavage reflects a posttranslationally modified, and hence partially protected peptide.
Figure 5.
Presentation by HLA-E of lipid-enriched antigen of Mtb. (A) 20,000 T cell clones were coincubated with 20,000 DC in the presence of either mannose lipoarabinomannan (ManLAM), Triton X-114 lipoprotein fraction (TX-114), CFPs, cell wall fraction (CW), cell membrane fraction (MEM), cytosol fraction (CYT), mycolylarabinogalactan peptidoglycan (mAGP), or Mtb genomic DNA (gen. DNA) at the concentrations indicated. After 18 h of coculture, IFN-γ was assessed by ELISPOT. These data are representative of three experiments. (B) Using 20,000 nonclassical T cells and 20,000 autologous DCs, antigens were tested at 10 μg/ml for T cell–dependent release of IFN-γ by ELISPOT. Error bars denote the SEM. *P < 0.05, where the “Dunnett's test” has been used to compare each experimental mean with the control mean (No Antigen). (C) 250 T cells were coincubated with 20,000 APCs in the presence of 10 μg/ml of pronase-treated TX-114 antigen. IFN-γ release is assessed by ELISPOT. Results represent the mean of duplicate determinations. Error bars denote the SEM. *P < 0.05, where the “Dunnett's test” has been used to compare each experimental mean with the control mean (721.221 F). These data are representative of three experiments.
To determine whether or not this pronase fraction was presented in an HLA-E–dependent fashion, stably transfected APCs were tested for their ability to present Mtb-derived antigen as described for Fig. 3. The Mtb-derived antigenic activity could be preferentially presented by the cell lines that express cell-surface HLA-E (721.221-AEH and LCL; Fig. 5 c). Importantly, the NK target Daudi was not recognized. Finally, while not HLA-E specific, we have found antibodies to CD8 and pan-HLA I (W6/32) capable of inhibiting the T cell–dependent recognition of the antigenic fraction (unpublished data).
Discussion
Taken together, these data provide the first compelling evidence that the nonclassical, Mtb-specific clones 23 and 29 recognize pathogen-derived antigen in the context of HLA-E, and extends the function of this molecule beyond its known role as an NK inhibitor through the interaction with the NKG2A/CD94 heterodimer. It is very unlikely that T cell activation is occurring through the NKG2A/CD94 heterodimer. First, antibodies known to block NK recognition via HLA-E or CD94 have proven ineffective in blocking T cell–dependent recognition of Mtb-infected DCs (unpublished data). Additionally, HLA-E–deficient cell lines (Daudi), which serve as NK targets through the lack of CD94/NKG2A inhibition, are not recognized by the nonclassical T cells (Fig. 5 c). Instead, we postulate that recognition of Mtb-derived antigen is occurring through the TCR.
All of the human cell lines tested express HLA-E, with cell surface expression limited by the availability of the HLA-Ia leader peptides (C1R and 721.221). Thus, the preferential recognition of the 721.221-AEH, LCL, DCs, and to a lesser extent T2 cell lines may reflect the greater cell-surface expression of HLA-E, with subsequent displacement of the HLA-Ia peptide. Cell-surface stabilization of HLA-E was not observed at 37°C.
Earlier studies demonstrated that nonclassically restricted T cells comprised the majority of Mtb-specific CD8 T cells in two latently infected subjects (9). However, whether these cells are entirely HLA-E restricted, and whether they recognize a common, or similar class of antigens remains to be determined. The current data would support the hypothesis that the antigen is a hydrophobic peptide, possibly modified via either lipidation or glycosylation. This hypothesis is further supported by the recent observation that hydrophobic peptides can be presented in a proteasomal-dependent, but TAP-independent pathway (39). By analogy with other HLA-Ib presentation pathways, it is attractive to speculate that the modifications represent unique characteristics of Mtb or mycobacteria. Nonetheless, addressing these questions may have important implications for the design of a vaccine capable of eliciting CD8+ T cell specific immunity.
In summary, this report is the first demonstration of recognition of a pathogen-derived antigen being presented in the context of HLA-E. Three independent approaches antigen to human CD8+ T cells in this context. First, antigenic activity found within conditioned Mtb/DC supernatants was presented preferentially by the HLA-E–expressing 721.221 AEH cell line. Furthermore, these supernatants could stabilize the expression of HLA-E on the TAP-deficient T2 cell line at 27°C. Second, murine macrophage cell lines coexpressing human β2M and HLA-E acquired the ability to present to the nonclassical T cells after infection with Mtb. Third, lipoprotein enriched, Mtb-derived material could be presented in the context of HLA-E. Thus, HLA-E should be included as an HLA-Ib processing and presentation system.
Acknowledgments
The authors wish to thank Steve Fling of Corixa Corporation for the provision of retrovirally encoded human β2 microglobulin. Daniel Geraghty of the Fred Hutchinson Cancer Research Center has graciously provided anti–HLA-E antibodies and cell lines expressing HLA-E. Walter Storkus, University of Pittsburgh Medical Center and University of Pittsburgh Cancer Institute has kindly provided C1R cell lines expressing group 1 CD1 antigens. We thank Gavin Wilkinson and Peter Tomasec of the University of Wales College of Medicine for their trans-Atlantic provision of HLA-E expressing adenovirus.
This work was supported by the American Lung Association (to D.M. Lewinsohn), the National Institutes of Health (no. K08-AI1644, R01-AI48090NIH to D.M. Lewinsohn and NIAID contract NO1 AI-75320 “TB Research Materials and Vaccine Testing” to J.T. Belisle). The Portland VA Medical Center has provided laboratory space and partial salary support (to D.M. Lewinsohn).
This work has been presented in part at the ASM Conference on Immunity to Bacterial, Viral, and Protozoal Pathogens, March 20–24, 2002, in Savannah, GA, the American Thoracic Society, May 17–22, 2002, in Atlanta, GA, and the Fourth World Congress on TB, June 3–5, 2002, in Washington, D.C.
Footnotes
Abbreviations used in this paper: CFP, culture filtrate protein; DC, dendritic cell; Mtb, Mycobacterium tuberculosis; TAP, transporter associated with antigen processing; TB, tuberculosis; TX-114, Triton X-114 lipoprotein/membrane protein fraction.
References
- 1.World Health Organization. 2001. Global Tuberculosis Control. WHO Report 2001. Geneva, Switzerland, WHO/CDS/TB/2001.287. 181 pp.
- 2.Flynn, J.L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93–129. [DOI] [PubMed] [Google Scholar]
- 3.Raupach, B., and S.H. Kaufmann. 2001. Immune responses to intracellular bacteria. Curr. Opin. Immunol. 13:417–428. [DOI] [PubMed] [Google Scholar]
- 4.Flynn, J.L., M.M. Goldstein, K.J. Triebold, B. Koller, and B.R. Bloom. 1992. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA. 89:12013–12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mogues, T., M. Goodrich, L. Ryan, R. LaCourse, and R. North. 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behar, S.M., C.C. Dascher, M.J. Grusby, C.R. Wang, and M.B. Brenner. 1999. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J. Exp. Med. 189:1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stenger, S., D.A. Hanson, R. Teitelbaum, P. Dewan, K.R. Niazi, C.J. Froelich, T. Ganz, S. Thoma-Uszynski, A. Melian, C. Bogdan, et al. 1998. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 282:121–125. [DOI] [PubMed] [Google Scholar]
- 8.Lewinsohn, D.M., M.R. Alderson, A.L. Briden, S.R. Riddell, S.G. Reed, and K.H. Grabstein. 1998. Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen-presenting cells. J. Exp. Med. 187:1633–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewinsohn, D.M., A.L. Briden, S.G. Reed, K.H. Grabstein, and M.R. Alderson. 2000. Mycobacterium tuberculosis-reactive CD8+ T lymphocytes: the relative contribution of classical versus nonclassical HLA restriction. J. Immunol. 165:925–930. [DOI] [PubMed] [Google Scholar]
- 10.Smith, S.M., A.S. Malin, T. Pauline, Lukey, S.E. Atkinson, J. Content, K. Huygen, and H.M. Dockrell. 1999. Characterization of human Mycobacterium bovis bacille Calmette-Guerin-reactive CD8+ T cells. Infect. Immun. 67:5223–5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pathan, A.A., K.A. Wilkinson, R.J. Wilkinson, M. Latif, H. McShane, G. Pasvol, A.V. Hill, and A. Lalvani. 2000. High frequencies of circulating IFN-γ-secreting CD8 cytotoxic T cells specific for a novel MHC class I-restricted Mycobacterium tuberculosis epitope in M. tuberculosis-infected subjects without disease. Eur. J. Immunol. 30:2713–2721. [DOI] [PubMed] [Google Scholar]
- 12.Mohagheghpour, N., D. Gammon, L.M. Kawamura, A. van Vollenhoven, C.J. Benike, and E.G. Engleman. 1998. CTL response to Mycobacterium tuberculosis: identification of an immunogenic epitope in the 19-kDa lipoprotein. J. Immunol. 161:2400–2406. [PubMed] [Google Scholar]
- 13.Lewinsohn, D.M., L. Zhu, V.J. Madison, D.C. Dillon, S.P. Fling, S.G. Reed, K.H. Grabstein, and M.R. Alderson. 2001. Classically restricted human CD8+ T lymphocytes derived from Mycobacterium tuberculosis-infected cells: definition of antigenic specificity. J. Immunol. 166:439–446. [DOI] [PubMed] [Google Scholar]
- 14.Ulrichs, T., and S.A. Porcelli. 2000. CD1 proteins: targets of T cell recognition in innate and adaptive immunity. Rev. Immunogenet. 2:416–432. [PubMed] [Google Scholar]
- 15.Pamer, E.G., C.R. Wang, L. Flaherty, K.F. Lindahl, and M.J. Bevan. 1992. H-2M3 presents a Listeria monocytogenes peptide to cytotoxic T lymphocytes. Cell. 70:215–223. [DOI] [PubMed] [Google Scholar]
- 16.Chun, T., N.V. Serbina, D. Nolt, B. Wang, N.M. Chiu, J.L. Flynn, and C.R. Wang. 2001. Induction of M3-restricted cytotoxic T lymphocyte responses by N-formylated peptides derived from Mycobacterium tuberculosis. J. Exp. Med. 193:1213–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo, W.F., H. Ong, E.S. Metcalf, and M.J. Soloski. 1999. T cell responses to Gram-negative intracellular bacterial pathogens: a role for CD8+ T cells in immunity to Salmonella infection and the involvement of MHC class Ib molecules. J. Immunol. 162:5398–5406. [PubMed] [Google Scholar]
- 18.Lo, W.F., A.S. Woods, A. DeCloux, R.J. Cotter, E.S. Metcalf, and M.J. Soloski. 2000. Molecular mimicry mediated by MHC class Ib molecules after infection with gram-negative pathogens. Nat. Med. 6:215–218. [DOI] [PubMed] [Google Scholar]
- 19.Rolph, M.S., B. Raupach, H.H. Kobernick, H.L. Collins, B. Perarnau, F.A. Lemonnier, and S.H. Kaufmann. 2001. MHC class Ia-restricted T cells partially account for β2-microglobulin-dependent resistance to Mycobacterium tuberculosis. Eur. J. Immunol. 31:1944–1949. [DOI] [PubMed] [Google Scholar]
- 20.Lee, N., D.R. Goodlett, A. Ishitani, H. Marquardt, and D.E. Geraghty. 1998. HLA-E surface expression depends on binding of TAP-dependent peptides derived from certain HLA class I signal sequences. J. Immunol. 160:4951–4960. [PubMed] [Google Scholar]
- 21.Storkus, W.J., M. Wei, P. Cresswell, and J.R. Dawson. 1996. Class I-like CD1A-C do not protect target cells from NK-mediated cytolysis. Cell. Immunol. 167:154–156. [DOI] [PubMed] [Google Scholar]
- 22.Riddell, S.R., K.S. Watanabe, J.M. Goodrich, C.R. Li, M.E. Agha, and P.D. Greenberg. 1992. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 257:238–241. [DOI] [PubMed] [Google Scholar]
- 23.Romani, N., S. Gruner, D. Brang, E. Kampgen, A. Lenz, B. Trockenbacher, G. Konwalinka, P.O. Fritsch, R.M. Steinman, and G. Schuler. 1994. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 180:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lalvani, A., R. Brookes, S. Hambleton, W.J. Britton, A.V. Hill, and A.J. McMichael. 1997. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 186:859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radolf, J.D., and M.V. Norgard. 1988. Pathogen specificity of Treponema pallidum subsp. pallidum integral membrane proteins identified by phase partitioning with Triton X-114. Infect. Immun. 56:1825–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith, P.K., R.I. Krohn, G.T. Hermanson, A.K. Mallia, F.H. Gartner, M.D. Provenzano, E.K. Fujimoto, N.M. Goeke, B.J. Olson, and D.C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76–85. [DOI] [PubMed] [Google Scholar]
- 27.Folch, J., M. Lees, and G.A. Sloane-Stanley. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226:497–509. [PubMed] [Google Scholar]
- 28.Neyrolles, O., K. Gould, M.P. Gares, S. Brett, R. Janssen, P. O'Gaora, J.L. Herrmann, M.C. Prevost, E. Perret, J.E. Thole, and D. Young. 2001. Lipoprotein access to MHC class I presentation during infection of murine macrophages with live mycobacteria. J. Immunol. 166:447–457. [DOI] [PubMed] [Google Scholar]
- 29.Beatty, W.L., E.R. Rhoades, H.J. Ullrich, D. Chatterjee, J.E. Heuser, and D.G. Russell. 2000. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic. 1:235–247. [DOI] [PubMed] [Google Scholar]
- 30.Braud, V.M., D.S. Allan, C.A. O'Callaghan, K. Soderstrom, A. D'Andrea, G.S. Ogg, S. Lazetic, N.T. Young, J.I. Bell, J.H. Phillips, et al. 1998. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 391:795–799. [DOI] [PubMed] [Google Scholar]
- 31.Lee, N., M. Llano, M. Carretero, A. Ishitani, F. Navarro, M. Lopez-Botet, and D.E. Geraghty. 1998. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc. Natl. Acad. Sci. USA. 95:5199–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinozzi, S., R. Pacasova, H.J. Boulouis, M. Ulbrecht, E.H. Weiss, F. Sigaux, and M. Pla. 1999. Cutting edge: requirement of class I signal sequence-derived peptides for HLA-E recognition by a mouse cytotoxic T cell clone. J. Immunol. 162:5662–5665. [PubMed] [Google Scholar]
- 33.Stevens, J., E. Joly, J. Trowsdale, and G.W. Butcher. 2001. Peptide binding characteristics of the non-classical class Ib MHC molecule HLA-E assessed by a recombinant random peptide approach. BMC Immunol. 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pietra, G., C. Romagnani, M. Falco, M. Vitale, R. Castriconi, D. Pende, E. Millo, S. Anfossi, R. Biassoni, L. Moretta, and M.C. Mingari. 2001. The analysis of the natural killer-like activity of human cytolytic T lymphocytes revealed HLA-E as a novel target for TCR α/β-mediated recognition. Eur. J. Immunol. 31:3687–3693. [DOI] [PubMed] [Google Scholar]
- 35.García, P., M. Llano, A.B. de Heredia, C.B. Willberg, E. Caparrós, P. Aparicio, V.M. Braud, and M. López-Botet. 2002. Human T cell receptor-mediated recognition of HLA-E. Eur. J. Immunol. 32:936–944. [DOI] [PubMed] [Google Scholar]
- 36.Schumacher, T.N., M.T. Heemels, J.J. Neefjes, W.M. Kast, C.J. Melief, and H.L. Ploegh. 1990. Direct binding of peptide to empty MHC class I molecules on intact cells and in vitro. Cell. 62:563–567. [DOI] [PubMed] [Google Scholar]
- 37.Tomasec, P., V.M. Braud, C. Rickards, M.B. Powell, B.P. McSharry, S. Gadola, V. Cerundolo, L.K. Borysiewicz, A.J. McMichael, and G.W. Wilkinson. 2000. Surface expression of HLA-E, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science. 287:1031. [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson, G.W., and A. Akrigg. 1992. Constitutive and enhanced expression from the CMV major IE promoter in a defective adenovirus vector. Nucleic Acids Res. 20:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lautscham, G., S. Mayrhofer, G. Taylor, T. Haigh, A. Leese, A. Rickinson, and N. Blake. 2001. Processing of a multiple membrane spanning Epstein-Barr virus protein for CD8+ T cell recognition reveals a proteasome-dependent, transporter associated with antigen processing-independent pathway. J. Exp. Med. 194:1053–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fling, S.P., R.A. Sutherland, L.N. Steele, B. Hess, S.E. D'Orazio, J. Maisonneuve, M.F. Lampe, P. Probst, and M.N. Starnbach. 2001. CD8+ T cells recognize an inclusion membrane-associated protein from the vacuolar pathogen Chlamydia trachomatis. Proc. Natl. Acad. Sci. USA. 98:1160–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]