Abstract
Melanosomes and premelanosomes are lysosome-related organelles with a unique structure and cohort of resident proteins. We have positioned these organelles relative to endosomes and lysosomes in pigmented melanoma cells and melanocytes. Melanosome resident proteins Pmel17 and TRP1 localized to separate vesicular structures that were distinct from those enriched in lysosomal proteins. In immunogold-labeled ultrathin cryosections, Pmel17 was most enriched along the intralumenal striations of premelanosomes. Increased pigmentation was accompanied by a decrease in Pmel17 and by an increase in TRP1 in the limiting membrane. Both proteins were largely excluded from lysosomal compartments enriched in LAMP1 and cathepsin D. By kinetic analysis of fluid phase uptake and immunogold labeling, premelanosomal proteins segregated from endocytic markers within an unusual endosomal compartment. This compartment contained Pmel17, was accessed by BSA–gold after 15 min, was acidic, and displayed a cytoplasmic planar coat that contained clathrin. Our results indicate that premelanosomes and melanosomes represent a distinct lineage of organelles, separable from conventional endosomes and lysosomes within pigmented cells. Furthermore, they implicate an unusual clathrin-coated endosomal compartment as a site from which proteins destined for premelanosomes and lysosomes are sorted.
Keywords: melanosome, lysosome, endosome, sorting, organelle biogenesis
Introduction
Biosynthesis of melanins, the major pigments synthesized by mammals, is sequestered within unique membrane-enclosed structures of melanocytes and retinal pigment epithelial cells (RPEs) called melanosomes. Melanosomes and their precursors can be classified into four stages of development based on morphology (Seiji et al. 1963a). Early stages, or premelanosomes, lack melanin and consist of vesicular structures with internal membranes (stage I) or characteristic, elongated structures with internal striations of unknown composition (stage II). As it is synthesized, polymerized melanins accumulate on the striations, resulting in their thickening and blackening (stage III) until melanin fills the entire melanosome (stage IV). Consistent with their singular structure and function, melanosomes contain specific resident proteins that are expressed only in cells of the melanocytic and RPE lineage (King et al. 1995). Most of those characterized are integral membrane proteins, such as tyrosinase, tyrosine-related protein 1 (TRP1; gp75, Brown locus protein), and Pmel17 (gp100, Silver locus protein).
The singular nature of melanosomes and their limited expression in melanocytes and RPEs pose a challenge for organellogenesis. To form the melanosome, these cells must have either modified a ubiquitous organelle or developed a novel mechanism for sorting specific resident proteins from ubiquitous organelles. Observations from early immunocytochemical and subcellular fractionation studies suggest common features for melanosomes and late endosomes/lysosomes. These include the presence of internal vesicles like those in multivesicular bodies (Turner et al. 1975; Jimbow et al. 1979), lysosomal hydrolases and integral membrane proteins, apparent fusion with phagocytosed particles, and an acidic pH (for review see Orlow 1995). If this is true, melanosomes could merely be modified lysosomes and no specialized protein sorting pathways need to be invoked to generate them. That sorting pathways for lysosomal and melanosomal proteins are similar is supported by the presence of tyrosinase activity in TGN-associated clathrin-coated vesicles in melanocytes (Novikoff et al. 1968; Maul and Brumbaugh 1971) and by the localization of tyrosinase and TRP1 to late endosomes and lysosomes in transfected nonpigmented cells (Bouchard et al. 1989; Vijayasaradhi et al. 1995; Calvo et al. 1999; Simmen et al. 1999).
Other observations suggest that melanosomes are distinct from lysosomes. Although acid phosphatase activity can be detected histochemically in melanosomes of some melanoma cells, nonmelanosomal structures similar to lysosomes contain higher activity (Seiji and Kikuchi 1969), and activity is absent from melanosomes and premelanosomes of untransformed skin melanocytes (Boissy et al. 1987). If melanosomes and lysosomes coexist within melanocytes, then their segregation must be maintained by specialized protein sorting pathways. In support of this view, sorting signals within the tyrosinase cytoplasmic domain have distinct properties from lysosomal sorting signals in nonpigmented cells (Calvo et al. 1999) and can mediate partial localization to synaptic vesicle–like vesicles in PC12 cells (Blagoveshchenskaya et al. 1999). Such a distinction in nonmelanogenic cells may reflect the use of conserved recognition mechanisms to mediate unique sorting activities in melanocytes, similar to the use of conserved recognition elements by polarized cells to differentially sort proteins to apical and basolateral surfaces (Yoshimori et al. 1996; Wilson and Colton 1997). Further support for a distinct melanosomal sorting pathway comes from patients with Hermansky-Pudlak syndrome (HPS). These patients have primary defects in specialized cell types such as melanocytes and megakaryocytes/platelets, suggesting specificity in biogenesis of lysosome-related organelles (Shotelersuk and Gahl 1998; Dell'Angelica et al. 2000b). Finally, the development of melanosomes in distinct stages indicates that still uncharacterized sorting steps must exist to ensure the stepwise development of a mature melanosome.
Here, we have morphologically characterized the localization and movement of melanosomal proteins through the endosomal pathway of highly pigmented melanocytic cells. Our data suggest the existence of unique sorting pathways permitting the biogenesis of melanosomal compartments in these cells and of unique mechanisms to maintain compartment identity within the endosomal pathway.
Materials and Methods
Cell Lines and Cell Culture
MNT-1 cells (gift of V. Hearing, National Cancer Institute, National Institutes of Health, Bethesda, MD) were maintained in DME containing 10% AIM-V medium (Life Technologies), 20% FBS, nonessential amino acids, sodium pyruvate, and antibiotics. Primary melanocytes (gift of M. Herlyn, Wistar Institute, Philadelphia, PA) were maintained for up to 10 passages in MCDB-131 medium containing 2% FBS, 10% chelated FBS, 0.44 nM basic FGF, 100 nM endothelin-3, 10 ng/ml human stem cell factor, 20 pM cholera toxin, antibiotics, and fungizone.
Antibodies, Endocytic Tracers, and Electron Dense Probes
We used the following primary antibodies: TA99 (Mel-5), mouse anti-TRP1 mAb (American Type Culture Collection; Thomson et al. 1985); HMB50, mouse anti-Pmel17 mAb (gift of C. Figdor, University Hospital, Nijmegen, The Netherlands; Thomson and MacKie 1989); HMB45, mouse anti-Pmel17 mAb (ENZO Diagnostics; Thomson and MacKie 1989); αPEP13h, affinity-purified rabbit antiserum to the Pmel17 cytoplasmic domain; anti-EEA1, rabbit antiserum to EEA1 (gift of M. Clague, University of Liverpool, UK; Mills et al. 1998); anti-LAMP1, mouse mAb (PharMingen); 100-3, mouse anti–γ-adaptin mAb (Sigma-Aldrich; Ahle et al. 1988); anticlathrin, mouse mAb (Transduction Laboratories). Rabbit polyclonal antisera to FITC and DNP were from Molecular Probes. Rabbit anti–mouse IgG was from Dakopatt. Iron-loaded human FITC-conjugated transferrin (Tf-FITC) was from Molecular Probes. BSA conjugated to 5-nm gold particles (BSAG) and protein A gold conjugates were purchased from J.W. Slot (Utrecht Medical School, Utrecht, The Netherlands).
Conventional Electron Microscopy
MNT-1 cells were fixed with a mixture of 2% (wt/vol) paraformaldehyde, 1% (wt/vol) glutaraldehyde in 0.2 M phosphate buffer (PB), pH 7.4, postfixed with 1% (wt/vol) OsO4 supplemented with 1.5% (wt/vol) ferrocyanide, dehydrated in ethanol, and embedded in epon. Ultrathin sections were prepared with a Reichert UltracutS ultramicrotome (Leica) and viewed with a TEM CM120 Philips electron microscope after counterstaining with uranyl acetate and lead citrate.
Ultracryomicrotomy and Immunogold Labeling
MNT-1 cells or primary melanocytes were fixed with 2% (wt/vol) paraformaldehyde or with a mixture of 2% (wt/vol) paraformaldehyde and 0.2% (wt/vol) glutaraldehyde in 60 mM Pipes, 25 mM Hepes, 2 mM MgCl2, 10 mM EGTA, pH 6.9, or in PB. Cell pellets were washed with PB, embedded in 10% (wt/vol) gelatin and infused in 2.3 M sucrose (Raposo et al. 1997). Mounted gelatin blocks were frozen in liquid nitrogen and ultrathin sections were prepared with an Ultracut FCS ultracryomicrotome (Leica). Ultrathin cryosections were collected with 2% (vol/vol) methylcellulose, 2.3 M sucrose (Liou et al. 1996) and single, double, or triple immunogold labeled with antibodies and protein A coupled to 5, 10, or 15 nm gold (protein A–gold [PAG] 5, PAG 10, and PAG 15).
Quantitation of Immunogold Labeling
Relative quantitation of the distribution of Pmel17 and TRP1, Pmel17 and EEA1, or Pmel17, TRP1, and LAMP1 was performed on double or triple immunogold-labeled cryosections by counting the number of gold particles labeling each protein in each of the defined compartments taken randomly in 30 cell profiles. Melanosome stages were defined by morphology (see Results), lysosomes were defined by morphology and/or dense labeling for LAMP1, and early endosomes were defined as tubulovesicular structures labeling densely for EEA1. Results are presented as a percentage of the total number of gold particles for each protein in each compartment and represent a mean and standard deviation of two independent experiments.
Uptake of Endocytic Tracers, Antibodies, and DAMP
Uptake of the endocytic tracer BSAG, Tf-FITC, anti-Pmel17 mAbs, and the weak base 3-(2,4-dinitroanilino)-3′-amino-N-methyldipropylamine (DAMP) were performed on living cells before fixation and processing.
Uptake of BSAG.
After washing cells with serum-free RPMI-1640, cells were pulsed for 5 min at 37°C with BSAG (OD 520 nm = 5). Cells were washed with ice-cold medium/5% FBS, and the endocytic tracer was chased for 5, 10, 25, or 115 min. At each time point cells were fixed as indicated above.
Internalization of Tf-FITC.
Cells were washed with serum-free medium and starved for 45 min before incubation with Tf-FITC (60 μg/ml) continuously for 10 or 20 min. After washing with ice-cold medium, cells were fixed.
Internalization of HMB50.
Cells were washed with serum-free medium and incubated for 1 h at 4°C with HMB50 (10 μg/ml) diluted in medium/ 5% FBS. After washing with ice-cold medium, cells were incubated at 37°C for 30 min before fixation.
Incubation with DAMP.
Cells were washed with serum-free medium and incubated with DAMP (30 μM) for 30 min at 37°C. Cells were washed with ice-cold medium and fixed.
Online Supplemental Material
Online supplemental Figure S1 shows the distribution of TRP1 and Pmel17 in MNT-1 cells by immunofluorescence microscopy (IFM). Online supplemental Figure S2 shows the ultrastructural overview of TRP1 and Pmel17 distribution in MNT-1 cells by immunoelectron microscopy (IEM). Online supplemental Figure S3 shows the differential immunolabeling of the Golgi apparatus, coated endosome, and stage II premelanosome with antibodies to the cytoplasmic and lumenal domains of Pmel17. Online supplemental Figure S4 shows the localization of TRP1 and Pmel17 relative to Lamp 1 in MNT-1 cells by IFM. Supplemental figures are available at http://www.jcb.org/cgi/content/full/152/4/809/DC1.
Results
MNT-1 Cells As a Model for Melanosome Development
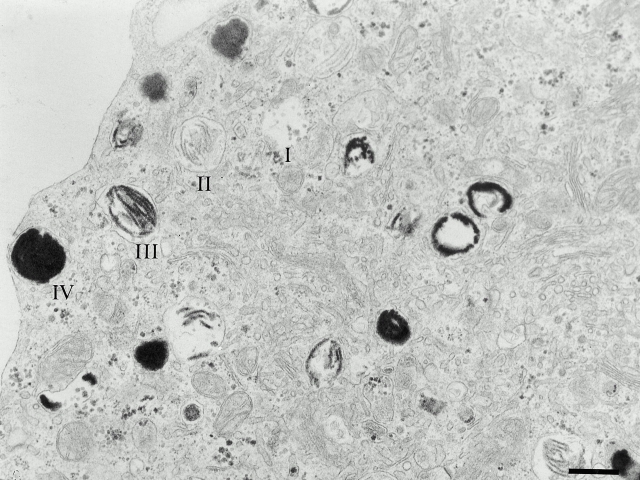
First, we set out to establish a model in which melanosome development could be monitored and manipulated. Melanoma cells vary in pigmentation, protein expression, and morphology. Screening of several pigmented human melanoma cell lines revealed that one of these cell lines, MNT-1, harbored melanosomes and premelanosomes that closely resembled those of untransformed melanocytes by ultrastructure (Fig. 1). These compartments could be classified into the melanosomal stages I–IV, based on morphology and melanin content, that have been defined in primary melanocytes and murine melanoma models (Seiji et al. 1963b). To facilitate description of these compartments, we refer to stage I and II structures as premelanosomes, and stage III and IV structures as mature melanosomes.
Figure 1.
Ultrastructure of MNT-1 cells. IEM analysis of ultrathin sections of epon-embedded MNT-1 cells reveal numerous melanosomes at different stages of maturation throughout the cytoplasm. Structures corresponding to stages I–IV melanosomes are indicated. Bar, 400 nm.
Localization of Pmel17 and TRP1 to Distinct Melanosomal Stages
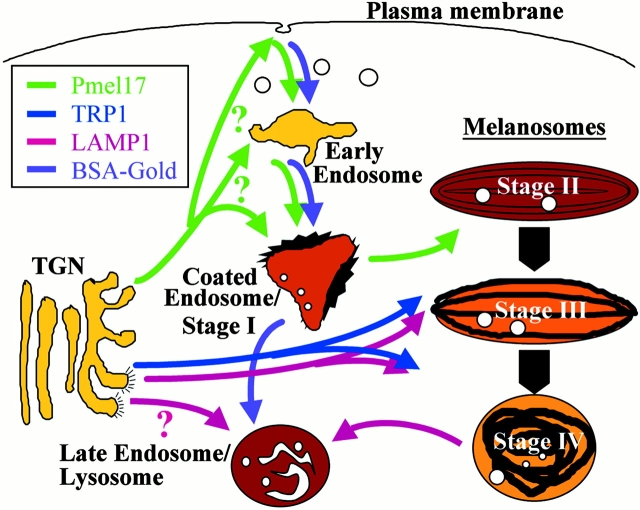
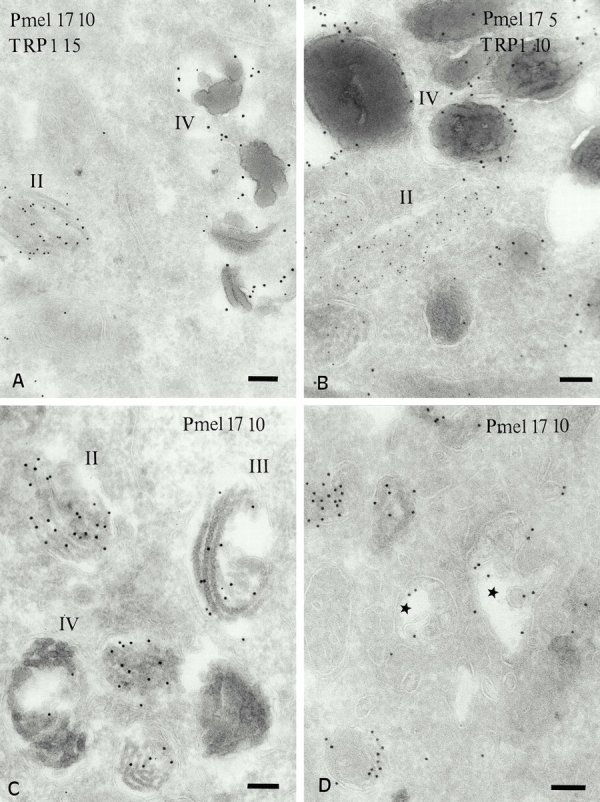
We next analyzed the distribution of melanosomal integral membrane proteins in pigmented cells. IFM analyses of MNT-1 (Figure S1, available at http://www.jcb.org/cgi/content/full/152/4/809/DC1), and other pigmented melanoma cells and melanocytes (not shown) demonstrated that TRP1 and Pmel17 localized to vesicular structures that only partially overlapped. TRP1 staining was brightest in the cell periphery, whereas vesicles containing Pmel17 were evenly distributed throughout the cell. To correlate these structures with morphologically defined melanosomal stages, we analyzed immunogold-labeled ultrathin cryosections of MNT-1 cells by IEM. The anti-Pmel17 mAb HMB50 primarily labels the lumenal space of hypopigmented compartments similar to stage II premelanosomes (Fig. 2; see also Figure S2 A, available at http://www.jcb.org/cgi/content/full/152/4/809/DC1). Gold particles detecting HMB50 (Fig. 2, A–C) or another anti-Pmel17 mAb, HMB45 (Fig. 2 D), often line the striations and underlying membrane vesicles in these compartments. Additional electron-lucent vesicular structures are labeled to a lesser extent with these antibodies (Fig. 2 D; see below), as are Golgi stacks and small vesicles (see below). In contrast, the anti-TRP1 mAb TA99 labels predominantly stage III and IV melanosomes (Fig. 2A and Fig. B; see also Figure S2 B, available at http://www.jcb.org/cgi/content/full/152/4/809/DC1), mostly on the limiting membrane but occasionally on internal membranes. Labeling for TRP1 is also observed in Golgi stacks and associated vesicles; in contrast to Pmel17, which is distributed randomly throughout the Golgi apparatus (see below), TRP1 is frequently enriched on the trans side, particularly in tubular structures that are often coated (Fig. 3). Most of these coated structures are labeled with antibodies to γ-adaptin (Fig. 3B and Fig. C). Tyrosinase is poorly labeled in MNT-1 cells but codistributes with TRP1, predominantly in stage IV melanosomes (not shown). Similar labeling patterns for TRP1 and Pmel17 are observed in other melanoma cells and melanocytes, as exemplified in primary melanocytes from a pigmented nevus (Fig. 2 B). These results extend previous observations suggesting that Pmel17 is a matrix protein of premelanosomes (Kobayashi et al. 1994; Lee et al. 1996), whereas TRP1 is a component of mature melanosomes.
Figure 2.
Distribution of Pmel17 and TRP1 in MNT-1 cells and primary melanocytes. Ultrathin cryosections of MNT-1 cells (A) or cultured primary melanocytes derived from a highly pigmented nevus (B) were double immunogold labeled with HMB50 (anti-Pmel17) and TA99 (anti-TRP1). (C and D) MNT-1 cells were immunogold labeled with the anti-Pmel17 mAbs HMB50 and HMB45, respectively. (A) Labeling for Pmel17 (PAG 10) in internal striations of stage II melanosomes, and labeling for TRP1 (PAG 15) in the limiting membrane of a stage IV melanosome. (B) In primary melanocytes, Pmel17 (PAG 5) is enriched in the lumen of a premelanosome, whereas TRP1 (PAG 10) localizes mainly to mature melanosomes (IV). (C) Labeling for Pmel17 (PAG 10) decreases during progression from stage II to IV. Note the deposits of melanin over the internal striations in the stage III melanosome. (D) Pmel17 (PAG 10) is labeled in compartments morphologically similar to multivesicular endosomes (stars). Bars, 100 nm.
Figure 3.
Distribution of TRP1 in the Golgi and associated vesicles. (A) Ultrathin cryosections of MNT-1 cells were labeled with anti-TRP1 and PAG 5. TRP1 is often enriched in one side of the Golgi apparatus. (B and C) Double labeling of MNT-1 cells for TRP1 (PAG 5) and γ-adaptin (PAG 10). TRP1 positive tubules and vesicles are labeled for γ-adaptin (arrowheads). Bars, 100 nm.
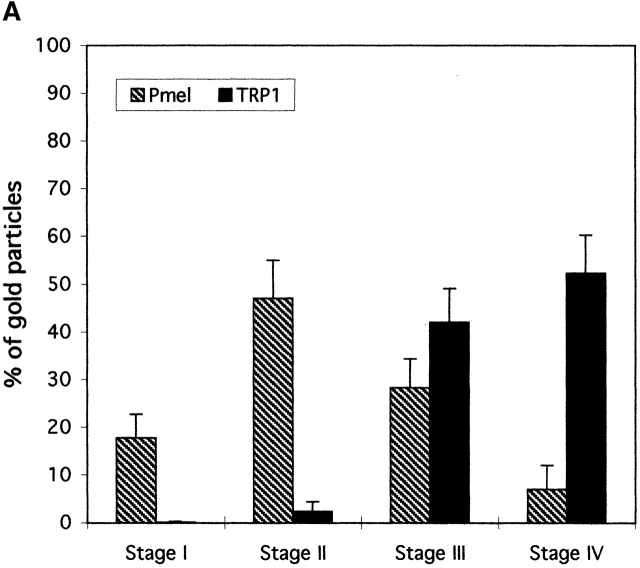
Although Pmel17 and TRP1 are highly enriched in stage II and IV melanosomes, respectively, both proteins can be detected in all melanosomal stages. Therefore, we quantitated the immunolabeling for Pmel17 and TRP1 in melanosomes of different stages (see Fig. 7 A, bar graph). Pmel17 is first enriched in stage I–like structures, as described in more detail below, and accumulates to a higher extent in stage II premelanosomes. In contrast, TRP1 is almost undetectable in stage I structures, and only a small percentage of labeling is observed in stage II. Although stage III and particularly stage IV melanosomes are densely labeled for TRP1, the levels of detectable Pmel17 strongly decrease in these structures. These observations stress that premelanosomes and mature melanosomes are enriched in different subsets of resident melanogenic proteins, but that they may represent endpoints of a progression involving gradual loss of some components, such as Pmel17, and acquisition of other components, such as TRP1 and tyrosinase.
Figure 7.
Quantitative analysis of the distribution of Pmel17 relative to TRP1, EEA1, and LAMP1 in MNT-1 cells. Gold particles labeling either Pmel17 or (A) TRP1, (B) EEA1, or (C) TRP1 or LAMP1 were counted in the different endosomal, melanosomal, or lysosomal compartments in double- (A and B) or triple- (C) labeled ultrathin cryosections of MNT-1 cells. For each set, 30 cell profiles were counted in each of two experiments. Results represent a percentage of the total number of counted gold particles for each marker in the distinct morphologically defined compartments. Only the compartments shown in each experiment were taken into account.
Relationship of Premelanosomes with the Early Endocytic System
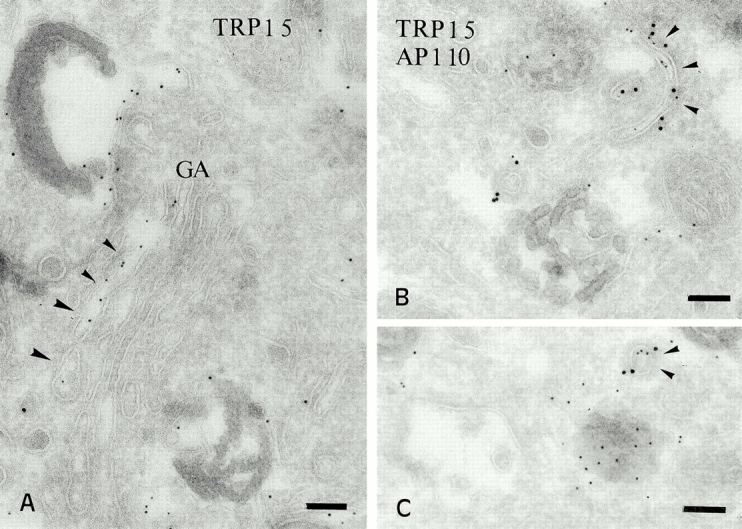
Although most enriched in stage II melanosomes, Pmel17 is also detected in electron-lucent compartments displaying variable amounts of internal membrane vesicles (Fig. 2 D). These structures correspond to stage I premelanosomes and are reminiscent of multivesicular compartments of the endocytic system (Trowbridge et al. 1993). Therefore, we investigated the relationship between these structures and endocytic compartments of MNT-1 cells.
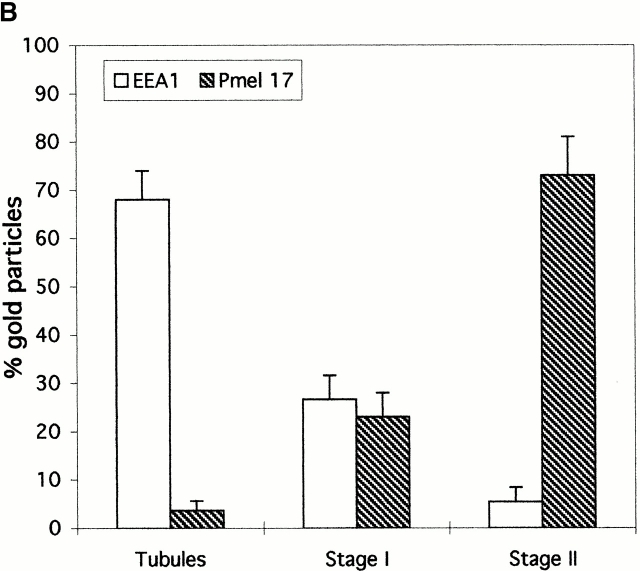
Double immunogold labeling of ultrathin cryosections of MNT-1 cells reveals that Pmel17 and the early endosomal marker, EEA1 (Mu et al. 1995), are both present within two types of electron-lucent compartments (Fig. 4A and Fig. B). Small vesicles and tubules that are strongly labeled for EEA1 are weakly labeled for Pmel17 (Fig. 4A and Fig. B). These structures likely represent early sorting endosomes, as they were also labeled with Tf-FITC and fluid phase tracers internalized for 10 and 5 min, respectively (see below). Labeling for Pmel17 is more intense in the larger stage I–like structures (Fig. 4A and Fig. B), which are observed in all pigmented melanoma cell lines and those primary melanocytes analyzed and are distinguished by the presence of a planar cytoplasmic coat on their surface (Fig. 4 B; see below). Among these coated compartments, those with fewer internal membranes are often also labeled for EEA1; however, the labeling for Pmel17 and EEA1 is often segregated (Fig. 4 A). Those coated compartments with more internal vesicles and the characteristic stage II premelanosomes (containing the bulk of Pmel17) show only sparse labeling for EEA1 (Fig. 4 A). Quantitation of the immunogold labeling (see Fig. 7 B, bar graph) emphasizes that progressive enrichment for Pmel17 correlates with depletion of EEA1, and suggests that the coated Pmel17–EEA1-positive compartment is an intermediate between early endosomes and stage II premelanosomes. We will refer to this stage I–like compartment as the coated endosome.
Figure 4.
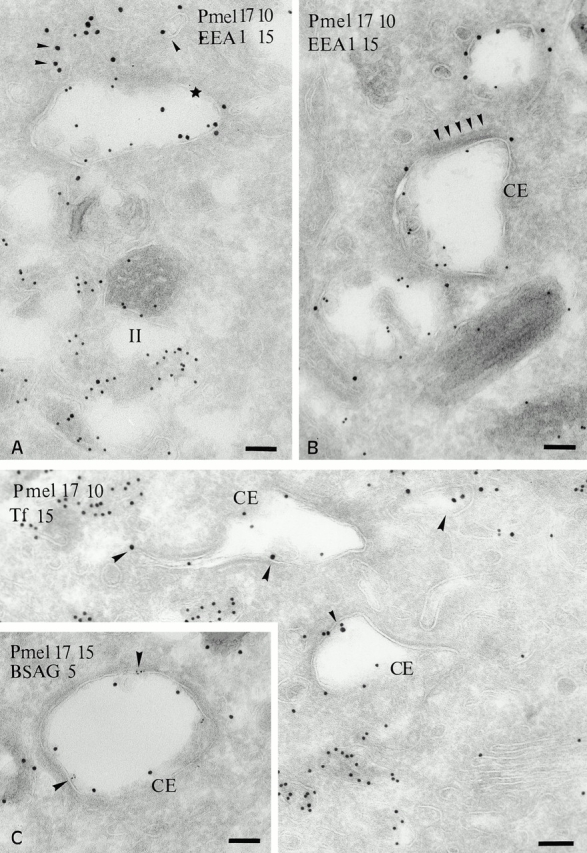
Localization of Pmel17 relative to EEA1 and endocytic tracers. (A and B) Ultrathin cryosections of MNT-1 cells were double labeled with HMB50 (anti-Pmel17; PAG 10) and anti-EEA1 (PAG 15). (A) EEA1 is detected in small vesicles (arrowheads) and on the limiting membrane of a compartment that also contains Pmel17 (star). Note that labeling for Pmel17 and EEA1 is segregated (B) EEA1 (PAG 15) is enriched in electron lucent vesicular compartments that are poorly labeled for Pmel17 (PAG 10). Note the unusual morphology of the endosomal compartments containing the bulk of Pmel17 (CE), which are poorly labeled for EEA1 and display on their cytosolic side an electron dense coat (arrowheads). (C) Cryosection of MNT-1 cells exposed to Tf-FITC for 20 min at 37°C, labeled with anti-FITC antibodies and PAG 15. Tf-FITC is present in small tubulovesicular structures (arrowheads) and in Pmel17-positive (PAG 10) coated endosomes (CE). (Inset in C) MNT-1 cells pulsed with BSAG for 5 min and chased for 10 min. BSAG (5 nm) at this time point is mainly detected in the Pmel17-positive coated endosome (CE). Pmel17 was labeled with HMB50 and PAG 15. Bars, 100 nm.
To confirm the position of the coated endosome within the endocytic pathway, we examined its accessibility to fluid phase endocytic tracers. MNT-1 cells were pulsed for 5 min with BSAG, and then washed and chased for 5 or 10 min. After 5 min of internalization, BSAG is detected in small vesicles and tubules that label only poorly for Pmel17; these structures correspond to the tubules that are densely labeled for EEA1 (not shown), confirming their identity as early endosomes. Practically no BSAG is detected in the coated endosome at this time. In contrast, after a 10-min chase, the coated endosome is accessed by BSAG (Fig. 4 C, inset). This timing correlates with the labeling of recycling endosomes in other cells (Gruenberg and Maxfield 1995); indeed, the coated endosome is accessed by Tf-FITC after 20 min, but not 10 min, of internalization (Fig. 4 C). Although the coated endosome was labeled with markers of the early endocytic pathway, it was poorly labeled for late endosomal proteins, such as cathepsin D and LAMP1 (see below). These results indicate that Pmel17 is first enriched in an early endocytic compartment that is downstream of peripheral tubulovesicular endosomes and accessed by material bound for both late (i.e., BSAG) and recycling (i.e., Tf-FITC) endosomes.
The Coated Endosome Is a Clathrin-coated Intermediate in Premelanosome Formation
Its involvement in the early endocytic pathway and its similarity to stage I premelanosomes suggested that the coated endosome may be an intermediate in melanosome biogenesis and a precursor to stage II premelanosomes. To address this, first, we tested whether Pmel17 accesses endocytic compartments en route to the premelanosome by following the fate of internalized anti-Pmel17 mAb. MNT-1 cells with bound HMB50 at 4°C were washed and incubated for 30 min at 37°C to allow internalization of the Pmel17-bound mAb. Subsequent labeling of ultrathin cryosections with anti-Ig demonstrates that the internalized mAb accumulates in EEA1-positive early endosomal tubules and the coated endosome (Fig. 5 A). Thus, Pmel17 cycles through the cell surface, early endosomes, and the coated endosome during its lifetime. Also, we compared the intercompartmental and subcompartmental distribution of Pmel17 labeled with two antibodies, HMB45 and αPEP13h, that preferentially recognize different subsets of Pmel17 molecules. αPEP13h labels Pmel17 early in the secretory pathway but not in stage II premelanosomes, whereas HMB45 labels Pmel17 only late in the secretory pathway, including stage II premelanosomes (Figure S3, available at http://www.jcb.org/cgi/content/full/152/4/809/DC1; and data not shown). Importantly, both antibodies label the limiting membrane and internal vesicles of the coated endosome (Fig. 2 D; see also Figure S3, available at http://www.jcb.org/cgi/content/full/152/4/809/DC1). The simplest explanation for this pattern is that Pmel17 localization to the coated endosome precedes that to the stage II premelanosome.
Figure 5.
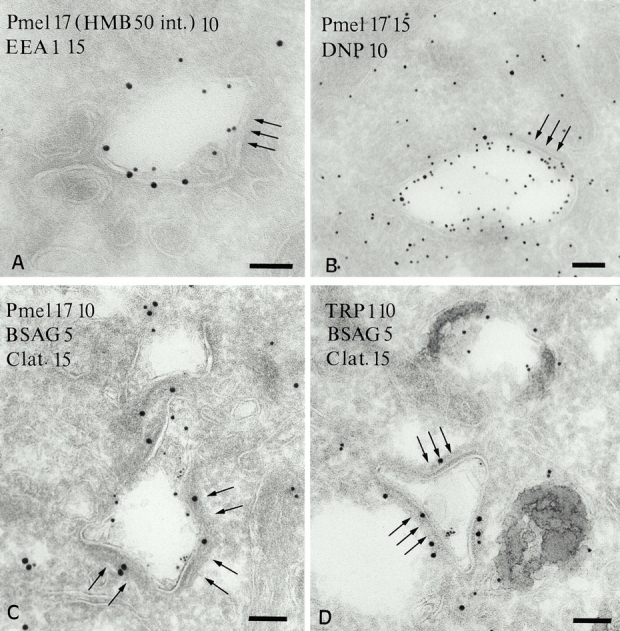
Characterization of the coated endosome in MNT-1 cells. (A) HMB50 (anti-Pmel17) was bound to the cell surface at 4°C and internalized for 30 min at 37°C. Cryosections were labeled with rabbit anti–mouse IgG (PAG 10) to visualize internalized mAb and anti-EEA1 (PAG 15). Internalized Pmel17 accesses the EEA1-positive–coated endosome (arrows) during this time period. (B) Cells were incubated with DAMP for 30 min. Accumulated DAMP was detected with anti-DNP antibodies (PAG 10); Pmel17 was detected with HMB50 (PAG 15). Note the accumulation of DAMP in the coated endosome, indicating that it is acidic. (C) The coated endosome containing Pmel17 (PAG 10) and BSAG (5 nm) internalized for 15 min is labeled on its cytosolic side with an anticlathrin antibody (PAG 15; arrows). (D) TRP1 (PAG 10) is present in mature melanosomes but is not consistently detected in the clathrin-positive coated endosome accessed by BSAG after 15 min (arrows). Bars, 100 nm.
A striking morphological feature of the coated endosome is a dense coat on one or two flat surfaces. This coat consists of planar lattices, since we observe no budding profiles common to endosomal compartments (Stoorvogel et al. 1996). The coated regions often show an inward curvature (Fig. 4A and Fig. C; see also Figure S3 A, available at http://www.jcb.org/cgi/content/full/152/4/809/DC1) and an unusually electron-dense region beneath the coat, reflecting enrichment for peripheral membrane proteins. They are reminiscent of planar clathrin lattices (Maupin and Pollard 1983; Miller et al. 1991; Robinson et al. 1992; Prekeris et al. 1999) or “filamentous plaques” (Futter et al. 1996) observed in some cells. Indeed, coated compartments that labeled for internalized BSAG and Pmel17, but not for TRP1, were labeled for clathrin heavy chain (Fig. 5C and Fig. D) and light chain (not shown) in regions overlapping the coat, as were other vesicular structures. This confirms that the planar coat contains clathrin. Finally, upon probing its relative pH, we found that the coated endosome efficiently accumulates the weak base DAMP (Anderson et al. 1984), indicating that it is an acidic compartment (Fig. 5 B).
Relationship of Melanosomes to the Late Endocytic Pathway
The identification of coated endosomes/stage I premelanosomes as early endocytic compartments raises the popular possibility that melanosome maturation parallels that of lysosomes. Therefore, we tested whether melanosomes and lysosomes could be distinguished in pigmented cells. By IFM, both Pmel17 and TRP1 accumulate in compartments that are distinct from those enriched in the lysosomal integral membrane protein LAMP1 in MNT-1 cells (Figure S4, available at http://www.jcb.org/cgi/content/full/152/4/809/DC1) and other pigmented melanoma cells and primary melanocytes (not shown). LAMP1- and Pmel17-enriched compartments were distributed throughout the cytoplasm, often in close proximity and distinguished from TRP1-enriched compartments which accumulated in the periphery. Often, TRP1 colocalized with weak staining for LAMP1. These data show that pigmented melanocytic cells contain a nonmelanosomal compartment enriched in a lysosomal integral membrane protein.
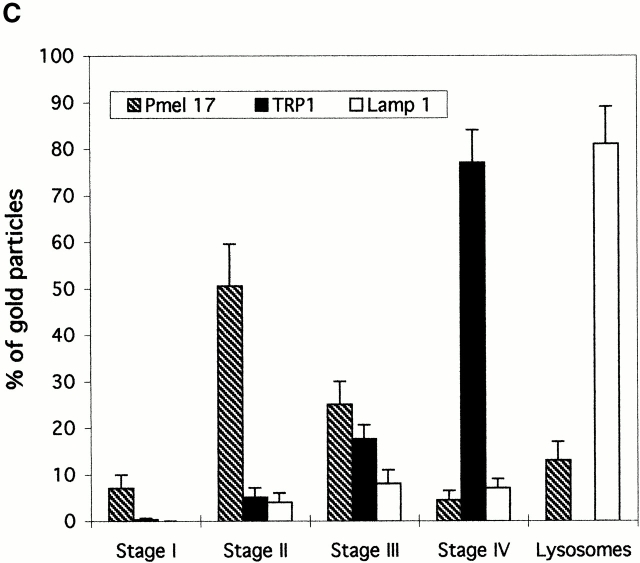
To confirm and extend these results, we qualitatively and quantitatively analyzed the distribution of Pmel17 and TRP1 relative to LAMP1 by immunogold labeling and IEM (Fig. 6, A–C). Labeling for LAMP1 is rarely detected in Pmel17-enriched stage II premelanosomes (Fig. 6 A), indicating that premelanosomes differ from lysosomes. Consistent with the weak staining by IFM, LAMP1 is present to some extent in the TRP1-enriched stage III and IV compartments (Fig. 6, A–C). However, the bulk of LAMP1 is observed in TRP1-depleted compartments that contain internal membranes or electron-dense content distinguishable from polymerized melanin (Fig. 6B and Fig. C). These LAMP1-enriched compartments are morphologically similar to lysosomes (Kornfeld and Mellman 1989), are also enriched in the lysosomal hydrolase cathepsin D (not shown), and are highly acidic as determined by DAMP labeling (not shown; see Fig. 8). They are occasionally labeled for Pmel17 (Fig. 6 B) and rarely for TRP1. Quantitation of these data is shown in Fig. 7 C, and emphasizes that (a) compartments most enriched in LAMP1 are distinct from melanosomes, and (b) the amount of LAMP1, like TRP1, increases during melanosome maturation. Labeling of the lysosomal hydrolase, cathepsin D, largely parallels that of LAMP1 (not shown), indicating that LAMP1 is representative of lysosomal proteins. In addition to differential protein enrichment, stage III and IV melanosomes accumulate DAMP to a lower extent than stage II premelanosomes or morphologically defined lysosomes (Fig. 8A and Fig. B). Together, these observations demonstrate that although mature melanosomes harbor low levels of lysosomal proteins, they do not correspond to the lysosomal compartment in pigmented melanocytic cells.
Figure 6.
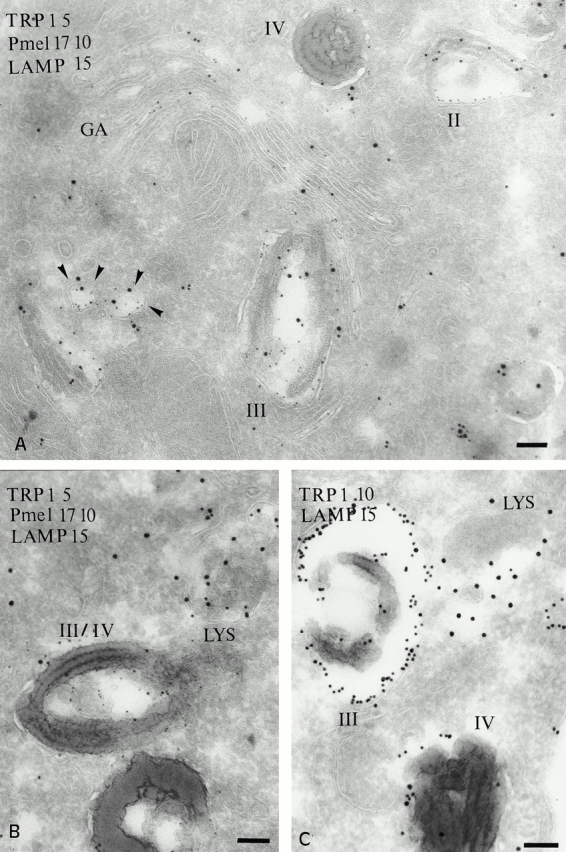
Localization of Pmel17 and TRP1 relative to the lysosomal protein LAMP1. (A and B) Ultrathin cryosections of MNT-1 cells were triple immunogold labeled for LAMP1 (PAG 15), Pmel17 (PAG 10) and TRP 1 (PAG 5). (A) Overview of the Golgi area. Stage II premelanosomes (II) displaying characteristic Pmel17-positive striations do not contain LAMP1. TRP1 is detected in the Golgi apparatus but mainly restricted to the trans-side. Vesicles containing both TRP1 and LAMP1 (arrowheads) are present near the TGN and melanosomes and to some extent in stage III/IV melanosomes (III). (B) Stage III/IV melanosomes (III/IV) are poorly labeled for LAMP1. LAMP1 is highly enriched in electron-dense compartments distinct from melanosomes (LYS). (C) Ultrathin cryosections of MNT-1 cells were double labeled with mAbs to TRP1 (PAG 10) and LAMP1 (PAG 15). TRP1 is present on the limiting membrane of stage III and IV melanosomes. LAMP1 is also detected in the limiting membrane of the mature melanosomes, but the majority of the labeling is observed in less electron-dense lysosomal compartments (LYS). Bars, 100 nm.
Figure 8.
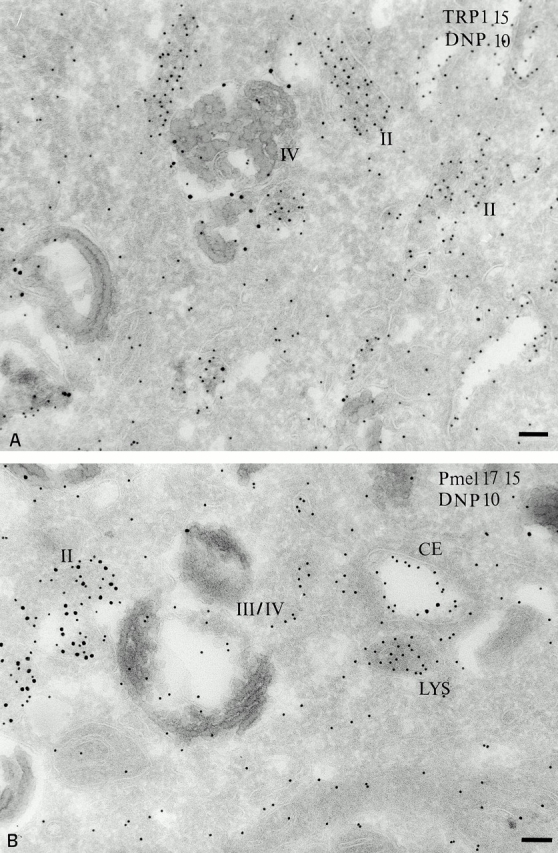
Acidity of melanosomal compartments probed with DAMP. MNT-1 cells were incubated with DAMP for 30 min at 37°C before fixation and processing. (A) DAMP, visualized with anti-DNP antibodies (PAG 10), accumulates primarily in stage II melanosomes displaying characteristic striations. TRP1-positive stage IV melanosomes show less labeling. (B) Double immunogold localization of DAMP (PAG 10) and Pmel17 (PAG 15). DAMP is distributed over the coated endosome, stage II and IV melanosomes, and lysosomes. Bars, 100 nm.
Interestingly, small vesicular structures bearing both TRP1 and LAMP1 are often observed in the Golgi area near melanosomes (Fig. 6 A, arrowheads). Nevertheless, TRP1 is not detected in lysosomes; this is not likely due to degradation within lysosomes, because metabolic pulse–chase analyses indicate that TRP1 in these cells is very long lived (not shown). The LAMP1+ dense lysosomes are generally physically very close to compartments enriched in Pmel17 and/or TRP1 (Fig. 6B and Fig. C), which may facilitate persistent protein sorting between them.
Although the data indicate that melanosomes are not terminal lysosomes, they do not rule out that melanosomes are specialized late endosomes. To define the relationship between melanosomes and the late endocytic pathway, we assessed the accessibility of melanosomes to internalized BSAG at late time points. Similar to the early time points, BSAG could not be detected in melanosomes of any stage even after 25 min or ∼2 h of chase (Fig. 9A and Fig. B), which are the known kinetics of internalization of fluid phase tracers into late endosomes and lysosomes in most cells (Gruenberg and Maxfield 1995). At these time points, BSAG is clearly visualized in late endosomes and lysosomes that are often closely apposed to melanosomes (Fig. 9 B). We considered the possibility that melanosomes are stable organelles, and thus that the majority of them do not access the endocytic pathway during the internalization procedure. However, even after 6 h of internalization, when BSAG is primarily detected in lysosomes, no BSAG is detected in mature melanosomes (not shown). These observations suggest that melanosomal organelles have limited access to fluid phase markers, are not primary endocytic organelles, and have diverged from the late endocytic pathway.
Figure 9.
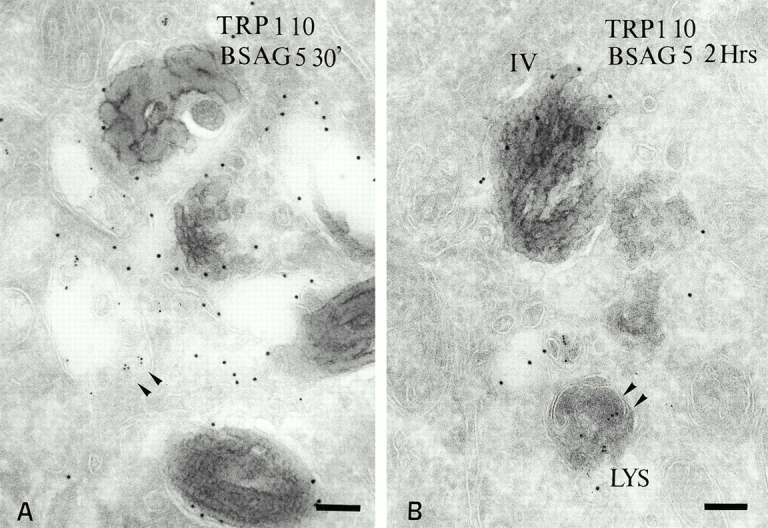
Mature melanosomes in MNT-1 cells are not accessed by endocytic tracers. (A) BSAG (5-nm) was internalized for 30 min. BSAG is present in coated endosomes and multivesicular bodies (arrowheads) situated near mature melanosomes labeled for TRP1 (PAG 10) but does not label the melanosomes. (B) BSAG (5-nm) internalized for 2 h accumulates in electron-dense lysosomes but not in TRP1-positive (PAG 10) melanosomes (IV). Bars, 100 nm.
Discussion
Melanosomes and lysosomes share common characteristics (Orlow 1995; Stinchcombe and Griffiths 1999; Dell'Angelica et al. 2000b), but the relationship between the different melanosomal stages and endocytic organelles of melanocytes has never been fully defined. Our data show that premelanosomes and melanosomes in highly pigmented melanocytic cells are distinct from conventional endosomal organelles but integrated with the endocytic pathway. The differential enrichment of Pmel17 and TRP1 during progressive development of the premelanosome to the mature melanosome suggests that pigmented melanocytes use multiple sorting processes to deliver resident proteins to melanosomes of different stages. Furthermore, these sorting processes must be distinct from those used to deliver material to the late endosomal–lysosomal pathway, since lysosomal proteins accumulate in nonmelanosomal structures. Finally, endocytic cargo destined for premelanosomal and lysosomal compartments are distinguished within a specialized coated endosome that is positioned downstream of early endosomes. The segregation of the melanosomal system from classical endocytic organelles in melanocytes has important implications for the etiology of pigment-related disorders that also affect lysosomal biogenesis.
Differential Enrichment of Proteins in Premelanosomes and Melanosomes
Our data show that progression through the different stages of melanosomal development is accompanied not only by increased melanin deposition but also by differential enrichment in distinct populations of resident proteins (Fig. 7 A). This implies that diverse sorting processes govern the steady state localization of proteins to premelanosomes and mature melanosomes. At least two models may account for this phenomenon. In one model, similar to some models of Golgi stack polarity (Rothman and Wieland 1996), all melanosomal proteins are delivered to a common entry point, but differential rates of anterograde and retrograde traffic establish a gradient of expression across the organelle system. Although not inconsistent with the data, this model does not best explain several observations that support the involvement of discrete transport intermediates in the sorting of premelanosomal and melanosomal proteins. These include (a) the presence of significant levels of Pmel17, but not of TRP1, in early endosomes (Fig. 4 and see below); (b) the enrichment of TRP1, but not Pmel17, in trans-Golgi– and TGN-associated vesicles (Fig. 3 and Fig. 6); and (c) the absence of Pmel17 from coated vesicle fractions that contain TRP1 and tyrosinase (Kobayashi et al. 1994). Although these observations could be explained by differential kinetics of Pmel17 and TRP1 transport through similar intermediates, we favor a second model in which components of the mature melanosome, such as TRP1, are delivered from the TGN, as initially suggested for tyrosinase (Novikoff et al. 1968; Maul and Brumbaugh 1971), once the premelanosome has been established by prior delivery of other components, such as Pmel17, from the endocytic pathway (Fig. 10). The restriction of Pmel17 from late stage melanosomes by this model could be due to epitope masking upon melanin deposition (Berson, J.F., D. Harper, D. Tenza, G. Raposo, M.S. Marks, manuscript submitted for publication; Kobayashi et al. 1994; Donatien and Orlow 1995), degradation in late stage melanosomes, or retrograde transport. More stringent testing will be needed to distinguish between these models.
Figure 10.
Model for protein sorting steps within the endocytic and melanosomal systems in pigmented melanocytic cells. Schematic representation of the endocytic and melanosomal organelles and a working model for the sorting pathways for proteins distributed among them. We propose that Pmel17 (green) is transported to stage II premelanosomes via early endosomes and the stage I premelanosome/coated endosome; transport to the coated endosome may also be direct from the TGN. In contrast, TRP1 (blue) is proposed to be targeted directly from the TGN to a preestablished stage II or III melanosome; continual transport of TRP1 and tyrosinase results in maturation, indicated by broad arrows, of the premelanosome to the stage III and IV melanosome. The deposition of black melanin in these compartments is schematized. Internalized fluid phase markers such as BSAG (violet) are targeted to lysosomes from the coated endosome after passage through early endosomes. LAMP1 (rose) may be sorted to late endosomes/lysosomes directly from the TGN or indirectly via melanosomes; at least a fraction of LAMP1 progresses through this latter pathway. Degree of acidity is indicated by yellow/brown color, with yellow being least acidic and dark brown being most acidic.
Relationship of Melanosomes to the Endocytic Pathway
Our data show for the first time a direct link between the premelanosome and early endocytic compartments. This is supported by the presence of a significant fraction of Pmel17 in early and coated endosomes. The coated endosome, structurally equivalent to the stage I premelanosome (Seiji et al. 1963b), is a post–early endosome precursor to stage II premelanosomes based on (a) the correlation of progressive loss of EEA1 and progressive increase of Pmel17 density from early endosome to coated endosome to stage II premelanosome (Fig. 7 B); (b) the progressive access to BSAG and Tf-FITC from early endosome to coated endosome and the exclusion of these markers from the stage II premelanosome (Fig. 4 and Fig. 5); and (c) labeling of the early and coated endosomes, but not the stage II premelanosome, by the αPEP13h antibody (Figure S3, available at http://www.jcb.org/cgi/content/full/152/4/809/DC1). αPEP13h recognizes the cytoplasmic domain of Pmel17, which becomes inaccessible to antibodies in fixed cells only within late endosomal compartments (Berson, J.F., unpublished observation; not shown). Together, these observations suggest that Pmel17 passes through the early endosome and the coated endosome before its localization in the stage II premelanosome (Fig. 10) and indicate that premelanosomes derive from the endocytic pathway rather than from “smooth endoplasmic reticulum membranes” as interpreted from early morphological observations (Maul 1969).
The colocalization of BSAG and Pmel17 in the coated endosome at early time points and their segregation at later time points (Fig. 4, Fig. 5, and Fig. 9) suggests that this compartment is a critical sorting point between the endocytic and premelanosomal pathways (Fig. 10). This sorting may be required for the segregation of proteins required for the morphogenesis and function of specialized endosomal organelles like the melanosome. The segregation from endosomes, rather than from the TGN, may reflect the requirement for acidification through a vacuolar ATPase in organelles such as premelanosomes, as supported by the efficient DAMP accumulation in coated endosomes and stage II premelanosomes (Fig. 5 and Fig. 8) and the disruption of melanosome biogenesis by inhibitors of the vacuolar ATPase (data not shown). The segregation from endosomes may also reflect a requirement for the formation of multivesicular bodies in the biogenesis of the melanosome, as suggested by several studies (Turner et al. 1975; Jimbow et al. 1979; Berson, J.F., D. Harper, D. Tenza, G. Raposo, M.S. Marks, submittedmanuscript for publication).
The flat clathrin-containing lattices of the coated endosome are similar to those observed under some conditions at the cell surface (Maupin and Pollard 1983; Heuser 1989; Miller et al. 1991) and on syntaxin 7–positive endosomes (Prekeris et al. 1999), but are particularly well organized in melanocytic cells. They are distinguished by an electron-dense region between the coat and the membrane. Although we could not detect coat-associated AP1 or AP3 adaptors, the lattices may have interfered with their detection. These specialized lattices likely facilitate sorting of lysosome- and premelanosome-bound cargo, perhaps through adaptors. Since Pmel17 localizes to the lumen of the coated endosome and downstream compartments, we speculate that the coats do not bind to Pmel17. Rather, they may either retain and mask components of the premelanosomal limiting membrane, permitting other cargo to be released to late endosomes and lysosomes, and/or facilitate the clustering and extraction of proteins bound for these other compartments, permitting concentration of Pmel17 and other melanogenic proteins in a forming premelanosome, as suggested for nascent secretory granules (Arvan and Castle 1998) and maturing transcytotic vesicles (Gibson et al. 1998). By analogy to similar structures proposed to participate in fusion at the junction of multivesicular endosomes and lysosomes in HEp-2 cells (Futter et al. 1996), the coats may also temporally restrict interactions of the forming premelanosome with conventional late endosomes or lysosomes until premelanosome formation is complete before removal of lysosome-bound cargo.
Are all melanosomal proteins sorted through early endosomes? TRP1 can be secreted and detected at the plasma membrane (Xu et al. 1997), and tyrosinase mediates internalization of surface-bound antibodies (Blagoveshchenskaya et al. 1999; Calvo et al. 1999; Simmen et al. 1999), suggesting that some of these molecules access the endocytic pathway. Nevertheless, we detected only Pmel17, and not TRP1, in endocytic compartments. In contrast, TRP1, like LAMP1 but unlike Pmel17, accumulated at the TGN and AP1-coated vesicles (Fig. 3 and Fig. 6). Although these observations may reflect a limitation of our TRP1- and γ-adaptin–specific mAbs or a failure to retain TRP1 within coated endosomes during transport, they are consistent with the notion (Kobayashi et al. 1994) that Pmel17 and TRP1 are delivered to melanosomes via different pathways (Fig. 10). Cell surface and internalized TRP1 and tyrosinase may reflect either a small fraction of missorted molecules or molecules awaiting reinternalization after melanin release.
Melanosomes in Highly Pigmented Melanocytes and Melanomas Are Not Lysosomes
Perhaps our most surprising finding is that melanosomes and lysosomes were not the same in pigmented melanocytic cells. LAMP1 and cathepsin D, although present to a small extent in melanosomes, were largely found at steady state in separate structures with morphological characteristics of lysosomes. These compartments were more acidic than mature melanosomes based on DAMP accumulation, were the destination for internalized BSAG, and contained insignificant levels of TRP1 and tyrosinase and only low levels of Pmel17. Our findings indicate that melanosomes are not merely modified lysosomes, and that sorting mechanisms within pigmented melanocytes distinguish between lysosomal and melanosomal cargo.
Our conclusions contrast with those drawn from several earlier studies (summarized in Orlow 1995). However, the data are not necessarily discordant. For example, acid phosphatase, a lysosomal enzyme, was detected histochemically within melanosomes in numerous studies; since these studies used nonquantitative techniques, however, they did not address whether the bulk of activity was present in distinct structures (e.g., see Seiji and Kikuchi 1969), which would be consistent with our data, or whether a melanosome-specific protein possessed acid phosphatase activity. Furthermore, many of these studies analyzed heterogeneous cell populations, and thus the activity detected may have represented phagolysosomes with phagocytosed melanosomes in keratinocytes, macrophages, or even melanocytes (Seiji and Iwashia 1965). In other studies, tyrosinase or TRP1 cofractionated with lysosomal enzyme activity by subcellular fractionation (Seiji and Iwashia 1965; Diment et al. 1995; Orlow et al. 1993), but the “melanosomal” fractions were not assessed for purity and may have contained late endosomes and/or lysosomes. Future studies will be devoted to biochemical fractionation of the morphologically distinct compartments that we observe by microscopy.
In addition to the considerations above, it is likely that the segregation of melanosomal and lysosomal proteins is regulated by melanocyte differentiation and/or by differences in cell type, tissue of origin, or degree or type of pigmentation. In support of this notion, melanosomal enzymes like tyrosinase and TRP1 localize to late endosomes/lysosomes when expressed in nonpigmented cells (Bouchard et al. 1989; Vijayasaradhi et al. 1995; Calvo et al. 1999; Simmen et al. 1999). Furthermore, different cohorts of melanosomal proteins are expressed in melanocytes during synthesis of pheomelanins and eumelanins (Kobayashi et al. 1995; Furumura et al. 1998), and hypopigmented melanoma cells or brown melanocytes seem less efficient at segregating these from lysosomal proteins (Marks, M.S., unpublished data). RPE have morphologically distinct melanosomes (for review see Schraermeyer and Heimann 1999) that may fuse more readily with lysosomes. A developmental difference in lysosome/melanosome segregation could explain why phagocytosed latex particles could be observed in melanosomes of RPE and choroidal melanocytes (Schraermeyer and Heimann 1999), whereas BSAG failed to access premelanosomes or melanosomes in MNT-1 cells after endocytosis (Fig. 4, Fig. 5, and Fig. 9). These differing results may also, however, be due to differences in the technique. For example, autophagic events or kiss-and-run fusion between melanosomes and lysosomes may permit transfer of material during the long chase times used in many of the earlier studies, consistent with poor preservation of MNT-1 cells after long chase times with BSAG (Raposo, G., unpublished observation; data not shown).
Our data suggest that lysosomal sorting occurs from at least two sites in these cells. One is the coated endosome, from which internalized cargo such as BSAG is sorted to lysosomes (Fig. 10). LAMP1, however, is not observed in this compartment at steady state, although we cannot rule out its rapid transport through it. Thus, we suspect that LAMP1 is sorted to late endosomes/lysosomes directly from the TGN (Fig. 10). LAMP1 and TRP1 likely cosegregate during post-Golgi sorting, since they often colocalize in vesicular structures near the Golgi apparatus or melanosomes, and some LAMP1 is found in melanosomes at steady state (Fig. 6). Thus, both lysosomal proteins (like LAMP1) and melanosomal proteins (like TRP1) may be targeted from the TGN (or endosomes) to maturing melanosomes, implicating the melanosome as the second site of lysosomal/melanosomal protein sorting. If this were true, then either LAMP1 contains additional information for sorting from melanosomes to lysosomes, or TRP1 contains retention information (Fig. 10)—hypotheses that can be distinguished by analysis of chimeric proteins with distinct lysosomal and melanosomal protein domains. The close proximity that we have observed of lysosomes to melanosomes may facilitate removal of lysosomal components from a maturing melanosome, perhaps by a kiss-and-run type of mechanism proposed for late endosome–lysosome fusion (Luzio et al. 2000). It is tempting to speculate that HPS1, a protein localized to vesicular structures near the Golgi apparatus and on the membrane of mature melanosomes (Oh et al. 2000), plays a role in such sorting at the level of the melanosome.
In addition to the differences in accumulated proteins, melanosomes and lysosomes differed in pH. Using DAMP accumulation as an indicator, progression from premelanosomes to mature melanosomes was accompanied by a decrease in acidity (Fig. 5 and Fig. 8). This alkalization may be driven by the progressive removal of a vacuolar ATPase during melanosome maturation, as proposed above for LAMP1, or by inactivation of a premelanosome-specific proton pump, such as the P transporter (Puri et al. 2000). A progressive increase in pH might favor higher activity of tyrosinase and other melanogenic enzymes in the low pH environment of early stage melanosomes (Devi et al. 1987), facilitating generation of melanin, and then lower activity as product accumulates in more mature melanosomal structures. One caveat to our conclusions is that detection of DAMP might be hindered by melanin polymerization or high levels of melanin intermediates in the melanosome. Indeed, melanosomal fractions have a very low pH (Bhatnagar et al. 1993), although these preparations may also contain lysosomes. Further work will be needed to rectify these conflicting conclusions.
Implications for Lysosomal Transport Diseases
Our data have important implications for the etiology of diseases affecting the morphogenesis and function of melanosomes and lysosomes. Patients with diseases such as Chediak-Higashi syndrome (CHS; Introne et al. 1999) and HPS (Shotelersuk and Gahl 1998) show primary defects in the function of particular lysosome-related organelles (Dell'Angelica et al. 2000b), including melanosomes. The products of the genes affected by these diseases, CHS1 (lyst and beige) in CHS and HPS1 or AP3 complex subunits in HPS, are thought to influence protein transport routes to lysosomes and lysosome-like compartments (Faigle et al. 1998; Kantheti et al. 1998; Dell'Angelica et al. 1999; Lem et al. 1999; Dell'Angelica et al. 2000a). Our data now provide multiple sites at which protein sorting contributes to melanosome biogenesis, any one of which could be directly affected by these gene defects. It will be important to analyze the functional effects of HPS- and CHS-related mutations at each sorting step to determine the precise role of CHS- and HPS-related gene products in melanogenesis, to understand their role in the biogenesis of other lysosome-like organelles and to begin to define treatments for these diseases.
Supplemental Material
Acknowledgments
We thank M. Clague, I. Mills, V.J. Hearing, C. Figdor, M. Herlyn, R. Finko, and W. Storkus for providing reagents; V.J. Hearing and E. Appella for helpful discussions; E. Dell'Angelica for critical comments on the manuscript; T. Coudert and D. Morineau for help with electron micrographs; and D. Louvard for continuing support.
This work was supported by National Institutes of Health (NIH) grant R01 EY-12207 from the National Eye Institute to M.S. Marks and by Association pour la Recherche sur le Cancer subvention number 9013 to G. Raposo. J.F. Berson was partly supported by NIH training grant T32 CA-09140 and by a fellowship from the American Cancer Society (number PF-99-336-01-CIM).
Footnotes
The online version of this article contains supplemental material.
Diane M. Murphy's present address is National Institute of Mental Health, National Institutes of Health, 6001 Executive Blvd., Rockville, MD 20852.
Abbreviations used in this paper: CHS, Chediak-Higashi syndrome; HPS, Hermansky-Pudlak syndrome; IEM, immunoelectron microscopy; IFM, immunofluorescence microscopy; PAG, protein A–gold; RPE, retinal pigment epithelial cell; Tf-FITC, FITC-conjugated transferrin.
References
- Ahle S., Mann A., Eichelsbacher U., Ungewickell E. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO (Eur. Mol. Biol. Organ.) J. 1988;7:919–929. doi: 10.1002/j.1460-2075.1988.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.G., Falck J.R., Goldstein J.L., Brown M.S. Visualization of acidic organelles in intact cells by electron microscopy. Proc. Natl. Acad. Sci. USA. 1984;81:4838–4842. doi: 10.1073/pnas.81.15.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvan P., Castle D. Sorting and storage during secretory granule biogenesislooking backward and looking forward. Biochem. J. 1998;332:593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar V., Anjaiah S., Puri N., Darshanam N.A., Ramaiah A. pH of melanosomes of B16 murine melanoma is acidicits physiological importance in the regulation of melanin biosynthesis. Arch. Biochem. Biophys. 1993;307:183–192. doi: 10.1006/abbi.1993.1577. [DOI] [PubMed] [Google Scholar]
- Blagoveshchenskaya A.D., Hewitt E.W., Cutler D.F. Di-leucine signals mediate targeting of tyrosinase and synaptotagmin to synaptic-like microvesicles within PC12 cells. Mol. Biol. Cell. 1999;10:3979–3990. doi: 10.1091/mbc.10.11.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissy R.E., Moellmann G.E., Halaban R. Tyrosinase and acid phosphatase activities in melanocytes from avian albinos. J. Invest. Dermatol. 1987;88:292–300. doi: 10.1111/1523-1747.ep12466164. [DOI] [PubMed] [Google Scholar]
- Bouchard B., Fuller B.B., Vijayasaradhi S., Houghton A.N. Induction of pigmentation in mouse fibroblasts by expression of human tyrosinase cDNA. J. Exp. Med. 1989;169:2029–2042. doi: 10.1084/jem.169.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo P.A., Frank D.W., Bieler B.M., Berson J.F., Marks M.S. A cytoplasmic sequence in human tyrosinase defines a second class of di-leucine-based sorting signals for late endosomal and lysosomal delivery. J. Biol. Chem. 1999;274:12780–12789. doi: 10.1074/jbc.274.18.12780. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica E.C., Shotelersuk V., Aguilar R.C., Gahl W.A., Bonifacino J.S. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the β3A subunit of the AP-3 adaptor. Mol. Cell. 1999;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica E.C., Aguilar R.C., Wolins N., Hazelwood S., Gahl W.A., Bonifacino J.S. Molecular characterization of the protein encoded by the Hermansky-Pudlak syndrome type 1 gene J. Biol. Chem. 275 2000. 1300 1306a [DOI] [PubMed] [Google Scholar]
- Dell'Angelica E.C., Mullins C., Caplan S., Bonifacino J.S. Lysosome-related organelles FASEB J. 14 2000. 1265 1278b [DOI] [PubMed] [Google Scholar]
- Devi C.C., Tripathi R.K., Ramaiah A. pH-dependent interconvertible allosteric forms of murine melanoma tyrosinase. Physiological implications. Eur. J. Biochem. 1987;166:705–711. doi: 10.1111/j.1432-1033.1987.tb13569.x. [DOI] [PubMed] [Google Scholar]
- Diment S., Eidelman M., Rodriguez G.M., Orlow S.J. Lysosomal hydrolases are present in melanosomes and are elevated in melanizing cells. J. Biol. Chem. 1995;270:4213–4215. doi: 10.1074/jbc.270.9.4213. [DOI] [PubMed] [Google Scholar]
- Donatien P.D., Orlow S.J. Interaction of melanosomal proteins with melanin. Eur. J. Biochem. 1995;232:159–164. doi: 10.1111/j.1432-1033.1995.tb20794.x. [DOI] [PubMed] [Google Scholar]
- Faigle W., Raposo G., Tenza D., Pinet V., Vogt A.B., Kropshofer H., Fischer A., de Saint-Basile G., Amigorena S. Deficient peptide loading and MHC class II endosomal sorting in a human genetic immunodeficiency diseasethe Chediak-Higashi syndrome. J. Cell Biol. 1998;141:1121–1134. doi: 10.1083/jcb.141.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumura M., Sakai C., Potterf S.B., Vieira W.D., Barsh G.S., Hearing V.J. Characterization of genes modulated during pheomelanogenesis using differential display. Proc. Natl. Acad. Sci. USA. 1998;95:7374–7378. doi: 10.1073/pnas.95.13.7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter C.E., Pearse A., Hewlett L.J., Hopkins C.R. Multivesicular endosomes containing internalized EGF–EGF receptor complexes mature and then fuse directly with lysosomes. J. Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Futter C.E., Maxwell S., Allchin E.H., Shipman M., Kraehenbuhl J.P., Domingo D., Odorizzi G., Trowbridge I.S., Hopkins C.R. Sorting mechanisms regulating membrane protein traffic in the apical transcytotic pathway of polarized MDCK cells. J. Cell Biol. 1998;143:81–94. doi: 10.1083/jcb.143.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J., Maxfield F.R. Membrane transport in the endocytic pathway. Curr. Opin. Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Heuser J. Effects of cytoplasmic acidification on clathrin lattice morphology. J. Cell Biol. 1989;108:401–411. doi: 10.1083/jcb.108.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Introne W., Boissy R.E., Gahl W.A. Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol. Genet. Metab. 1999;68:283–303. doi: 10.1006/mgme.1999.2927. [DOI] [PubMed] [Google Scholar]
- Jimbow K., Oikawa O., Sugiyama S., Takeuchi T. Comparison of eumelanogenesis in retinal and follicular melanocytes; role of vesiculo-globular bodies in melanosome differentiation. J. Invest. Dermatol. 1979;73:278–284. doi: 10.1111/1523-1747.ep12531650. [DOI] [PubMed] [Google Scholar]
- Kantheti P., Qiao X., Diaz M.E., Peden A.A., Meyer G.E., Carskadon S.L., Kapfhamer D., Sufalko D., Robinson M.S., Noebels J.L., Burmeister M. Mutation in AP-3δ in the mocha mouse links endosomal transport to storage deficiency in platelets, melanosomes, and synaptic vesicles. Neuron. 1998;21:111–122. doi: 10.1016/s0896-6273(00)80519-x. [DOI] [PubMed] [Google Scholar]
- King R.A., Hearing V.J., Creel D.J., Oetting W.S. Albinism. In: Scriver C.R., Beaudet A.L., Sly W.S., Valle D., editors. The Metabolic and Molecular Bases of Inherited Disease. Vol. 3. McGraw-Hill, Inc; New York: 1995. pp. 4353–4392. [Google Scholar]
- Kobayashi T., Urabe K., Orlow S.J., Higashi K., Imokawa G., Kwon B.S., Potterf B., Hearing V.J. The Pmel 17/silver locus protein. Characterization and investigation of its melanogenic function. J. Biol. Chem. 1994;269:29198–29205. [PubMed] [Google Scholar]
- Kobayashi T., Vieira W.D., Potterf B., Sakai C., Imokawa G., Hearing V.J. Modulation of melanogenic protein expression during the switch from eu- to pheomelanogenesis. J. Cell Sci. 1995;108:2301–2309. doi: 10.1242/jcs.108.6.2301. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Mellman I. The biogenesis of lysosomes. Annu. Rev. Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Lee Z.H., Hou L., Moellmann G., Kuklinska E., Antol K., Fraser M., Halaban R., Kwon B.S. Characterization and subcellular localization of human Pmel 17/silver, a 100-kDa (pre)melanosomal membrane protein associated with 5,6,-dihydroxyindole-2-carboxylic acid (DHICA) converting activity. J. Invest. Dermatol. 1996;106:605–610. doi: 10.1111/1523-1747.ep12345163. [DOI] [PubMed] [Google Scholar]
- Lem L., Riethof D.A., Scidmore-Carlson M., Griffiths G.M., Hackstadt T., Brodsky F.M. Enhanced interaction of HLA-DM with HLA-DR in enlarged vacuoles of hereditary and infectious lysosomal diseases. J. Immunol. 1999;162:523–532. [PubMed] [Google Scholar]
- Liou W., Geuze H.J., Slot J.W. Improving structural integrity of cryosections for immunogold labeling. Histochem. Cell Biol. 1996;106:41–58. doi: 10.1007/BF02473201. [DOI] [PubMed] [Google Scholar]
- Luzio J.P., Rous B.A., Bright N.A., Pryor P.R., Mullock B.M., Piper R.C. Lysosome-endosome fusion and lysosome biogenesis. J. Cell Sci. 2000;113:1515–1524. doi: 10.1242/jcs.113.9.1515. [DOI] [PubMed] [Google Scholar]
- Maul G.G. Golgi-melanosome relationship in human melanoma in vitro. J. Ultrastruct. Res. 1969;26:163–176. doi: 10.1016/s0022-5320(69)90042-2. [DOI] [PubMed] [Google Scholar]
- Maul G.G., Brumbaugh J.A. On the possible function of coated vesicles in melanogenesis of the regenerating fowl feather. J. Cell Biol. 1971;48:41–48. doi: 10.1083/jcb.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupin P., Pollard T.D. Improved preservation and staining of HeLa cell actin filaments, clathrin-coated membranes, and other cytoplasmic structures by tannic acid-glutaraldehyde-saponin fixation. J. Cell Biol. 1983;96:51–62. doi: 10.1083/jcb.96.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K., Shipman M., Trowbridge I.S., Hopkins C.R. Transferrin receptors promote the formation of clathrin lattices. Cell. 1991;65:621–632. doi: 10.1016/0092-8674(91)90094-f. [DOI] [PubMed] [Google Scholar]
- Mills I.G., Jones A.T., Clague M.J. Involvement of the endosomal autoantigen EEA1 in homotypic fusion of early endosomes. Curr. Biol. 1998;8:881–884. doi: 10.1016/s0960-9822(07)00351-x. [DOI] [PubMed] [Google Scholar]
- Mu F.T., Callaghan J.M., Steele-Mortimer O., Stenmark H., Parton R.G., Campbell P.L., McCluskey J., Yeo J.P., Tock E.P., Toh B.H. EEA1, an early endosome-associated protein. EEA1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J. Biol. Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- Novikoff A.B., Albala A., Biempica L. Ultrastructural and cytochemical observations on B-16 and Harding-Passey mouse melanomas. The origin of premelanosomes and compound melanosomes. J. Histochem. Cytochem. 1968;16:299–319. doi: 10.1177/16.5.299. [DOI] [PubMed] [Google Scholar]
- Oh J., Liu Z.X., Feng G.H., Raposo G., Spritz R.A. The Hermansky-Pudlak syndrome (HPS) protein is part of a high molecular weight complex involved in biogenesis of early melanosomes. Hum. Mol. Genet. 2000;9:375–385. doi: 10.1093/hmg/9.3.375. [DOI] [PubMed] [Google Scholar]
- Orlow S.J. Melanosomes are specialized members of the lysosomal lineage of organelles. J. Invest. Dermatol. 1995;105:3–7. doi: 10.1111/1523-1747.ep12312291. [DOI] [PubMed] [Google Scholar]
- Orlow S.J., Boissy R.E., Moran D.J., Pifko-Hirst S. Subcellular distribution of tyrosinase and tyrosinase-related protein-1implications for melanosomal biogenesis. J. Invest. Dermatol. 1993;100:55–64. doi: 10.1111/1523-1747.ep12354138. [DOI] [PubMed] [Google Scholar]
- Prekeris R., Yang B., Oorschot V., Klumperman J., Scheller R.H. Differential roles of syntaxin 7 and syntaxin 8 in endosomal trafficking. Mol. Biol. Cell. 1999;10:3891–3908. doi: 10.1091/mbc.10.11.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri N., Gardner J.M., Brilliant M.H. Aberrant pH of melanosomes in Pink-Eyed Dilution (p) mutant melanocytes. J. Invest. Dermatol. 2000;115:607–613. doi: 10.1046/j.1523-1747.2000.00108.x. [DOI] [PubMed] [Google Scholar]
- Raposo G., Kleijmeer M.J., Posthuma G., Slot J.W., Geuze H.J. Immunogold labeling of ultrathin cryosectionsapplication in immunology. In: Herzenberg L.A., Weir D., Herzenberg L.A., Blackwell C., editors. Handbook of Experimental Immunology. Vol. 4. Blackwell Science, Inc; Cambridge, MA: 1997. pp. 1–11. [Google Scholar]
- Robinson L.J., Pang S., Harris D.S., Heuser J., James D.E. Translocation of the glucose transporter (GLUT4) to the cell surface in permeabilized 3T3-L1 adipocyteseffects of ATP insulin, and GTPγS and localization of GLUT4 to clathrin lattices. J. Cell Biol. 1992;117:1181–1196. doi: 10.1083/jcb.117.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J.E., Wieland F.T. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Schraermeyer U., Heimann K. Current understanding on the role of retinal pigment epithelium and its pigmentation. Pigment Cell Res. 1999;12:219–236. doi: 10.1111/j.1600-0749.1999.tb00755.x. [DOI] [PubMed] [Google Scholar]
- Seiji M., Iwashia S. Intracellular localization of tyrosinase and site of melanin formation in melanocyte. J. Invest. Dermatol. 1965;45:305–314. doi: 10.1038/jid.1965.135. [DOI] [PubMed] [Google Scholar]
- Seiji M., Fitzpatrick T.M., Simpson R.T., Birbeck M.S.C. Chemical composition and terminology of specialized organelles (melanosomes and melanin granules) in mammalian melanocytes Nature 197 1963. 1082 1084a [DOI] [PubMed] [Google Scholar]
- Seiji M., Shimao K., Birbeck M.S.C., Fitzpatrick T.B. Subcellular localization of melanin biosynthesis Ann. NY Acad. Sci. 100 1963. 497 533b [PubMed] [Google Scholar]
- Seiji M., Kikuchi A. Acid phosphatase activity in melanosomes. J. Invest. Dermatol. 1969;52:212–216. doi: 10.1038/jid.1969.33. [DOI] [PubMed] [Google Scholar]
- Shotelersuk V., Gahl W.A. Hermansky-Pudlak syndromemodels for intracellular vesicle formation. Mol. Genet. Metab. 1998;65:85–96. doi: 10.1006/mgme.1998.2729. [DOI] [PubMed] [Google Scholar]
- Simmen T., Schmidt A., Hunziker W., Beermann F. The tyrosinase tail mediates sorting to the lysosomal compartment in MDCK cells via a di-leucine and a tyrosine-based signal. J. Cell Sci. 1999;112:45–53. doi: 10.1242/jcs.112.1.45. [DOI] [PubMed] [Google Scholar]
- Stinchcombe J.C., Griffiths G.M. Regulated secretion from hemopoietic cells. J. Cell Biol. 1999;147:1–5. doi: 10.1083/jcb.147.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W., Oorschot V., Geuze H.J. A novel class of clathrin-coated vesicles budding from endosomes. J. Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson T.M., Mattes M.J., Roux L., Old L.J., Lloyd K.O. Pigmentation-associated glycoprotein of human melanomas and melanocytesdefinition with a mouse monoclonal antibody. J. Invest. Dermatol. 1985;85:169–174. doi: 10.1111/1523-1747.ep12276608. [DOI] [PubMed] [Google Scholar]
- Thomson W., MacKie R.M. Comparison of five antimelanoma antibodies for identification of melanocytic cells on tissue sections in routine dermatopathology. J. Am. Acad. Dermatol. 1989;21:1280–1284. doi: 10.1016/s0190-9622(89)70344-3. [DOI] [PubMed] [Google Scholar]
- Trowbridge I.S., Collawn J.F., Hopkins C.R. Signal-dependent membrane protein trafficking in the endocytic pathway. Ann. Rev. Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- Turner W.A.J., Taylor J.D., Tchen T.T. Melanosome formation in the goldfishthe role of multivesicular bodies. J. Ultrastruct. Res. 1975;51:16–31. doi: 10.1016/s0022-5320(75)80004-9. [DOI] [PubMed] [Google Scholar]
- Vijayasaradhi S., Xu Y.Q., Bouchard B., Houghton A.N. Intracellular sorting and targeting of melanosomal membrane proteinsidentification of signals for sorting of the human brown locus protein, gp75. J. Cell Biol. 1995;130:807–820. doi: 10.1083/jcb.130.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J.M., Colton T.L. Targeting of an intestinal apical endosomal protein to endosomes in nonpolarized cells. J. Cell Biol. 1997;136:319–330. doi: 10.1083/jcb.136.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Setaluri V., Takechi Y., Houghton A.N. Sorting and secretion of a melanosome membrane protein, gp75/TRP1. J. Invest. Dermatol. 1997;109:788–795. doi: 10.1111/1523-1747.ep12340971. [DOI] [PubMed] [Google Scholar]
- Yoshimori T., Keller P., Roth M.G., Simons K. Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J. Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.